Fading into Obscurity: Impact of Climate Change on Suitable Habitats for Two Lesser-Known Giant Flying Squirrels (Sciuridae: Petaurista) in Northeastern India
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
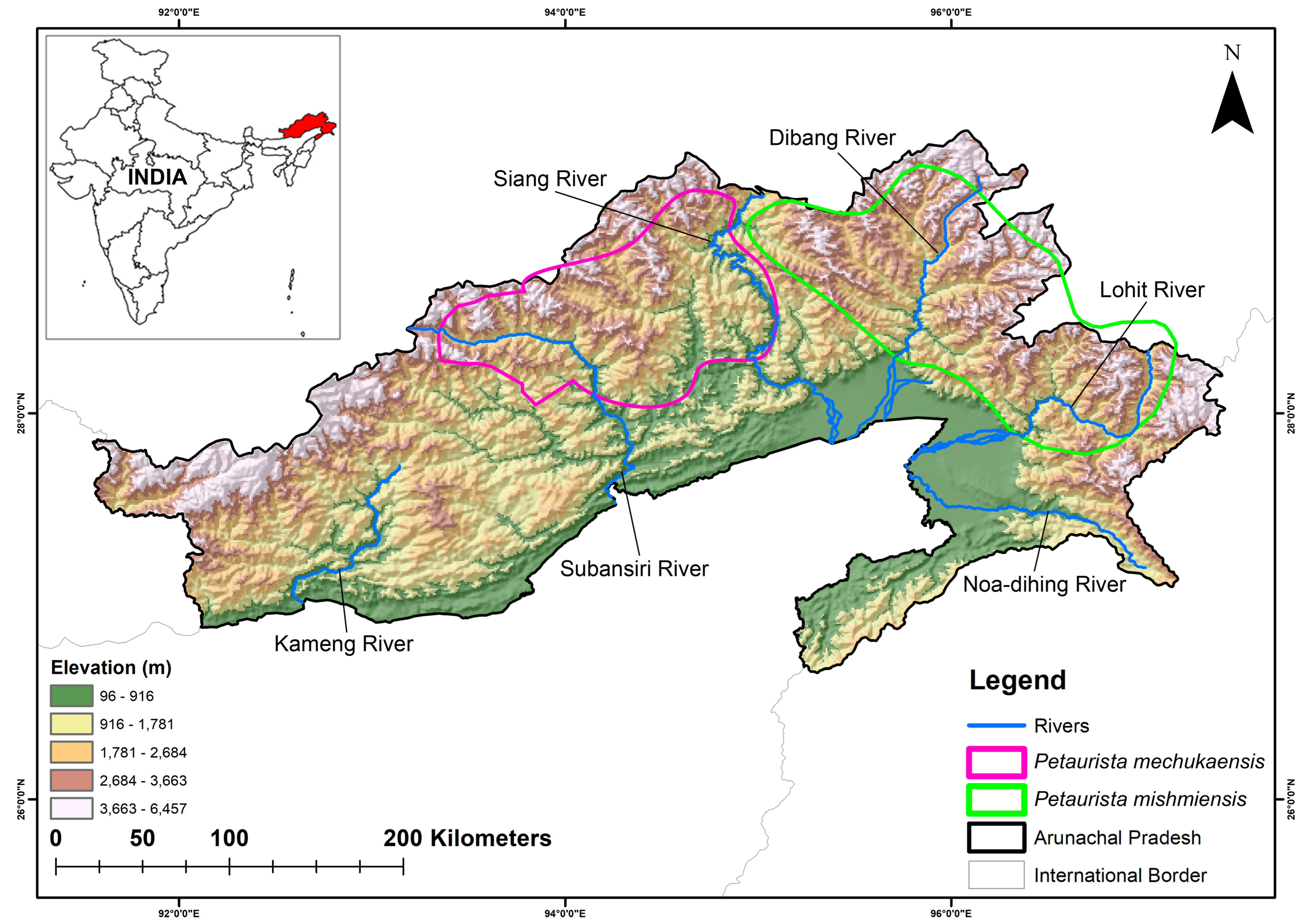
2.1. Study Area
2.2. Habitat Requirements
2.3. Selection of Covariates
2.4. Model Building and Evaluation
2.5. Assessment of Habitat Quality
3. Results
3.1. Evaluation of Models and Variable Importance
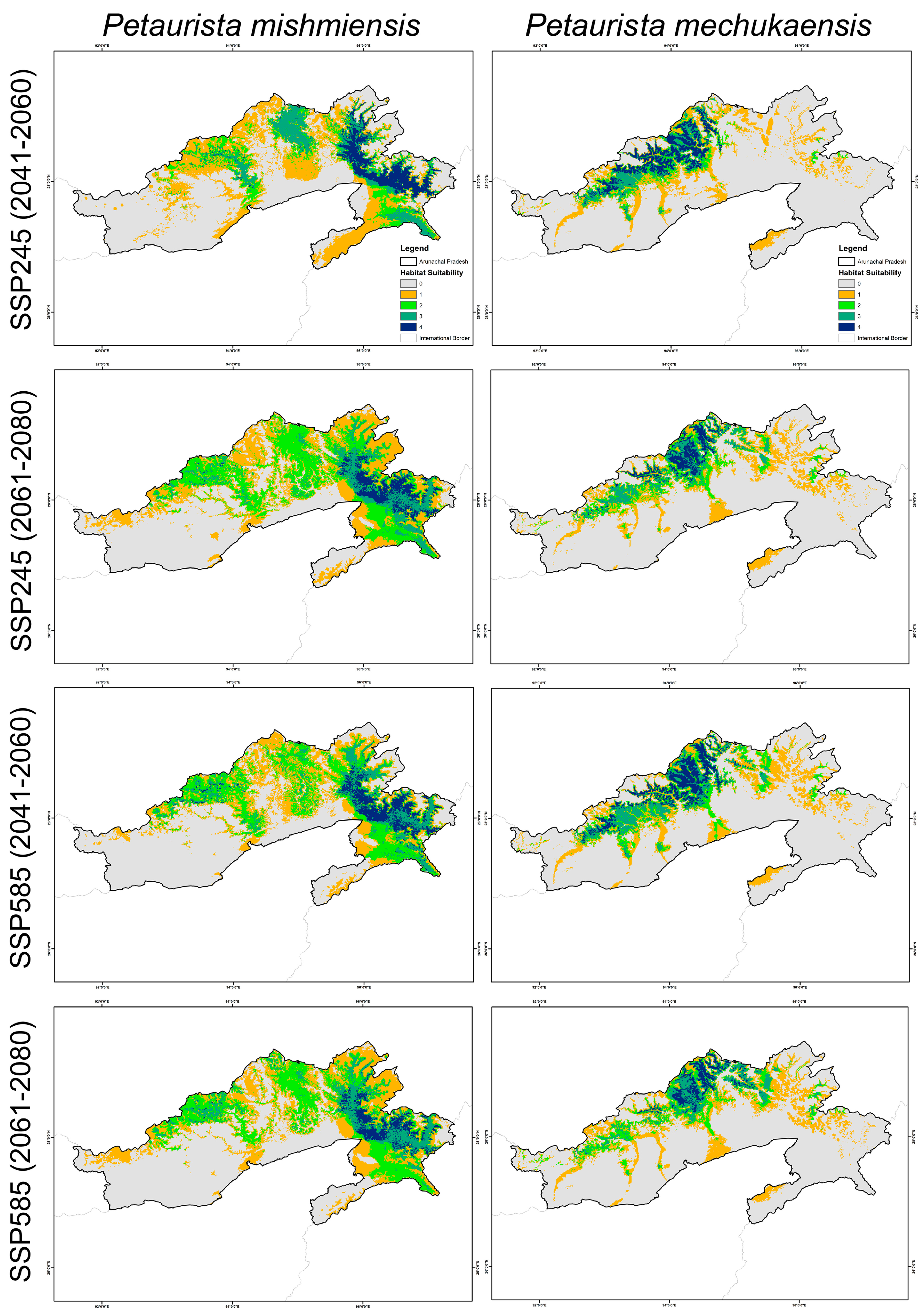
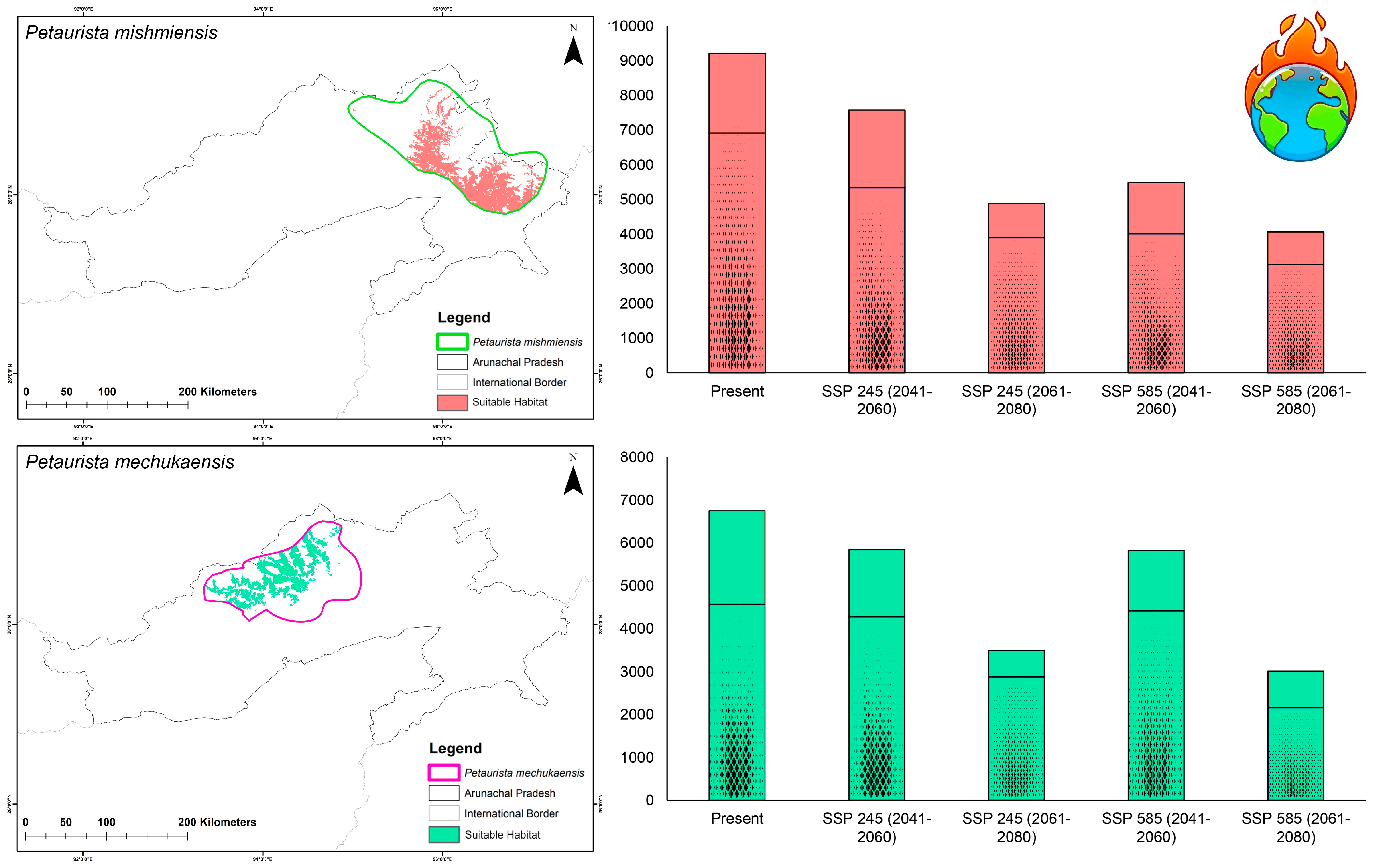
3.2. Habitat Suitability in Present and Future Climate Scenarios
3.3. Assessment of Habitat Shape Geometry Dynamics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wingard, G.L.; Schneider, C.L.; Dietl, G.P.; Fordham, D.A. Turning Setbacks into Stepping-Stones for Growth in Conservation Paleobiology. Front. Ecol. Evol. 2024, 12, 1384291. [Google Scholar] [CrossRef]
- Farnsworth, A.; Lo, Y.T.E.; Valdes, P.J.; Buzan, J.R.; Mills, B.J.W.; Merdith, A.S.; Scotese, C.R.; Wakeford, H.R. Climate Extremes Likely to Drive Land Mammal Extinction during Next Supercontinent Assembly. Nat. Geosci. 2023, 16, 901–908. [Google Scholar] [CrossRef]
- Crooks, K.R.; Burdett, C.L.; Theobald, D.M.; King, S.R.B.; Di Marco, M.; Rondinini, C.; Boitani, L. Quantification of habitat fragmentation reveals extinction risk in terrestrial mammals. Proc. Natl. Acad. Sci. USA 2017, 114, 7635–7640. [Google Scholar] [CrossRef]
- Ahmad, S.; Yang, L.; Khan, T.U.; Wanghe, K.; Li, M.; Luan, X. Using an ensemble modelling approach to predict the potential distribution of Himalayan gray goral (Naemorhedus goral bedfordi) in Pakistan. Glob. Ecol. Conserv. 2020, 21, e00845. [Google Scholar] [CrossRef]
