GRAMMAR-Lambda Delivers Efficient Understanding of the Genetic Basis for Head Size in Catfish
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Profile and Data Preparation
2.2. Statistical Analysis
2.3. GRAMMAR-Lambda Implementation
2.4. Multi-Trait GWAS and Epistasis Analysis Using GMAT Software
3. Results
3.1. Genome-Wide Association Study Analysis
3.2. Multi-Trait Genome-Wide Association Study Analysis
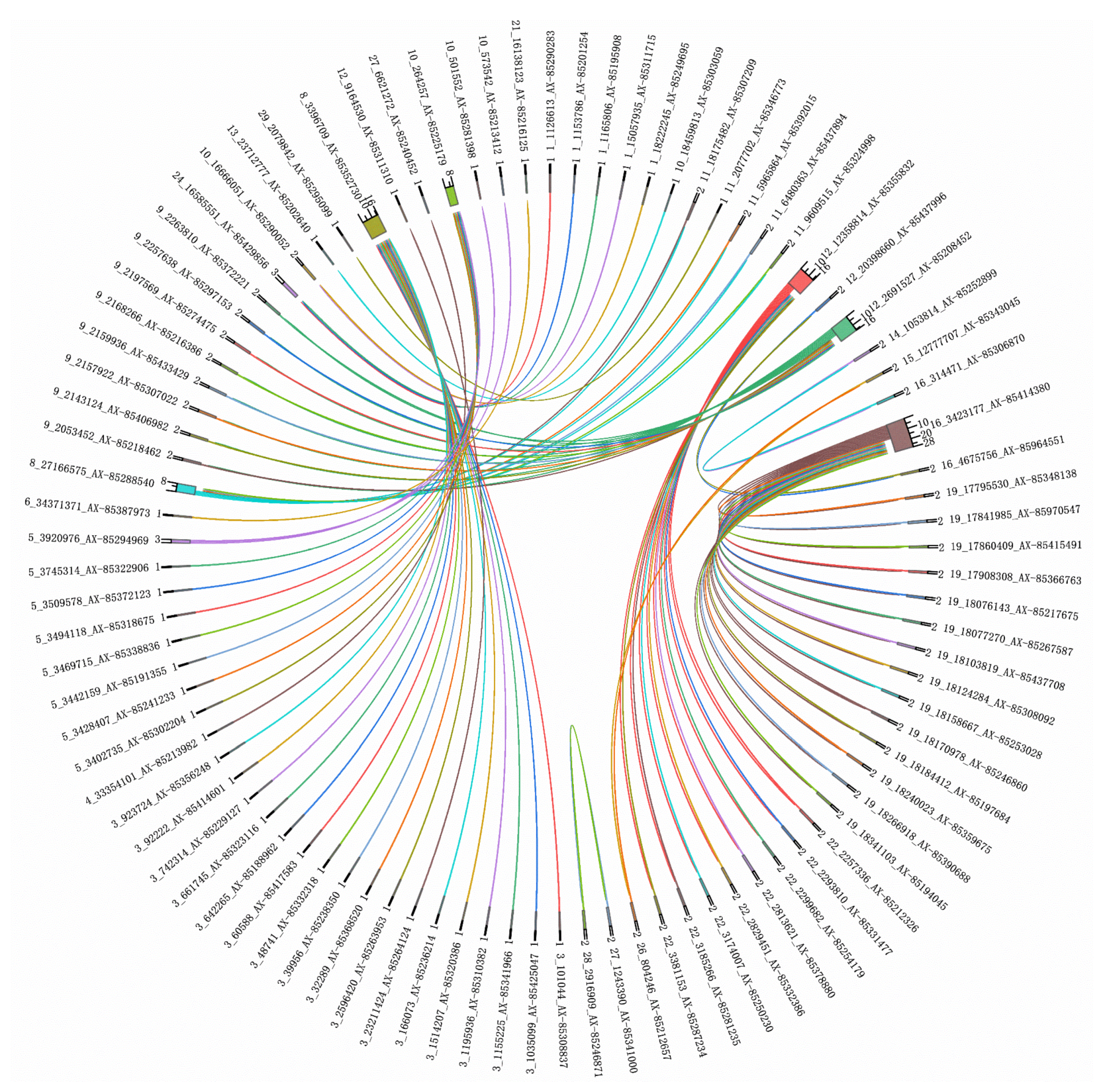
3.3. Genome-Wide Epistatic Association Analysis
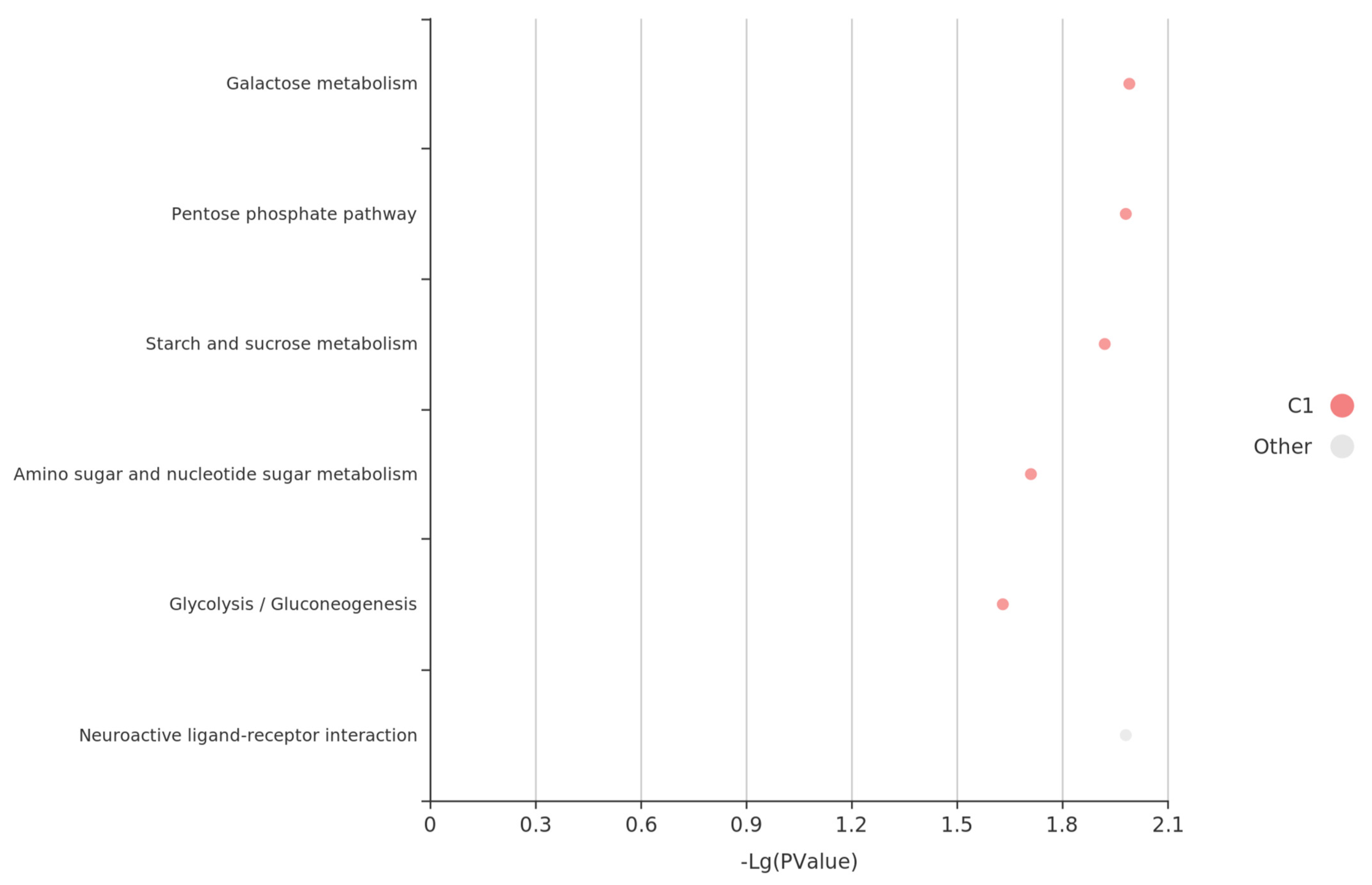
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albertson, R.C.; Streelman, J.T.; Kocher, T.D. Directional selection has shaped the oral jaws of Lake Malawi cichlid fishes. Proc. Natl. Acad. Sci. USA 2003, 100, 5252–5257. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Peng, W.; Kong, S.; Pu, F.; Chen, B.; Zhou, Z.; Feng, J.; Li, X.; Xu, P. Genetic mapping of head size related traits in common carp (Cyprinus carpio). Front. Genet. 2018, 9, 448. [Google Scholar] [CrossRef]
- Wünnenberg-Stapleton, K.; Blitz, I.L.; Hashimoto, C.; Cho, K.W. Involvement of the small GTPases XRhoA and XRnd1 in cell adhesion and head formation in early Xenopus development. Development 1999, 126, 5339–5351. [Google Scholar] [CrossRef] [PubMed]
- Schoenebeck, J.J.; Ostrander, E.A. The genetics of canine skull shape variation. Genetics 2013, 193, 317–325. [Google Scholar] [CrossRef]
- Schoenebeck, J.J.; Hutchinson, S.A.; Byers, A.; Beale, H.C.; Carrington, B.; Faden, D.L.; Rimbault, M.; Decker, B.; Kidd, J.M.; Sood, R. Variation of BMP3 contributes to dog breed skull diversity. PLoS Genet. 2012, 8, e1002849. [Google Scholar] [CrossRef]
- Maga, A.M.; Navarro, N.; Cunningham, M.L.; Cox, T.C. Quantitative trait loci affecting the 3D skull shape and size in mouse and prioritization of candidate genes in-silico. Front. Physiol. 2015, 6, 92. [Google Scholar] [CrossRef]
- Su, S.; Raouf, B.; He, X.; Cai, N.; Li, X.; Yu, J.; Li, J.; Yu, F.; Wang, M.; Tang, Y. Genome wide analysis for growth at two growth stages in a new fast-growing common carp strain (Cyprinus carpio L.). Sci. Rep. 2020, 10, 7259. [Google Scholar] [CrossRef]
- Gonzalez-Pena, D.; Gao, G.; Baranski, M.; Moen, T.; Cleveland, B.M.; Kenney, P.B.; Vallejo, R.L.; Palti, Y.; Leeds, T.D. Genome-wide association study for identifying loci that affect fillet yield, carcass, and body weight traits in rainbow trout (Oncorhynchus mykiss). Front. Genet. 2016, 7, 203. [Google Scholar] [CrossRef]
- Horn, S.S.; Ruyter, B.; Meuwissen, T.H.; Moghadam, H.; Hillestad, B.; Sonesson, A.K. GWAS identifies genetic variants associated with omega-3 fatty acid composition of Atlantic salmon fillets. Aquaculture 2020, 514, 734494. [Google Scholar] [CrossRef]
- Cáceres, G.; López, M.E.; Cádiz, M.I.; Yoshida, G.M.; Jedlicki, A.; Palma-Véjares, R.; Travisany, D.; Díaz-Domínguez, D.; Maass, A.; Lhorente, J.P. Fine mapping using whole-genome sequencing confirms anti-Müllerian hormone as a major gene for sex determination in farmed Nile tilapia (Oreochromis niloticus L.). G3 Genes Genomes Genet. 2019, 9, 3213–3223. [Google Scholar] [CrossRef]
- Geng, X.; Liu, S.; Yao, J.; Bao, L.; Zhang, J.; Li, C.; Wang, R.; Sha, J.; Zeng, P.; Zhi, D. A genome-wide association study identifies multiple regions associated with head size in catfish. G3 Genes Genomes Genet. 2016, 6, 3389–3398. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wang, J.; Zhou, Y.; Pang, M.; Yu, X.; Tong, J. Dynamic mRNA and miRNA expression of the head during early development in bighead carp (Hypophthalmichthys nobilis). BMC Genom. 2022, 23, 168. [Google Scholar] [CrossRef] [PubMed]
