Impacts of Urbanization and Habitat Characteristics on the Human Risk of West Nile Disease in the United States
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
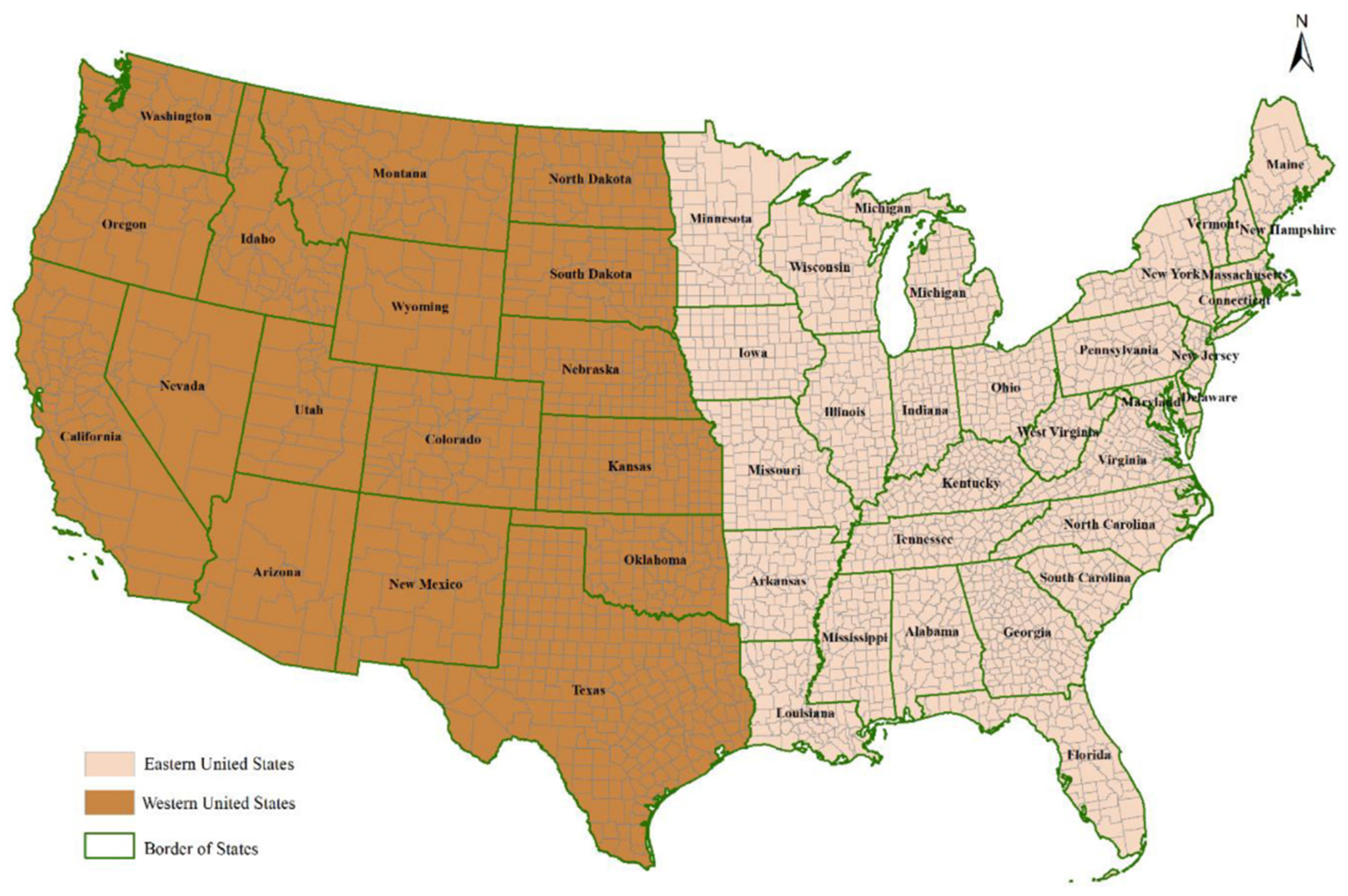
2.1. Study Area and Disease Data
2.2. Data of Predictors
2.3. Statistical Analysis
3. Results
3.1. Single-Variable Regression Analyses
3.2. Multi-Variable Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kramer, L.D.; Ciota, A.T.; Kilpatrick, A.M. Introduction, spread, and establishment of West Nile virus in the Americas. J. Med. Entomol. 2019, 56, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Ronca, S.E.; Ruff, J.C.; Murray, K.O. A 20-year historical review of West Nile virus since its initial emergence in North America: Has West Nile virus become a neglected tropical disease? PLoS Negl. Trop. Dis. 2021, 15, e0009190. [Google Scholar] [CrossRef] [PubMed]
- George, T.L.; Harrigan, R.J.; LaManna, J.A.; DeSante, D.F.; Saracco, J.F.; Smith, T.B. Persistent impacts of West Nile virus on North American bird populations. Proc. Natl. Acad. Sci. USA 2015, 112, 14290–14294. [Google Scholar] [CrossRef] [PubMed]
- Gould, C.V.; Staples, J.E.; Huang, C.Y.-H.; Brault, A.C.; Nett, R.J. Combating west nile virus disease—Time to revisit vaccination. N. Engl. J. Med. 2023, 388, 1633–1636. [Google Scholar] [CrossRef]
- Kaiser, J.A.; Barrett, A.D. Twenty years of progress toward West Nile virus vaccine development. Viruses 2019, 11, 823. [Google Scholar] [CrossRef]
- Tolsá, M.J.; García-Peña, G.E.; Rico-Chávez, O.; Roche, B.; Suzán, G. Macroecology of birds potentially susceptible to West Nile virus. Proc. R. Soc. B 2018, 285, 20182178. [Google Scholar] [CrossRef]
- Andreadis, T.G. The contribution of Culex pipiens complex mosquitoes to transmission and persistence of West Nile virus in North America. J. Am. Mosq. Control Assoc. 2012, 28, 137–151. [Google Scholar] [CrossRef]
- Bauer, A.M.; Guralnick, R.P.; Whitehead, S.A.; Barve, N.; Allen, J.M.; Campbell, L.P. Land use predicts proportion of West Nile virus vector-competent mosquitoes. Ecosphere 2024, 15, e4771. [Google Scholar] [CrossRef]
- Hahn, M.B.; Monaghan, A.J.; Hayden, M.H.; Eisen, R.J.; Delorey, M.J.; Lindsey, N.P.; Nasci, R.S.; Fischer, M. Meteorological conditions associated with increased incidence of West Nile virus disease in the United States, 2004–2012. Am. J. Trop. Med. Hyg. 2015, 92, 1013. [Google Scholar] [CrossRef]