- Jablonski, D. Extinction and the spatial dynamics of biodiversity. Proc. Natl. Acad. Sci. USA 2008, 105, 11528–11535. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Roy, S.; Suman, A.; Ray, S.; Saikia, S.K. Use of species distribution models to study habitat suitability for sustainable management and conservation in the Indian subcontinent: A decade’s retrospective. Front. Sustain. Resour. Manag. 2022, 1, 1031646. [Google Scholar] [CrossRef]
- Santangeli, A.; Mammola, S.; Lehikoinen, A.; Rajasärkkä, A.; Lindén, A.; Saastamoinen, M. The effects of protected areas on the ecological niches of birds and mammals. Sci. Rep. 2022, 12, 11601. [Google Scholar] [CrossRef]
- Meehl, G.A.; Covey, C.; Delworth, T.; Latif, M.; McAvaney, B.; Mitchell, J.F.B.; Stouffer, R.J.; Taylor, K.E. The WCRP CMIP3 Multimodel Dataset: A New Era in Climate Change Research. Bull. Am. Meteorol. Soc. 2007, 88, 1383–1394. [Google Scholar] [CrossRef]
- Knight, J. Scientists’ warning of the impacts of climate change on mountains. PeerJ 2022, 10, e14253. [Google Scholar] [CrossRef]
- Belden, G.; Stuebing, R.; Nyegang, M. Small carnivores in mixed-use forest in Bintulu Division, Sarawak, Malaysia. Small Carnivore Conserv. 2007, 36, 35–37. [Google Scholar]
- Datta, A.; Naniwadekar, R.; Anand, M.O. Occurrence and conservation status of small carnivores in two protected areas in Arunachal Pradesh, north-east India. Small Carnivore Conserv. 2008, 39, 1–10. [Google Scholar]
- Chutipong, W.; Lynam, A.J.; Steinmetz, R.; Savini, T.; Gale, G.A. Sampling mammalian carnivores in western Thailand: Issues of rarity and detectability. Raffles Bull. Zool. 2014, 62, 521–535. [Google Scholar]
- Abedin, I.; Mukherjee, T.; Kim, A.R.; Kim, H.-W.; Lee, S.R.; Kundu, S. Shifting shadows: Assessing the habitat and climate change response of binturong (Arctictis binturong) in the conservation landscape of the Asian continent. Ecol. Inform. 2025, 85, 102941. [Google Scholar] [CrossRef]