- Argue, B.J.; Liu, Z.; Dunham, R.A. Dress-out and fillet yields of channel catfish, Ictalurus punctatus, blue catfish, Ictalurus furcatus, and their F1, F2 and backcross hybrids. Aquaculture 2003, 228, 81–90. [Google Scholar] [CrossRef]
- Dunham, R.A.; Umali, G.M.; Beam, R.; Kristanto, A.H.; Trask, M. Comparison of production traits of NWAC103 channel catfish, NWAC103 channel catfish× blue catfish hybrids, Kansas Select 21 channel catfish, and blue catfish grown at commercial densities and exposed to natural bacterial epizootics. N. Am. J. Aquac. 2008, 70, 98–106. [Google Scholar] [CrossRef]
- Yang, R.; Gao, J.; Song, Y.; Hao, Z.; Xu, P. GRAMMAR-Lambda: An Extreme Simplification for Genome-wide Mixed Model Association Analysis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kim, S.; Sohn, K.-A.; Xing, E.P. A multivariate regression approach to association analysis of a quantitative trait network. Bioinformatics 2009, 25, i204–i212. [Google Scholar] [CrossRef] [PubMed]
- Bolormaa, S.; Pryce, J.; Hayes, B.; Goddard, M. Multivariate analysis of a genome-wide association study in dairy cattle. J. Dairy Sci. 2010, 93, 3818–3833. [Google Scholar] [CrossRef] [PubMed]
- Paaby, A.B.; Rockman, M.V. The many faces of pleiotropy. Trends Genet. 2013, 29, 66–73. [Google Scholar] [CrossRef]
- Stephens, M. A unified framework for association analysis with multiple related phenotypes. PLoS ONE 2013, 8, e65245. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Teng, J.; Zhao, C.; Zhang, X.; Tang, H.; Fan, X.; Xu, S.; Zhang, Q.; Ning, C. An Improved Linear Mixed Model for Multivariate Genome-Wide Association Studies. bioRxiv 2022. [Google Scholar] [CrossRef]
- Mackay, T.; Moore, J. Why epistasis is important for tackling complex human disease genetics. Genome Med. 2014, 6, 124, Erratum in Genome Med. 2015, 7, 85. [Google Scholar] [CrossRef]
- Upton, A.; Trelles, O.; Cornejo-García, J.A.; Perkins, J.R. High-performance computing to detect epistasis in genome scale data sets. Brief. Bioinform. 2016, 17, 368–379. [Google Scholar] [CrossRef]
- Fisher, R.A. XV.—The correlation between relatives on the supposition of Mendelian inheritance. Earth Environ. Sci. Trans. R. Soc. Edinb. 1919, 52, 399–433. [Google Scholar] [CrossRef]
- Zhang, F.; Boerwinkle, E.; Xiong, M. Epistasis analysis for quantitative traits by functional regression model. Genome Res. 2014, 24, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Wang, D.; Kang, H.; Mrode, R.; Zhou, L.; Xu, S.; Liu, J.-F. A rapid epistatic mixed-model association analysis by linear retransformations of genomic estimated values. Bioinformatics 2018, 34, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, L.; Li, Y.; Sun, F.; Jiang, Y.; Zhang, Y.; Zhang, J.; Feng, J.; Kaltenboeck, L.; Kucuktas, H. Development of the catfish 250K SNP array for genome-wide association studies. BMC Res. Notes 2014, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tang, H.; Liu, J.-F.; Xu, S.; Zhang, Q.; Ning, C. Rapid epistatic mixed-model association studies by controlling multiple polygenic effects. Bioinformatics 2020, 36, 4833–4837. [Google Scholar] [CrossRef] [PubMed]
- Catela, C.; Bilbao-Cortes, D.; Slonimsky, E.; Kratsios, P.; Rosenthal, N.; Welscher, P.T. Multiple congenital malformations of Wolf-Hirschhorn syndrome are recapitulated in null mice. Dis. Model. Mech. 2009, 2, 283–294. [Google Scholar] [CrossRef]
- Krantz, I.D.; McCallum, J.; DeScipio, C.; Kaur, M.; Gillis, L.A.; Yaeger, D.; Jukofsky, L.; Wasserman, N.; Bottani, A.; Morris, C.A. Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster Nipped-B. Nat. Genet. 2004, 36, 631–635. [Google Scholar] [CrossRef]