- Rochlin, I.; Faraji, A.; Healy, K.; Andreadis, T.G. West Nile virus mosquito vectors in North America. J. Med. Entomol. 2019, 56, 1475–1490. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; LaDeau, S.L.; Marra, P.P. Ecology of West Nile virus transmission and its impact on birds in the western hemisphere. Auk 2007, 124, 1121–1136. [Google Scholar] [CrossRef]
- Bowden, S.E.; Magori, K.; Drake, J.M. Regional differences in the association between land cover and West Nile virus disease incidence in humans in the United States. Am. J. Trop. Med. Hyg. 2011, 84, 234. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L.; Barker, C.M.; Moore, C.G.; Pape, W.J.; Winters, A.M.; Cheronis, N. Irrigated agriculture is an important risk factor for West Nile virus disease in the hyperendemic Larimer-Boulder-Weld area of north central Colorado. J. Med. Entomol. 2010, 47, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.-W.; Hildreth, M.B.; Vanroekel, D.L.; Wimberly, M.C. Weather and land cover influences on mosquito populations in Sioux Falls, South Dakota. J. Med. Entomol. 2011, 48, 669–679. [Google Scholar] [CrossRef]
- Heidecke, J.; Lavarello Schettini, A.; Rocklöv, J. West Nile virus eco-epidemiology and climate change. PLoS Clim. 2023, 2, e0000129. [Google Scholar] [CrossRef]
- Thomson, M.C.; Stanberry, L.R. Climate change and vectorborne diseases. N. Engl. J. Med. 2022, 387, 1969–1978. [Google Scholar] [CrossRef]
- Rogers, D.; Randolph, S. Climate change and vector-borne diseases. Adv. Parasitol. 2006, 62, 345–381. [Google Scholar]
- Bell, J.A.; Brewer, C.M.; Mickelson, N.J.; Garman, G.W.; Vaughan, J.A. West Nile virus epizootiology, central Red River Valley, North Dakota and Minnesota, 2002–2005. Emerg. Infect. Dis. 2006, 12, 1245. [Google Scholar] [CrossRef]
- Danforth, M.E.; Reisen, W.K.; Barker, C.M. The impact of cycling temperature on the transmission of West Nile virus. J. Med. Entomol. 2016, 53, 681–686. [Google Scholar] [CrossRef]
- Reisen, W.K.; Fang, Y.; Martinez, V.M. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae). J. Med. Entomol. 2006, 43, 309–317. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Kramer, L.D.; Jones, M.J.; Marra, P.P.; Daszak, P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006, 4, e82. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.M.; Meola, M.A.; Moudy, R.M.; Kramer, L.D. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008, 4, e1000092. [Google Scholar] [CrossRef]