- Koli, V.K. Biology and conservation status of flying squirrels (Pteromyini, Sciuridae, Rodentia) in India: An update and review. Proc. Zool. Soc. 2016, 69, 9–21. [Google Scholar] [CrossRef]
- Carey, A.B.; Harrington, C.A. Small mammals in young forests: Implications for management for sustainability. For. Ecol. Manag. 2001, 154, 289–309. [Google Scholar] [CrossRef]
- Nandini, R.; Parthasarathy, N. Food habits of the Indian giant flying squirrel (Petaurista philippensis) in a rain forest fragment, Western Ghats. J. Mammal. 2008, 89, 1550–1556. [Google Scholar] [CrossRef]
- Burgin, C.J.; Colella, J.P.; Kahn, P.L.; Upham, N.S. How many species of mammals are there? J. Mammal. 2018, 99, 1–14. [Google Scholar] [CrossRef]
- Thorington, R.W.; Pitassy, D.; Jansa, S.A. Phylogenies of flying squirrels (Pteromyinae). J. Mamm. Evol. 2002, 9, 99–133. [Google Scholar] [CrossRef]
- Dudley, R.; Byrnes, G.; Yanoviak, S.P.; Borrell, B.; Brown, R.M.; McGuire, J.A. Gliding and the functional origins of flight: Biomechanical novelty or necessity? Annu. Rev. Ecol. Evol. Syst. 2007, 38, 179–201. [Google Scholar] [CrossRef]
- Chaitanya, R.; McGuire, J.A.; Karanth, P.; Meiri, S. Their fates intertwined: Diversification patterns of the Asian gliding vertebrates may have been forged by dipterocarp trees. Proc. R. Soc. B 2023, 290, 20231379. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, G.; Spence, A.J. Ecological and biomechanical insights into the evolution of gliding in mammals. Integr. Comp. Biol. 2011, 51, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- McGuire, J.A.; Dudley, R. The biology of gliding in flying lizards (genus Draco) and their fossil and extant analogs. Integr. Comp. Biol. 2011, 51, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Progulske, D.R.; Lee, P.F.; Day, Y.T. Bibliography of Petauristinae (Rodentia, Sciuridae). J. Taiwan Mus. 1985, 38, 49–57. [Google Scholar]