- Muto, A.; Ikeda, S.; Lopez-Burks, M.E.; Kikuchi, Y.; Calof, A.L.; Lander, A.D.; Schilling, T.F. Nipbl and mediator cooperatively regulate gene expression to control limb development. PLoS Genet. 2014, 10, e1004671. [Google Scholar] [CrossRef]
- Tarantino, U.; Greggi, C.; Cariati, I.; Visconti, V.V.; Gasparini, M.; Cateni, M.; Gasbarra, E.; Botta, A.; Salustri, A.; Scimeca, M. The Role of PTX3 in Mineralization Processes and Aging-Related Bone Diseases. Front. Immunol. 2021, 11, 622772. [Google Scholar] [CrossRef] [PubMed]
- Parente, R.; Sobacchi, C.; Bottazzi, B.; Mantovani, A.; Grčevic, D.; Inforzato, A. The Long Pentraxin PTX3 in Bone Homeostasis and Pathology. Front Immunol 2019, 10, 2628. [Google Scholar] [CrossRef]
- Grčević, D.; Sironi, M.; Valentino, S.; Deban, L.; Cvija, H.; Inforzato, A.; Kovačić, N.; Katavić, V.; Kelava, T.; Kalajzić, I. The long pentraxin 3 plays a role in bone turnover and repair. Front. Immunol. 2018, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E. Osteoclastogenesis and osteogenesis. Int. J. Mol. Sci. 2022, 23, 6659. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H.; Zhou, X.-Z.; Li, N.; Guo, Y.-C.; Chen, T.-P. Pentraxin 3 promotes the osteoblastic differentiation of MC3T3-E1 cells through the PI3K/Akt signaling pathway. Biosci. Rep. 2020, 40, BSR20201165. [Google Scholar] [CrossRef]
- Green, J.; Taylor, J.J.; Hindes, A.; Johnson, S.L.; Goldsmith, M.I. A gain of function mutation causing skeletal overgrowth in the rapunzel mutant. Dev. Biol. 2009, 334, 224–234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wangler, M.F.; Yamamoto, S.; Chao, H.-T.; Posey, J.E.; Westerfield, M.; Postlethwait, J.; Members of the Undiagnosed Diseases Network (UDN); Hieter, P.; Boycott, K.M.; Campeau, P.M.; et al. Model Organisms Facilitate Rare Disease Diagnosis and Therapeutic Research. Genetics 2017, 207, 9–27. [Google Scholar] [CrossRef]
- Bai, M.; Han, Y.; Wu, Y.; Liao, J.; Li, L.; Wang, L.; Li, Q.; Xing, W.; Chen, L.; Zou, W.; et al. Targeted genetic screening in mice through haploid embryonic stem cells identifies critical genes in bone development. PLoS Biol. 2019, 17, e3000350. [Google Scholar] [CrossRef]
- Cain, C.J.; Gaborit, N.; Lwin, W.; Barruet, E.; Ho, S.; Bonnard, C.; Hamamy, H.; Shboul, M.; Reversade, B.; Kayserili, H. Loss of Iroquois homeobox transcription factors 3 and 5 in osteoblasts disrupts cranial mineralization. Bone Rep. 2016, 5, 86–95. [Google Scholar] [CrossRef]
- Kong, S.H.; Yoon, J.W.; Kim, J.H.; Park, J.; Choi, J.; Lee, J.H.; Hong, A.R.; Cho, N.H.; Shin, C.S. Identification of Novel Genetic Variants Related to Trabecular Bone Score in Community-Dwelling Older Adults. Endocrinol. Metab. 2020, 35, 801–810. [Google Scholar] [CrossRef]
- Farmer, D.J.T.; Patel, P.; Choi, R.; Liu, C.-Y.; Crump, J.G. A comprehensive series of Irx cluster mutants reveals diverse roles in facial cartilage development. Development 2021, 148, dev197244. [Google Scholar] [CrossRef] [PubMed]
- Besio, R.; Maruelli, S.; Gioia, R.; Villa, I.; Grabowski, P.; Gallagher, O.; Bishop, N.J.; Foster, S.; De Lorenzi, E.; Colombo, R. Lack of prolidase causes a bone phenotype both in human and in mouse. Bone 2015, 72, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Ishii, M.; Ohshima, S.; Miyatake, K.; Saeki, Y. Expression and function of transmembrane-4 superfamily (tetraspanin) proteins in osteoclasts: Reciprocal roles of Tspan-5 and NET-6 during osteoclastogenesis. Allergol. Int. 2007, 56, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Gataulin, D.; Kuperman, Y.; Tsoory, M.; Biton, I.E.; Nataniel, T.; Palty, R.; Karbat, I.; Meshcheriakova, A.; Reuveny, E. Store-operated Ca2+ entry regulatory factor alters murine metabolic state in an age-dependent manner via hypothalamic pathways. PNAS Nexus 2023, 2, pgad068. [Google Scholar] [CrossRef]