- Landesman, W.J.; Allan, B.F.; Langerhans, R.B.; Knight, T.M.; Chase, J.M. Inter-annual associations between precipitation and human incidence of West Nile virus in the United States. Vector Borne Zoonotic Dis. 2007, 7, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Diuk-Wasser, M.A.; VanAcker, M.C.; Fernandez, M.P. Impact of land use changes and habitat fragmentation on the eco-epidemiology of tick-borne diseases. J. Med. Entomol. 2021, 58, 1546–1564. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.A.; Marshall, J.C.; French, N.P.; Hayman, D.T. Habitat fragmentation, biodiversity loss and the risk of novel infectious disease emergence. J. R. Soc. Interface 2018, 15, 20180403. [Google Scholar] [CrossRef]
- Johnson, B.J.; Munafo, K.; Shappell, L.; Tsipoura, N.; Robson, M.; Ehrenfeld, J.; Sukhdeo, M.V. The roles of mosquito and bird communities on the prevalence of West Nile virus in urban wetland and residential habitats. Urban Ecosyst. 2012, 15, 513–531. [Google Scholar] [CrossRef]
- Rochlin, I.; Turbow, D.; Gomez, F.; Ninivaggi, D.V.; Campbell, S.R. Predictive mapping of human risk for West Nile virus (WNV) based on environmental and socioeconomic factors. PLoS ONE 2011, 6, e23280. [Google Scholar] [CrossRef]
- Wickham, J.; Homer, C.; Vogelmann, J.; McKerrow, A.; Mueller, R.; Herold, N.; Coulston, J. The multi-resolution land characteristics (MRLC) consortium—20 years of development and integration of USA national land cover data. Remote Sens. 2014, 6, 7424–7441. [Google Scholar] [CrossRef]
- Pindral, S.; Kot, R.; Hulisz, P.; Charzyński, P. Landscape metrics as a tool for analysis of urban pedodiversity. Land Degrad. Dev. 2020, 31, 2281–2294. [Google Scholar] [CrossRef]
- Anderson, D.; Burnham, K. Model Selection and Multi-Model Inference; Springer: New York, NY, USA, 2004. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Grothendieck, G.; Green, P.; Bolker, M.B. Package ‘lme4’. R Package. 2015. Available online: https://github.com/lme4/lme4/ (accessed on 21 May 2024).
- Barton, K.; Barton, M.K. Package ‘mumin’. R Package. 2015. Available online: https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf (accessed on 2 May 2024).