- Lee, P.F.; Liao, C.Y. Species richness and research trend of flying squirrels. J. Taiwan Mus. 1998, 51, 1–20. [Google Scholar]
- Umapathy, G.; Kumar, A. The occurrence of arboreal mammals in the rain forest fragments in Anamalai Hills, south India. Biol. Conserv. 2000, 92, 311–319. [Google Scholar] [CrossRef]
- Kumara, H.N.; Singh, M. Distribution and relative abundance of giant squirrels and flying squirrels in Karnataka, India. Mammalia 2006, 70, 40–47. [Google Scholar] [CrossRef]
- Puyravaud, J.P.; Davidar, P.; Laurance, W.F. Cryptic destruction of India’s native forests. Conserv. Lett. 2010, 3, 390–394. [Google Scholar] [CrossRef]
- Sharma, G.; Kamalakannan, M.; Saikia, U.; Talmale, S.; Dam, D.; Banerjee, D. Checklist of Fauna of India: Chordata: Mammalia. Version 1.0; Zoological Survey of India: Kolkata, India, 2024. [CrossRef]
- Choudhury, A.U. A new flying squirrel of the genus Petaurista from Arunachal Pradesh in north-east India. Newsletter J. Rhino Found. Nature N. E. India 2007, 7, 26–32. [Google Scholar]
- Choudhury, A.U. One more new species of giant flying squirrel of the genus Petaurista Link, 1795 from Arunachal Pradesh in north-east India. Newsl. J. Rhino Found. Nat. N. E. India 2009, 8, 26–34. [Google Scholar]
- Choudhury, A.U. Description of a second specimen of Mechuka giant flying squirrel Petaurista mechukaensis from Arunachal Pradesh in North East India. Newsl. J. Rhino Found. Nat. N. E. India 2013, 9, 70–72. [Google Scholar]
- Choudhury, A.U.; Gazzard, A. Petaurista mechukaensis. The IUCN Red List of Threatened Species 2024, e.T45959013A258448634. Available online: https://www.iucnredlist.org/species/45959013/258448634 (accessed on 13 November 2024).
- Choudhury, A.U.; Gazzard, A. Petaurista mishmiensis. The IUCN Red List of Threatened Species 2024, e.T45959040A258448912. Available online: https://www.iucnredlist.org/species/45959040/258448912 (accessed on 13 November 2024).