- Zomot, E.; Achildiev Cohen, H.; Dagan, I.; Militsin, R.; Palty, R. Bidirectional regulation of calcium release-activated calcium (CRAC) channel by SARAF. J. Cell Biol. 2021, 220, e202104007. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Wang, D.; Hu, Y.; Wang, Q.; Zuo, Z.; Ye, B.; Lu, L.; Zhou, A.; Zou, J. Genome-wide comparative analysis between Cranoglanis bouderius and Pangasianodon hypophthalmus: Reveal the genes related to resistance to low-temperature stress. J. World Aquac. Soc. 2023, 54, 1367–1385. [Google Scholar] [CrossRef]
- Fels, L.; Marschall, Y.; Philipp, U.; Distl, O. Multiple loci associated with canine hip dysplasia (CHD) in German shepherd dogs. Mamm. Genome 2014, 25, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, N.; Zou, W.; Li, Y.; Cho, K.; Collins, P.L.; Tycksen, E.; Pandey, G.; DeSelm, C.J.; Patti, G.J.; Dey, A. BAP1 promotes osteoclast function by metabolic reprogramming. Nat. Commun. 2023, 14, 5923. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Meng, X.; Sun, C.; Yang, X.; Chen, G.; Li, W.; Chen, Z. Genome-wide DNA methylation profile analysis in thoracic ossification of the ligamentum flavum. J. Cell. Mol. Med. 2020, 24, 8753–8762. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Zhang, P.; Zhang, M.; Zhang, X.; Lv, L.; Liu, H.; Liu, Y.; Zhou, Y. Inhibition of SLC7A11 by sulfasalazine enhances osteogenic differentiation of mesenchymal stem cells by modulating BMP2/4 expression and suppresses bone loss in ovariectomized mice. J. Bone Miner. Res. 2017, 32, 508–521. [Google Scholar] [CrossRef]
- Singh, S.; McDonough, C.W.; Gong, Y.; Alghamdi, W.A.; Arwood, M.J.; Bargal, S.A.; Dumeny, L.; Li, W.Y.; Mehanna, M.; Stockard, B.; et al. Genome Wide Association Study Identifies the HMGCS2 Locus to be Associated With Chlorthalidone Induced Glucose Increase in Hypertensive Patients. J. Am. Heart Assoc. 2018, 7, e007339. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-R.V.; Hwang, Y.; Phadke, A.; Kang, H.; Hwang, N.S.; Caro, E.J.; Nguyen, S.; Siu, M.; Theodorakis, E.A.; Gianneschi, N.C. Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 990–995. [Google Scholar] [CrossRef]
- Beck, L. Expression and function of Slc34 sodium–phosphate co-transporters in skeleton and teeth. Pflügers Arch.-Eur. J. Physiol. 2019, 471, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Knöpfel, T.; Pastor-Arroyo, E.M.; Schnitzbauer, U.; Kratschmar, D.V.; Odermatt, A.; Pellegrini, G.; Hernando, N.; Wagner, C.A. The intestinal phosphate transporter NaPi-IIb (Slc34a2) is required to protect bone during dietary phosphate restriction. Sci. Rep. 2017, 7, 11018. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, M.J.; Mulari, M.T.; Buki, K.G.; Vihko, P.; Harkonen, P.L.; Vaananen, H.K. Rab13 is upregulated during osteoclast differentiation and associates with small vesicles revealing polarized distribution in resorbing cells. J. Histochem. Cytochem. 2012, 60, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Poncet, N.; Mitchell, F.E.; Ibrahim, A.F.M.; McGuire, V.A.; English, G.; Arthur, J.S.C.; Shi, Y.B.; Taylor, P.M. The Catalytic Subunit of the System L1 Amino Acid Transporter (slc7a5) Facilitates Nutrient Signalling in Mouse Skeletal Muscle. PLoS ONE 2014, 9, e89547. [Google Scholar] [CrossRef] [PubMed]