- Hartig, F.; Hartig, M.F. Package ‘dharma’. R Package. 2017. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 1 December 2024).
- Brown, H.; Diuk-Wasser, M.; Andreadis, T.; Fish, D. Remotely-sensed vegetation indices identify mosquito clusters of West Nile virus vectors in an urban landscape in the northeastern United States. Vector Borne Zoonotic Dis. 2008, 8, 197–206. [Google Scholar] [CrossRef]
- Deichmeister, J.M.; Telang, A. Abundance of West Nile virus mosquito vectors in relation to climate and landscape variables. J. Vector Ecol. 2011, 36, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Y.X.; Van Langevelde, F.; Estrada-Peña, A.; Suzán, G.; De Boer, W. The diversity–disease relationship: Evidence for and criticisms of the dilution effect. Parasitology 2016, 143, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Y.X.; de Boer, W.F.; van Langevelde, F.; Olson, V.; Blackburn, T.M.; Prins, H.H. Species’ life-history traits explain interspecific variation in reservoir competence: A possible mechanism underlying the dilution effect. PLoS ONE 2013, 8, e54341. [Google Scholar]
- Dong, Y.; Huang, Z.Y.X.; Zhang, Y.; Wang, Y.X.; La, Y. Comparing the climatic and landscape risk factors for Lyme disease cases in the Upper Midwest and Northeast United States. Int. J. Environ. Res. Public Health 2020, 17, 1548. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Ostfeld, R.S.; Peterson, A.T.; Poulin, R.; de la Fuente, J. Effects of environmental change on zoonotic disease risk: An ecological primer. Trends Parasitol. 2014, 30, 205–214. [Google Scholar] [CrossRef]
- Davis, J.K.; Vincent, G.; Hildreth, M.B.; Kightlinger, L.; Carlson, C.; Wimberly, M.C. Integrating environmental monitoring and mosquito surveillance to predict vector-borne disease: Prospective forecasts of a West Nile virus outbreak. PLoS Curr. 2017, 9. [Google Scholar] [CrossRef]
- Hess, A.; Davis, J.; Wimberly, M. Identifying environmental risk factors and mapping the distribution of West Nile virus in an endemic region of North America. GeoHealth 2018, 2, 395–409. [Google Scholar] [CrossRef]
- Chase, J.M.; Knight, T.M. Drought-induced mosquito outbreaks in wetlands. Ecol. Lett. 2003, 6, 1017–1024. [Google Scholar] [CrossRef]
- Wang, G.; Minnis, R.B.; Belant, J.L.; Wax, C.L. Dry weather induces outbreaks of human West Nile virus infections. BMC Infect. Dis. 2010, 10, 38. [Google Scholar] [CrossRef]
- Huang, Z.Y.X.; van Langevelde, F.; Honer, K.J.; Naguib, M.; de Boer, W.F. Regional level risk factors associated with the occurrence of African swine fever in West and East Africa. Parasites Vectors 2017, 10, 16. [Google Scholar] [CrossRef]
- Zhang, S.; Li, N.; Xu, M.; Huang, Z.Y.X.; Gu, Z.; Yin, S. Urbanization and Habitat Characteristics Associated with the Occurrence of Peste des Petits Ruminants in Africa. Sustainability 2022, 14, 8978. [Google Scholar] [CrossRef]