- Koli, V.K.; Jangid, A.K.; Singh, C.P. Habitat suitability mapping of the Indian giant flying squirrel (Petaurista philippensis Elliot, 1839) in India with ensemble modeling. Acta Ecol. Sin. 2023, 43, 644–652. [Google Scholar] [CrossRef]
- Searing, K.B.; Lomolino, M.V.; Rozzi, R. Melting climates shrink North American small mammals. Proc. Natl. Acad. Sci. USA 2023, 120, e2310855120. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J. Species distribution modelling supports the study of past, present and future biogeographies. J. Biogeogr. 2023, 50, 1485–1667. [Google Scholar] [CrossRef]
- Marston, C.; Raoul, F.; Rowland, C.; Quéré, J.-P.; Feng, X.; Lin, R.; Giraudoux, P. Mapping small mammal optimal habitats using satellite-derived proxy variables and species distribution models. PLoS ONE 2023, 18, e0289209. [Google Scholar] [CrossRef]
- Abedin, I.; Mukherjee, T.; Kim, A.R.; Kim, H.-W.; Kang, H.-E.; Kundu, S. Distribution Model Reveals Rapid Decline in Habitat Extent for Endangered Hispid Hare: Implications for Wildlife Management and Conservation Planning in Future Climate Change Scenarios. Biology 2024, 13, 198. [Google Scholar] [CrossRef]
- Benavides Rios, E.; Sadler, J.; Graham, L.; Matthews, T.J. Species distribution models and island biogeography: Challenges and prospects. Global Ecol. Conserv. 2024, 51, e02943. [Google Scholar] [CrossRef]
- Araújo, M.B.; Anderson, R.P.; Barbosa, A.M.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; Naimi, B.; et al. Standards for distribution models in biodiversity assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J. Species distribution modeling and ecological niche modeling: Getting the concepts right. Braz. J. Nat. Conserv. 2012, 10, 102–107. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Testing whether ensemble modelling is advantageous for maximising predictive performance of species distribution models. Ecography 2020, 43, 549–558. [Google Scholar] [CrossRef]
- Lamounier, W.L.; Silveira dos Santos, J.; Rodrigues, E.L.; Drumond, M.A. Defining priority areas for conservation based on multispecies functional connectivity. Biol. Conserv. 2024, 290, 110438. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, Z.; Zhuang, H.; Zhou, J.; Zhang, Y.; Lv, X.; Chen, M.; Krzton, A.; Xia, W. Identification of potential suitable areas and conservation priority areas for representative wild animals in the Greater and Lesser Khingan Mountains. Ecol. Evol. 2024, 14, e11600. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef] [PubMed]
- Lodrick, D.O. Arunachal Pradesh. Encyclopedia Britannica. 2025. Available online: https://www.britannica.com/place/Arunachal-Pradesh (accessed on 13 November 2024).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Karra, K.; Kontgis, C.; Statman-Weil, Z.; Mazzariello, J.C.; Mathis, M.; Brumby, S.P. Global Land Use/Land Cover with Sentinel-2 and Deep Learning. In Proceedings of the IGARSS 2021—2021 IEEE International Geoscience and Remote Sensing Symposium, Brussels, Belgium, 11–16 July 2021; IEEE: New York, NY, USA, 2021. [Google Scholar]
- SEDAC. Last of the Wild Project, Version 2, 2005 (LWP-2): Global Human Footprint Dataset (Geographic). Available online: https://www.earthdata.nasa.gov/data/catalog/sedac-ciesin-sedac-lwp2-hf-geog-2.00 (accessed on 13 November 2024).
- Morisette, J.T.; Jarnevich, C.S.; Holcombe, T.R.; Talbert, C.B.; Ignizio, D.; Talbert, M.K.; Silva, C.; Koop, D.; Swanson, A.; Young, N.E. VisTrails SAHM: Visualization and Workflow Management for Species Habitat Modeling. Ecography 2013, 36, 129–135. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A Toolbox for Comparative Studies of Environmental Niche Models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Andrews, M.B.; Ridley, J.K.; Wood, R.A.; Andrews, T.; Blockley, E.W.; Booth, B.; Burke, E.; Dittus, A.J.; Florek, P.; Gray, L.J.; et al. Historical Simulations with HadGEM3-GC3.1 for CMIP6. J. Adv. Model. Earth Syst. 2020, 12, e2019MS001995. [Google Scholar] [CrossRef]
- Li, L.; Xie, F.; Yuan, N. On the Long-Term Memory Characteristic in Land Surface Air Temperatures: How Well Do CMIP6 Models Perform? Atmos. Oceanic Sci. Lett. 2023, 16, 100291. [Google Scholar] [CrossRef]
- Gautam, S.; Shany, V.J. Navigating Climate Change in Southern India: A Study on Dynamic Dry-Wet Patterns and Urgent Policy Interventions. Geosyst. Geoenviron. 2024, 3, 100263. [Google Scholar] [CrossRef]
- Allen, B.J.; Hill, D.J.; Burke, A.M.; Clark, M.; Marchant, R.; Stringer, L.C.; Williams, D.R.; Lyon, C. Projected Future Climatic Forcing on the Global Distribution of Vegetation Types. Philos. Trans. R. Soc. B Biol. Sci. 2024, 379, 20230011. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E.; Elith, J.; Graham, C.H.; Phillips, S.; Peterson, A.T. What Matters for Predicting the Occurrences of Trees: Techniques, Data, or Species’ Characteristics? Ecol. Monogr. 2007, 77, 615–630. [Google Scholar] [CrossRef]
- Miller, J. Species Distribution Modeling. Geogr. Compass 2010, 4, 490–509. [Google Scholar] [CrossRef]
- Talbert, C.B.; Talbert, M.K. User Manual for SAHM Package for VisTrails. 2012. Available online: https://pubs.usgs.gov/publication/70118102 (accessed on 13 November 2024).