- Ni, F.; Zhang, T.; Xiao, W.; Dong, H.; Gao, J.; Liu, Y.; Li, J. IL-18-mediated SLC7A5 overexpression enhances osteogenic differentiation of human bone marrow mesenchymal stem cells via the c-MYC pathway. Front. Cell Dev. Biol. 2021, 9, 748831. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Yamada, T.; Horie, T.; Ishizaki, A.; Hiraiwa, M.; Iezaki, T.; Park, G.; Fukasawa, K.; Kamada, H.; Tokumura, K. The L-type amino acid transporter LAT1 inhibits osteoclastogenesis and maintains bone homeostasis through the mTORC1 pathway. Sci. Signal. 2019, 12, eaaw3921. [Google Scholar] [CrossRef] [PubMed]
- Handa, M.; Demura, S.; Yokogawa, N.; Hinoi, E.; Hiraiwa, M.; Kato, S.; Shinmura, K.; Annen, R.; Kobayashi, M.; Yamada, Y. Characteristics of Scoliosis in Mice Induced by Chondrocyte-specific Inactivation of L-type Amino Acid Transporter 1. Spine 2024, 49, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Schnur, R.E.; Yousaf, S.; Liu, J.; Chung, W.K.; Rhodes, L.; Marble, M.; Zambrano, R.M.; Sobreira, N.; Jayakar, P.; Pierpont, M.E. UBA2 variants underlie a recognizable syndrome with variable aplasia cutis congenita and ectrodactyly. Genet. Med. 2021, 23, 1624–1635. [Google Scholar] [CrossRef]
- Tønne, E.; Due-Tønnessen, B.J.; Vigeland, M.D.; Amundsen, S.S.; Ribarska, T.; Åsten, P.M.; Sheng, Y.; Helseth, E.; Gilfillan, G.D.; Mero, I.L. Whole-exome sequencing in syndromic craniosynostosis increases diagnostic yield and identifies candidate genes in osteogenic signaling pathways. Am. J. Med. Genet. Part A 2022, 188, 1464–1475. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, P.; Chen, Y.; Chen, Y.; Zhang, W.; Zhao, H.; Cao, Y.; Wang, F.; Jiang, N.; Lin, S. Foxp2 regulates anatomical features that may be relevant for vocal behaviors and bipedal locomotion. Proc. Natl. Acad. Sci. USA 2018, 115, 8799–8804. [Google Scholar] [CrossRef] [PubMed]
- Cesario, J.M.; Almaidhan, A.A.; Jeong, J. Expression of forkhead box transcription factor genes Foxp1 and Foxp2 during jaw development. Gene Expr. Patterns 2016, 20, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhou, W.; Yao, Z.; Wan, Y.; Cao, J.; Zhang, L.; Zhao, J.; Li, H.; Zhou, R.; Li, B. Foxp1/2/4 regulate endochondral ossification as a suppresser complex. Dev. Biol. 2015, 398, 242–254. [Google Scholar] [CrossRef]
- Rani, A. RAR-related orphan receptor alpha and the staggerer mice: A fine molecular story. Front. Endocrinol. 2023, 14, 1300729. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.C.; Xi, Y.; Pflugh, D.L.; Hesslein, D.G.; Schatz, D.G.; Lorenzo, J.A.; Bothwell, A.L. Pax5-deficient mice exhibit early onset osteopenia with increased osteoclast progenitors. J. Immunol. 2004, 173, 6583–6591. [Google Scholar] [CrossRef]
- Frost, V.; Grocott, T.; Eccles, M.R.; Chantry, A. Self-regulated Pax gene expression and modulation by the TGFβ superfamily. Crit. Rev. Biochem. Mol. Biol. 2008, 43, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; van der Lijn, F.; Schurmann, C.; Zhu, G.; Chakravarty, M.M.; Hysi, P.G.; Wollstein, A.; Lao, O.; de Bruijne, M.; Ikram, M.A.; et al. A genome-wide association study identifies five loci influencing facial morphology in Europeans. PLoS Genet 2012, 8, e1002932. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, K.; Fuentes-Guajardo, M.; Quinto-Sánchez, M.; Mendoza-Revilla, J.; Camilo Chacón-Duque, J.; Acuña-Alonzo, V.; Jaramillo, C.; Arias, W.; Lozano, R.B.; Pérez, G.M.; et al. A genome-wide association scan implicates DCHS2, RUNX2, GLI3, PAX1 and EDAR in human facial variation. Nat. Commun. 2016, 7, 11616. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, D.; Qiao, L.; Liu, Y.; Peng, Q.; Wu, S.; Zhang, M.; Yang, Y.; Tan, J.; Xu, S.; et al. A genome-wide association study of facial morphology identifies novel genetic loci in Han Chinese. J. Genet. Genom. 2021, 48, 198–207. [Google Scholar] [CrossRef]