| Predictors | Description | Unit |
|---|---|---|
| Landscape factors | ||
| PLAND X | The percentage of area covered by a land cover class X | / |
| FN X | The fragmentation index of a land cover class X | / |
| Climatic factors | ||
| PreMean | Annual mean precipitation | mm |
| TemMean | Annual mean temperature | °C |
| BIO9 | Mean temperature of the driest quarter | °C |
| BIO10 | Mean temperature of the warmest quarter | °C |
| BIO11 | Mean temperature of the coldest quarter | °C |
| BIO17 | Precipitation of the driest quarter | mm |
| BIO18 | Precipitation of the warmest quarter | mm |
| BIO19 | Precipitation of the coldest quarter | mm |
| Predictors | Western | Eastern | ||||
|---|---|---|---|---|---|---|
| b | Z | p-Value | b | Z | p-Value | |
| Landscape predictors 1 | ||||||
| PLAND21 | 0.211 | 10.01 | <0.001 *** | 0.059 | 3.24 | 0.001 ** |
| PLAND22 | 0.206 | 11.87 | <0.001 *** | 0.248 | 12.50 | <0.001 *** |
| PLAND23 | 0.188 | 11.97 | <0.001 *** | 0.357 | 18.99 | <0.001 *** |
| PLAND24 | 0.081 | 5.14 | <0.001 *** | 0.012 | 0.61 | 0.54 |
| PLAND41 | −0.164 | −7.55 | <0.001 *** | −0.323 | −14.31 | <0.001 *** |
| PLAND42 | −0.403 | −21.32 | <0.001 *** | 0.13 | 6.58 | <0.001 *** |
| PLAND43 | −0.257 | −13.80 | <0.001 *** | −0.129 | −6.20 | <0.001 *** |
| PLAND71 | 0.139 | 7.80 | <0.001 *** | 0.155 | 9.46 | <0.001 *** |
| PLAND82 | 0.274 | 13.32 | <0.001 *** | 0.101 | 4.36 | <0.001 *** |
| PLAND90 | −0.121 | −6.42 | <0.001 *** | 0.063 | 3.12 | 0.002 ** |
| FN21 | −0.073 | −3.35 | 0.001 ** | −0.292 | −12.36 | <0.001 *** |
| FN22 | 0.006 | 0.21 | 0.831 | −0.048 | −1.85 | 0.064 |
| FN23 | −0.123 | −4.19 | <0.001 *** | −0.23 | −7.39 | <0.001 *** |
| FN24 | 0.016 | 0.66 | 0.511 | −0.041 | −1.61 | 0.108 |
| FN41 | 0.158 | 10.22 | <0.001 *** | 0.083 | 5.17 | <0.001 *** |
| FN42 | 0.157 | 9.18 | <0.001 *** | 0.095 | 5.95 | <0.001 *** |
| FN43 | 0.186 | 11.32 | <0.001 *** | 0.14 | 8.41 | <0.001 *** |
| FN71 | −0.188 | −9.89 | <0.001 *** | −0.06 | −3.84 | <0.001 *** |
| FN82 | -0.078 | −4.40 | <0.001 *** | −0.005 | −0.37 | 0.711 |
| FN90 | 0.194 | 11.68 | <0.001 *** | 0.02 | 1.01 | 0.313 |
| Climatic predictors 2 | ||||||
| TemMean | 0.421 | 3.24 | 0.001 ** | 0.196 | 1.83 | 0.067 |
| PreMean | −0.45 | −20.89 | <0.001 *** | −0.177 | −5.33 | <0.001 *** |
| BIO9 | −0.094 | −2.58 | 0.01 * | 0.278 | 6.82 | <0.001 *** |
| BIO10 | 0.574 | 19.78 | <0.001 *** | 0.491 | 11.30 | <0.001 *** |
| BIO11 | −0.137 | −3.25 | 0.001 ** | 0.154 | 2.83 | 0.005 ** |
| BIO17 | −0.396 | −16.93 | <0.001 *** | −0.035 | −0.89 | 0.373 |
| BIO18 | −0.447 | −13.32 | <0.001 *** | −0.222 | −9.28 | <0.001 *** |
| BIO19 | −0.498 | −20.56 | <0.001 *** | 0.195 | 4.00 | <0.001 *** |