- Hayes, M.A.; Cryan, P.M.; Wunder, M.B. Seasonally-dynamic presence-only species distribution models for a cryptic migratory bat impacted by wind energy development. PLoS ONE 2015, 10, e0132599. [Google Scholar] [CrossRef]
- Lavazza, L.; Morasca, S.; Rotoloni, G. On the reliability of the area under the ROC curve in empirical software engineering. In Proceedings of the 27th International Conference on Evaluation and Assessment in Software Engineering, Oulu, Finland, 14–16 June 2023; pp. 93–100. [Google Scholar]
- Cohen, J. Weighted kappa: Nominal scale agreement provision for scaled disagreement or partial credit. Psychol. Bull. 1968, 70, 213–220. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Phillips, S.J.; Elith, J. POC Plots: Calibrating Species Distribution Models with Presence-Only Data. Ecology 2010, 91, 2476–2484. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Acevedo, P.; Barbosa, A.M.; Lobo, J.M.; Real, R. Discrimination Capacity in Species Distribution Models Depends on the Representativeness of the Environmental Domain. Glob. Ecol. Biogeogr. 2013, 22, 508–516. [Google Scholar] [CrossRef]
- McGarigal, K.; Marks, B.J. FRAGSTATS: Spatial Pattern Analysis Program for Quantifying Landscape Structure; Gen. Tech. Rep. PNW-GTR-351; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 1995; pp. 122–351. [CrossRef]
- Hesselbarth, M.H.K.; Sciaini, M.; With, K.A.; Wiegand, K.; Nowosad, J. landscapemetrics: An open-source R tool to calculate landscape metrics. Ecography 2019, 42, 1648–1657. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef]
- Kamalakannan, M.; Sivaperuman, C.; Kundu, S.; Gokulakrishnan, G.; Venkatraman, C.; Chandra, K. Discovery of a new mammal species (Soricidae: Eulipotyphla) from Narcondam volcanic island, India. Sci. Rep. 2021, 11, 9416. [Google Scholar] [CrossRef] [PubMed]
- Kaiho, K. Extinction magnitude of animals in the near future. Sci. Rep. 2022, 12, 19593. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S. Biodiversity conservation issues of Northeast India. Int. For. Rev. 2008, 10, 315–324. [Google Scholar] [CrossRef]