- Bergstrom, W.H.; Wallace, W.M. Bone as a sodium and potassium reservoir. J. Clin. Investig. 1954, 33, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, M.; Han, Y.-M.; Kwon, B.-M.; Kim, S.H. Butamben derivatives enhance BMP-2-stimulated commitment of C2C12 cells into osteoblasts with induction of voltage-gated potassium channel expression. Bioorg. Med. Chem. Lett. 2011, 21, 7363–7366. [Google Scholar] [CrossRef]
- Young, K.A.; Ivester, C.; West, J.; Carr, M.; Rodman, D.M. BMP signaling controls PASMC KV channel expression in vitro and in vivo. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2006, 290, L841–L848. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, K.; Otsuka, F.; Suzuki, J.; Kano, Y.; Takeda, M.; Miyoshi, T.; Otani, H.; Mimura, Y.; Ogura, T.; Makino, H. Involvement of Bone Morphogenetic Protein-6 in Differential Regulation of Aldosterone Production by Angiotensin II and Potassium in Human Adrenocortical Cells. Endocrinology 2006, 147, 2681–2689. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kobayashi-Sun, J.; Suzuki, N.; Hattori, A.; Yamaguchi, M.; Kobayashi, I. Melatonin suppresses both osteoblast and osteoclast differentiation through repression of epidermal Erk signaling in the zebrafish scale. Biochem. Biophys. Res. Commun. 2020, 530, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Kong, N.; Tian, R.; Cao, R.; Liu, G.; Li, Y.; Wei, Q.; Jiao, M.; Lei, Y.; Xing, F. Melatonin increases bone mass in normal, perimenopausal, and postmenopausal osteoporotic rats via the promotion of osteogenesis. J. Transl. Med. 2022, 20, 132. [Google Scholar] [CrossRef]
- Li, T.; Jiang, S.; Lu, C.; Yang, W.; Yang, Z.; Hu, W.; Xin, Z.; Yang, Y. Melatonin: Another avenue for treating osteoporosis? J. Pineal Res. 2019, 66, e12548. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Han, N.; Li, F.; Wang, L.; Liu, G.; Hu, M.; Wang, S.; Wei, X.; Guo, J.; Jiang, H. Melatonin enhances osteoblastogenesis of senescent bone marrow stromal cells through NSD2-mediated chromatin remodelling. Clin. Transl. Med. 2022, 12, e746. [Google Scholar] [CrossRef]
- Yang, M.; Guo, Q.; Peng, H.; Xiao, Y.-Z.; Xiao, Y.; Huang, Y.; Li, C.-J.; Su, T.; Zhang, Y.-L.; Lei, M.-X. Krüppel-like factor 3 inhibition by mutated lncRNA Reg1cp results in human high bone mass syndrome. J. Exp. Med. 2019, 216, 1944–1964. [Google Scholar] [CrossRef]
- You, M.; Ai, Z.; Zeng, J.; Fu, Y.; Zhang, L.; Wu, X. Bone mesenchymal stem cells (BMSCs)-derived exosomal microRNA-21-5p regulates Kruppel-like factor 3 (KLF3) to promote osteoblast proliferation in vitro. Bioengineered 2022, 13, 11933–11944. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, H.S.; Lee, S.; Min, K.Y.; Choi, W.S.; You, J.S. Hexosamine Biosynthetic Pathway-Derived O-GlcNAcylation Is Critical for RANKL-Mediated Osteoclast Differentiation. Int. J. Mol. Sci. 2021, 22, 8888. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Yin, W.; Han, H.; Miller, H.; Li, J.; Herrada, A.A.; Kubo, M.; Sui, Z.; Gong, Q. The regulation of DOCK family proteins on T and B cells. J. Leucoc. Biol. 2021, 109, 383–394. [Google Scholar] [CrossRef]