| Predictors | Western | Eastern | ||||
|---|---|---|---|---|---|---|
| b ± S.E. | Z | p-Value | b ± S.E. | Z | p-Value | |
| AREA | 0.29 ± 0.03 | 11.26 | <0.001 *** | 0.22 ± 0.02 | 9.62 | <0.001 *** |
| Population | 1.04 ± 0.04 | 27.73 | <0.001 *** | 0.75 ± 0.03 | 24.89 | <0.001 *** |
| PLAND22 | 0.191 ± 0.021 | 8.95 | <0.001 *** | / | / | / |
| PLAND23 | / | / | / | 0.24 ± 0.02 | 12.33 | <0.001 *** |
| PLAND24 | 0.054 ± 0.018 | 3.06 | 0.002 ** | / | / | / |
| PLAND41 | 0.055 ± 0.026 | 2.16 | 0.031 * | −0.19 ± 0.027 | −6.96 | <0.001 *** |
| PLAND42 | −0.027 ± 0.028 | −0.96 | 0.34 | −0.075 ± 0.027 | −3.29 | 0.001 ** |
| PLAND43 | −0.212 ± 0.025 | −8.66 | <0.001 *** | 0.002 ± 0.027 | 0.06 | 0.95 |
| PLAND71 | / | / | / | 0.146 ± 0.021 | 6.92 | <0.001 *** |
| PLAND82 | 0.279 ± 0.028 | 9.86 | <0.001 *** | / | / | / |
| PLAND90 | 0.266 ± 0.024 | 11.17 | <0.001 *** | 0.012 ± 0.023 | 0.53 | 0.59 |
| FN21 | 0.006 ± 0.024 | 0.24 | 0.814 | −0.23 ± 0.024 | −9.44 | <0.001 *** |
| FN23 | −0.033 ± 0.031 | −1.10 | 0.271 | −0.18 ± 0.031 | −5.86 | <0.001 *** |
| FN41 | 0.01 ± 0.02 | 0.52 | 0.603 | 0.047 ± 0.02 | 2.33 | 0.020 * |
| FN42 | −0.016 ± 0.02 | −0.83 | 0.404 | 0.023 ± 0.018 | 1.29 | 0.20 |
| FN43 | 0.048 ± 0.019 | 2.51 | 0.012 * | 0.11 ± 0.021 | 5.10 | <0.001 *** |
| FN71 | / | / | / | −0.048 ± 0.016 | −2.90 | 0.004 ** |
| FN82 | 0.044 ± 0.019 | 2.36 | 0.018 * | / | / | / |
| FN90 | 0.125 ± 0.019 | 6.45 | <0.001 *** | / | / | / |
| BIO10 | 0.317 ± 0.041 | 7.83 | <0.001 *** | 0.36 ± 0.05 | 7.50 | <0.001 *** |
| BIO18 | −0.365 ± 0.047 | −7.83 | <0.001 *** | −0.26 ± 0.03 | −9.97 | <0.001 *** |
| TemMean | 0.318 ± 0.14 | 2.27 | 0.023 * | 0.068 ± 0.09 | 0.77 | 0.41 |
| PreMean | −0.18 ± 0.033 | −5.38 | <0.001 *** | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Xu, N.; Xu, Y.; Huang, Z.Y.X.; Chen, C.; Wang, Y.X.G. Impacts of Urbanization and Habitat Characteristics on the Human Risk of West Nile Disease in the United States. Biology 2025, 14, 224. https://doi.org/10.3390/biology14030224
Ma J, Xu N, Xu Y, Huang ZYX, Chen C, Wang YXG. Impacts of Urbanization and Habitat Characteristics on the Human Risk of West Nile Disease in the United States. Biology. 2025; 14(3):224. https://doi.org/10.3390/biology14030224
Chicago/Turabian StyleMa, Jian, Nuo Xu, Ying Xu, Zheng Y. X. Huang, Chuanwu Chen, and Yingying X. G. Wang. 2025. "Impacts of Urbanization and Habitat Characteristics on the Human Risk of West Nile Disease in the United States" Biology 14, no. 3: 224. https://doi.org/10.3390/biology14030224
APA StyleMa, J., Xu, N., Xu, Y., Huang, Z. Y. X., Chen, C., & Wang, Y. X. G. (2025). Impacts of Urbanization and Habitat Characteristics on the Human Risk of West Nile Disease in the United States. Biology, 14(3), 224. https://doi.org/10.3390/biology14030224