- Jokinen, M.; Hanski, I.; Numminen, E.; Valkama, J.; Selonen, V. Promoting species protection with predictive modelling: Effects of habitat, predators, and climate on the occurrence of the Siberian flying squirrel. Biol. Conserv. 2019, 230, 37–46. [Google Scholar] [CrossRef]
- Selonen, V.; Hongisto, K.; Hänninen, M.; Turkia, T.; Korpimäki, E. Weather and biotic interactions as determinants of seasonal shifts in abundance measured through nest-box occupancy in the Siberian flying squirrel. Sci. Rep. 2020, 10, 14465. [Google Scholar] [CrossRef]
- Bedoya-Canas, L.E.; López-Hernández, F.; Cortés, A.J. Climate Change Responses of High-Elevation Polylepis Forests. Forests 2024, 15, 811. [Google Scholar] [CrossRef]
- Luo, W.; Sun, C.; Yang, S.; Chen, W.; Sun, Y.; Li, Z.; Liu, J.; Tao, W.; Tao, J. Contrasting Range Changes and Drivers of Four Forest Foundation Species under Future Climate Change in China. Sci. Total Environ. 2024, 942, 173784. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Jetz, W. Projected range contractions of montane biodiversity under global warming. Proc. R. Soc. B Biol. Sci. 2010, 277, 3401–3410. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Rowe, R.J.; Terry, R.C. Small mammal responses to environmental change: Integrating past and present dynamics. J. Mammal. 2014, 95, 1157–1174. [Google Scholar] [CrossRef]
- Payne, B.L.; Bro-Jørgensen, J. Disproportionate climate-induced range loss forecast for the most threatened African antelopes. Curr. Biol. 2016, 26, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Dubos, N.; Montfort, F.; Grinand, C.; Nourtier, M.; Deso, G.; Probst, J.M.; Razafimanahaka, J.H.; Andriantsimanarilafy, R.R.; Rakotondrasoa, E.F.; Razafindraibe, P.; et al. Are narrow-ranging species doomed to extinction? Projected dramatic decline in future climate suitability of two highly threatened species. Perspect. Ecol. Conserv. 2022, 20, 18–28. [Google Scholar] [CrossRef]