- Sundaravel, S.; Duggan, R.; Bhagat, T.; Ebenezer, D.L.; Liu, H.; Yu, Y.; Bartenstein, M.; Unnikrishnan, M.; Karmakar, S.; Liu, T.-C.; et al. Reduced DOCK4 expression leads to erythroid dysplasia in myelodysplastic syndromes. Proc. Natl. Acad. Sci. USA 2015, 112, E6359–E6368. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, J.A.; Wood, S.L.; Cairns, D.A.; McMahon, K.; Gahlaut, R.; Thygesen, H.; Shires, M.; Roberts, S.; Marshall, H.; Oliva, M.R.; et al. Identification and validation of DOCK4 as a potential biomarker for risk of bone metastasis development in patients with early breast cancer. J. Pathol. 2019, 247, 381–391. [Google Scholar] [CrossRef]
- Wu, C.; Wang, C.; Xiao, B.; Li, S.; Sheng, Y.; Wang, Q.; Tao, J.; Zhang, Y.; Jiang, X. Integration analysis of lncRNA and mRNA expression data identifies DOCK4 as a potential biomarker for elderly osteoporosis. BMC Med. Genom. 2024, 17, 70. [Google Scholar] [CrossRef]
- Tarroni, P.; Rossi, D.; Conti, A.; Sorrentino, V. Expression of the ryanodine receptor type 3 calcium release channel during development and differentiation of mammalian skeletal muscle cells. J. Biol. Chem. 1997, 272, 19808–19813. [Google Scholar] [CrossRef] [PubMed]
- Sonnleitner, A.; Conti, A.; Bertocchini, F.; Schindler, H.; Sorrentino, V. Functional properties of the ryanodine receptor type 3 (RyR3) Ca2+ release channel. EMBO J. 1998, 17, 2790–2798. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, J.; Guo, X.; Su, B.; Wang, H.; Yang, R.; Jiang, L. Gene-Based Genome-Wide Association Study Identified Genes for Agronomic Traits in Maize. Biology 2022, 11, 1649. [Google Scholar] [CrossRef] [PubMed]
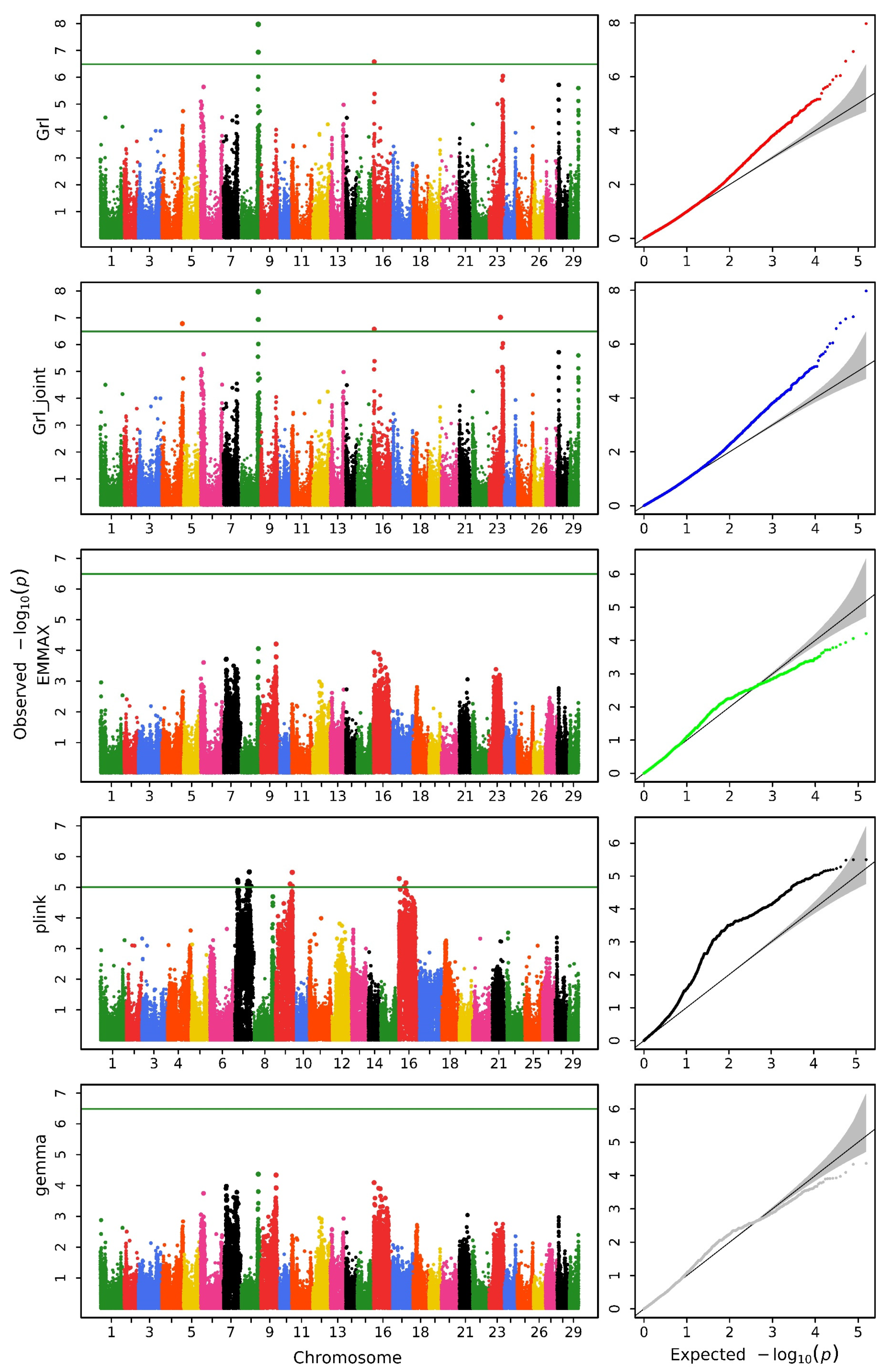

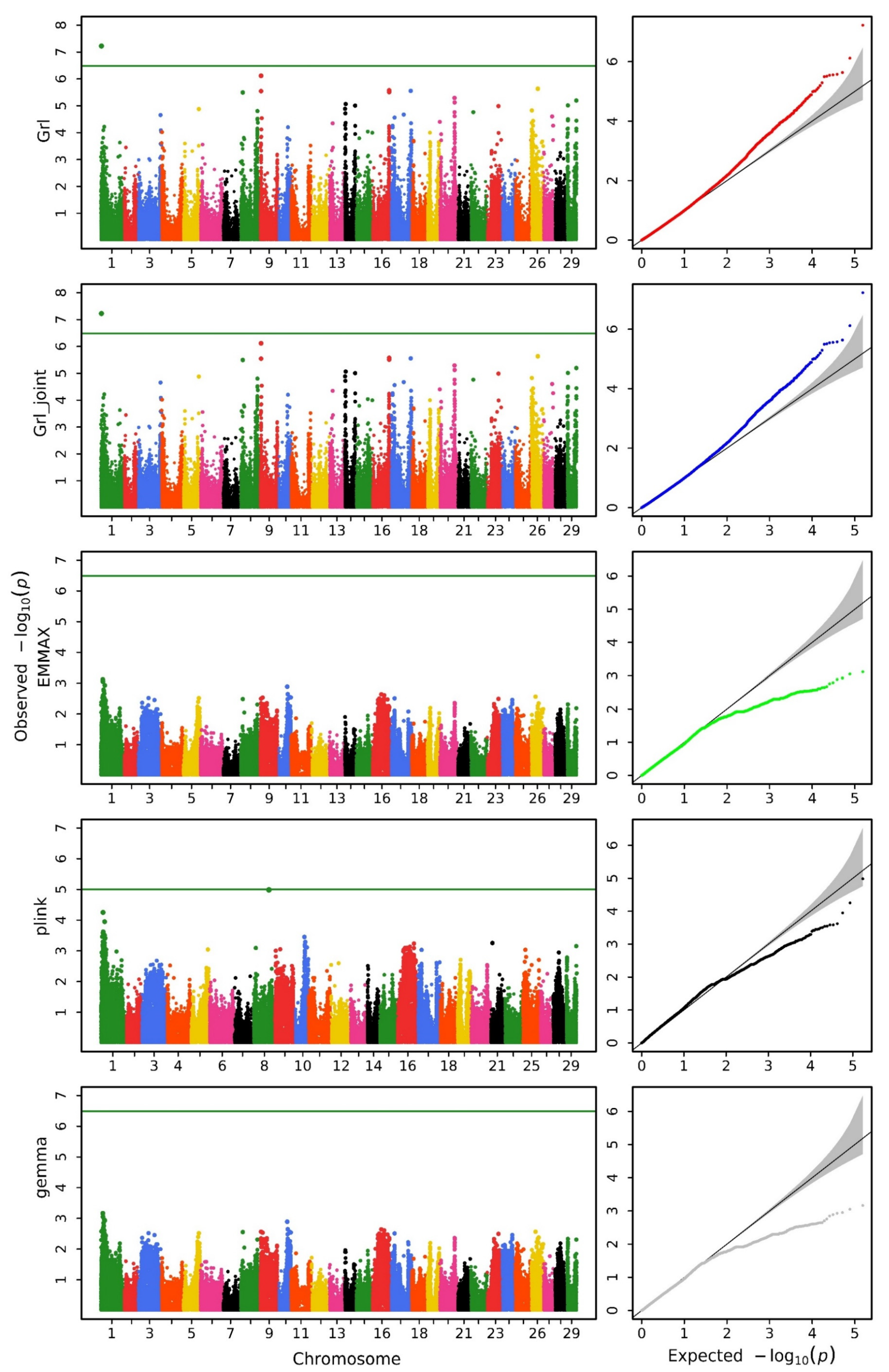


| Method | Head Length | Head Width | Head Depth |
|---|---|---|---|
| GRAMMAR-Lambda | 0.988 | 0.992 | 0.989 |
| GRAMMAR-Lambda-joint | 0.989 | 0.992 | 0.989 |
| EMMAX | 1.045 | 1.062 | 0.957 |
| QFAM (conducted in PLINK) | 1.199 | 1.312 | 0.934 |
| GEMMA | 1.028 | 1.022 | 0.959 |
| Trait | SNP ID | Chr | Position (bp) | Effect | Heritability (%) | −log10 (p) | Associated Gene (±150 kb) |
|---|---|---|---|---|---|---|---|
| length | AX-85293903 | 8 | 27,899,399 | −0.068 | 1.775 | 7.974 | fgfrl1b slc7a11 |
| AX-85344350 | 8 | 27,902,733 | −0.042 | 1.527 | 6.937 | fgfrl1b slc7a11 | |
| AX-85223744 | 16 | 1,445,591 | 0.040 | 1.442 | 6.577 | bmpr1bb cplane1 gdnfa nipblb nup155 opn4xa pdlim5b slc1a3b | |
| AX-85280970 | 4 | 33,281,317 | −0.027 | 0.843 | - * | ankrd67 ccnl1a pepd ptx3a | |
| AX-85220227 | 23 | 18,795,869 | 0.020 | 0.624 | - * | lamtor2 mex3a rab11al rab25b rpz4 rpz5 ubqln4 | |
| width | AX-85285315 | 29 | 693,486 | 0.025 | 1.597 | 6.863 | chic2 clta cplx2a fip1l1b gne mttp nansa rbpja saraf slc24a2 slc34a2a spink4 stim2a stra6l tdrd7a tmod1 trnai-aau tspan5b xpa |
| depth | AX-85396595 | 1 | 1,398,786 | 0.032 | 1.277 | 7.226 | irx2a irx4a nrsn1 slc6a3 |
| SNP ID | Chr | Position (bp) | Multivariate p-Value | Associated Gene (±150 kb) |
|---|---|---|---|---|
| AX-85401435 | 8 | 22,832,965 | 2.628 × 10−12 | ca5a rab33a slc7a5 uba2 |
| Trait | SNP ID | Chr | Position (bp) | Gene Containing the SNP | Chromosomes Affected |
|---|---|---|---|---|---|
| depth | AX-85329618 | 19 | 16,100,562 | dock4b | 29 |
| AX-85285929 | 19 | 16,812,298 | cdk17 | 29 | |
| AX-85329989 | 19 | 15,863,956 | / | 29 | |
| AX-85415036 | 19 | 15,947,222 | / | 29 | |
| AX-85249120 | 19 | 16,731,184 | / | 29 | |
| AX-85394139 | 22 | 5,866,945 | sra1 | 2 | |
| AX-85414246 | 22 | 5,754,759 | / | 2 | |
| width | AX-85290001 | 19 | 4,356,752 | grm8a | 3 |
| AX-85404624 | 19 | 4,440,623 | / | 3 | |
| AX-85318046 | 19 | 4,043,142 | LOC108279457 | 3 | |
| length | AX-85414380 | 16 | 3,423,177 | gramd1ba | 19 |
| AX-85352730 | 8 | 3,396,709 | / | 3 | |
| AX-85355832 | 12 | 12,358,814 | zeb2b | 22 | |
| AX-85208452 | 12 | 2,691,527 | LOC128634186 | 9 |
| Trait | SNP ID | Chr | Position (bp) | Gene Containing the SNP | SNP ID | Chr | Position (bp) | Gene Containing the SNP | Effect | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| depth | AX-85434516 | 5 | 25,232,076 | LOC108265909 | AX-85188891 | 18 | 195,408 | acsl6 | 0.313035 | 1.83 × 10−12 |
| AX-85323180 | 11 | 1,008,327 | / | AX-85342524 | 14 | 16,288,297 | roraa | −0.22966 | 5.85 × 10−13 | |
| AX-85323180 | 11 | 1,008,327 | / | AX-85394793 | 14 | 16,321,107 | / | −0.23027 | 4.23 × 10−13 | |
| AX-85396703 | 19 | 16,097,836 | dock4b | AX-85246106 | 29 | 1,782,517 | / | −0.16464 | 1.23 × 10−12 | |
| AX-85329618 | 19 | 16,100,562 | dock4b | AX-85313565 | 29 | 1,802,187 | mtnr1aa | −0.18043 | 1.71 × 10−12 | |
| AX-85329618 | 19 | 16,100,562 | dock4b | AX-85257177 | 29 | 1,897,225 | klf3 | −0.17712 | 1.71 × 10−12 | |
| AX-85329618 | 19 | 16,100,562 | dock4b | AX-85269499 | 29 | 1,910,208 | / | −0.17793 | 1.54 × 10−12 | |
| AX-85329618 | 19 | 16,100,562 | dock4b | AX-85227046 | 29 | 1,976,921 | pgm2 | −0.18352 | 2.98 × 10−13 | |
| AX-85329618 | 19 | 16,100,562 | dock4b | AX-85324535 | 29 | 2,060,488 | / | −0.18262 | 4.02 × 10−13 | |
| AX-85329618 | 19 | 16,100,562 | dock4b | AX-85320921 | 29 | 2,081,764 | pax5 | −0.18262 | 4.02 × 10−13 | |
| AX-85329618 | 19 | 16,100,562 | dock4b | AX-85325334 | 29 | 2,112,807 | pax5 | −0.18202 | 4.86 × 10−13 | |
| AX-85329618 | 19 | 16,100,562 | dock4b | AX-85336457 | 29 | 2,157,774 | / | −0.17848 | 1.54 × 10−12 | |
| AX-85329618 | 19 | 16,100,562 | dock4b | AX-85215120 | 29 | 2,414,791 | glrba | −0.18169 | 5.23 × 10−13 | |
| AX-85433924 | 19 | 16,333,930 | foxp2 | AX-85246106 | 29 | 1,782,517 | / | −0.17359 | 1.51 × 10−12 | |
| AX-85237071 | 19 | 16,406,535 | foxp2 | AX-85246106 | 29 | 1,782,517 | / | −0.18294 | 2.09 × 10−14 | |
| AX-85367637 | 22 | 8,744,028 | / | AX-85223510 | 11 | 2,136,759 | / | 0.15971 | 8.20 × 10−13 | |
| AX-85371989 | 22 | 16,303,493 | / | AX-85223510 | 11 | 2,136,759 | / | 0.197008 | 9.14 × 10−13 | |
| AX-85371989 | 22 | 16,303,493 | / | AX-85299031 | 11 | 2,213,073 | cntn4 | 0.208702 | 1.84 × 10−12 | |
| AX-85371989 | 22 | 16,303,493 | / | AX-85422684 | 11 | 2,228,063 | cntn4 | 0.208512 | 1.87 × 10−12 | |
| length | AX-85319825 | 3 | 31,181,550 | / | AX-86070899 | 6 | 3,844,599 | / | 0.264966 | 1.59 × 10−12 |
| AX-85439845 | 3 | 31,199,287 | / | AX-86070899 | 6 | 3,844,599 | / | 0.264978 | 1.51 × 10−12 | |
| width | AX-85250220 | 1 | 8,798,081 | efna3b | AX-85406367 | 20 | 5,741,046 | / | −0.1639 | 1.98 × 10−12 |
| AX-85340400 | 9 | 9,123,557 | RyR3 | AX-85311060 | 4 | 33,001,848 | kcnab1a | −0.48076 | 2.04 × 10−12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Gao, J.; Feng, H.; Jiang, L. GRAMMAR-Lambda Delivers Efficient Understanding of the Genetic Basis for Head Size in Catfish. Biology 2025, 14, 63. https://doi.org/10.3390/biology14010063
Zhao Y, Gao J, Feng H, Jiang L. GRAMMAR-Lambda Delivers Efficient Understanding of the Genetic Basis for Head Size in Catfish. Biology. 2025; 14(1):63. https://doi.org/10.3390/biology14010063
Chicago/Turabian StyleZhao, Yunfeng, Jin Gao, Hong Feng, and Li Jiang. 2025. "GRAMMAR-Lambda Delivers Efficient Understanding of the Genetic Basis for Head Size in Catfish" Biology 14, no. 1: 63. https://doi.org/10.3390/biology14010063
APA StyleZhao, Y., Gao, J., Feng, H., & Jiang, L. (2025). GRAMMAR-Lambda Delivers Efficient Understanding of the Genetic Basis for Head Size in Catfish. Biology, 14(1), 63. https://doi.org/10.3390/biology14010063