- Costa-Pinto, A.L.; Bovendorp, R.S.; Heming, N.M.; Malhado, A.C.; Ladle, R.J. Where could they go? Potential distribution of small mammals in the Caatinga under climate change scenarios. J. Arid Environ. 2024, 221, 105133. [Google Scholar] [CrossRef]
- Lindenmayer, D. Small patches make critical contributions to biodiversity conservation. Proc. Natl. Acad. Sci. USA 2019, 116, 717–719. [Google Scholar] [CrossRef]
- Wintle, B.A.; Kujala, H.; Whitehead, A.; Cameron, A.; Veloz, S.; Kukkala, A.; Moilanen, A.; Gordon, A.; Lentini, P.E.; Cadenhead, N.C.R.; et al. Global synthesis of conservation studies reveals the importance of small habitat patches for biodiversity. Proc. Natl. Acad. Sci. USA 2019, 116, 909–914. [Google Scholar] [CrossRef]
- Saha, S.S. A new genus and a new species of flying squirrel (Mammalia: Rodentia: Sciuridae) from northeastern India. Bull. Zool. Surv. India 1981, 4, 331–336. [Google Scholar]
- Choudhury, A. Description of a new species of giant flying squirrel of the genus Petaurista Link, 1795 from the Siang Basin, Arunachal Pradesh in North East India. Newsl. J. Rhino Found. Nat. N. E. India 2013, 9, 30–38. [Google Scholar]
- Molur, S. Biswamoyopterus biswasi (Errata Version Published in 2017). The IUCN Red List of Threatened Species 2016, e.T2816A115063959. Available online: https://www.iucnredlist.org/species/2816/115063959 (accessed on 13 November 2024).
- Molur, S.; Srinivasulu, C.; Srinivasulu, B.; Walker, S.; Nameer, P.O.; Ravikumar, L. Status of Non-Volant Small Mammals: Conservation Assessment and Management Plan (C.A.M.P); Workshop Report; Zoo Outreach Organisation/CBSG-South Asia: Coimbatore, India, 2005. [Google Scholar]
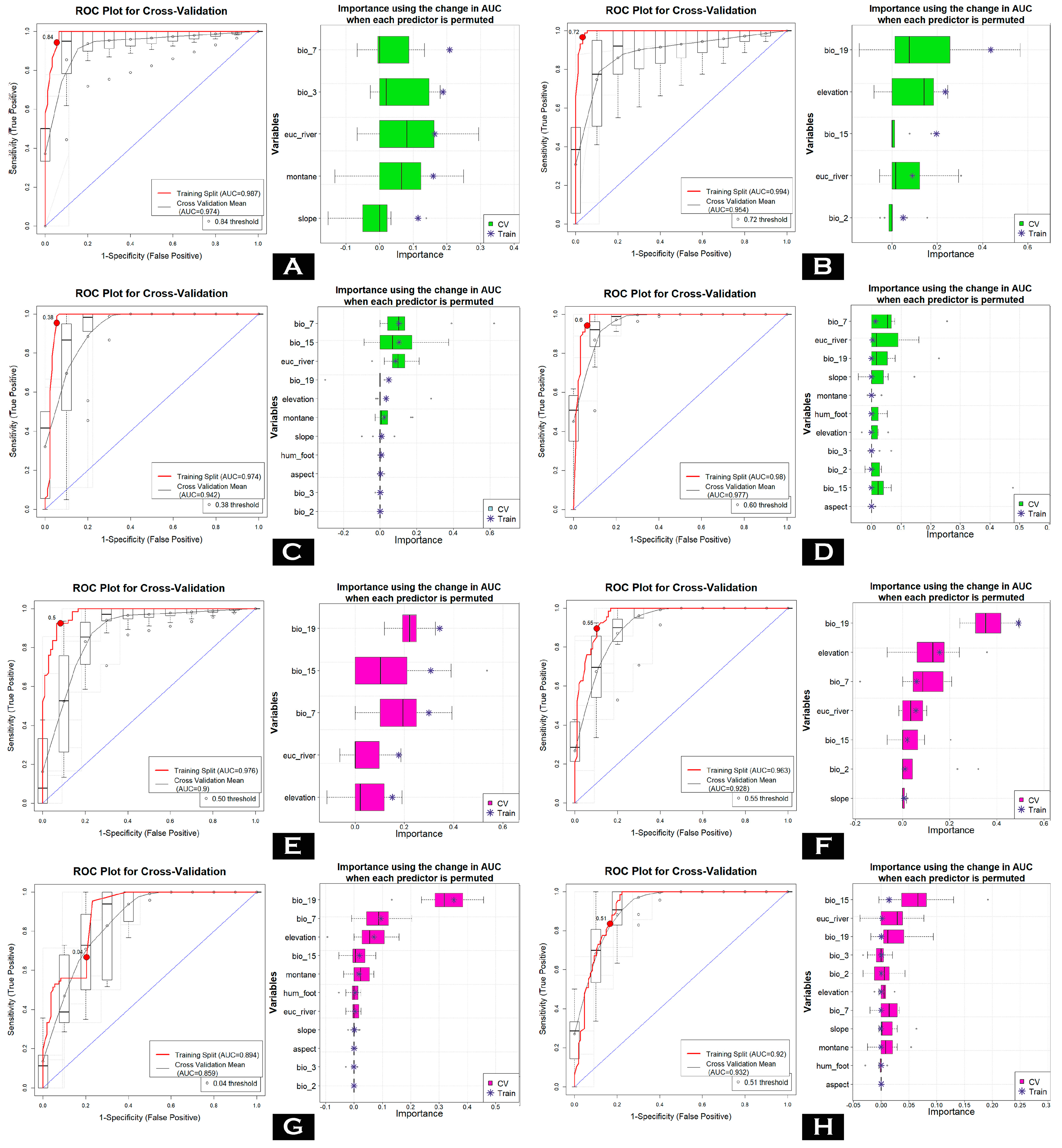
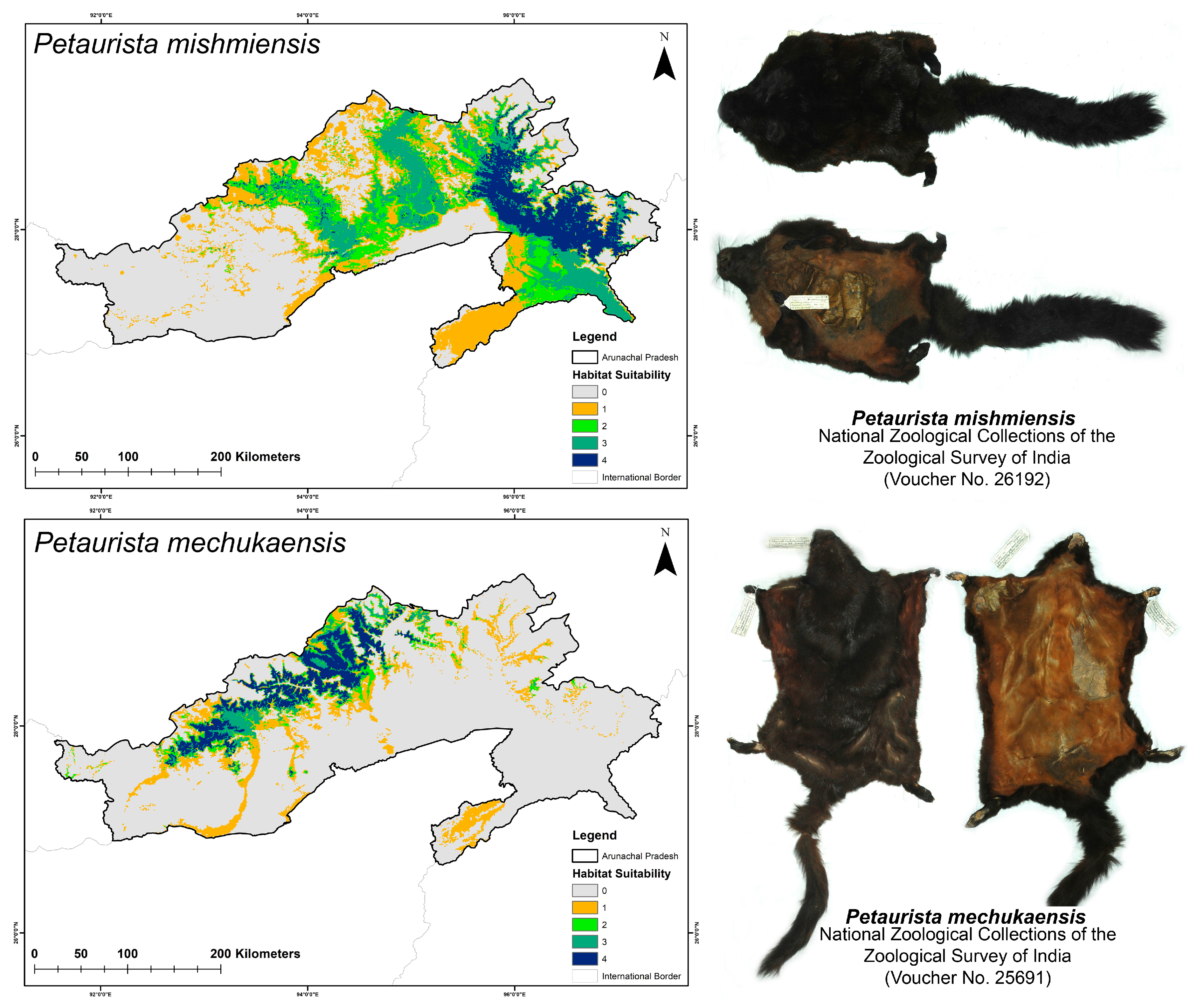
| Species | Model | Dataset | AUC | ΔAUC | PCC | TSS | Kappa | Specificity | Sensitivity |
|---|---|---|---|---|---|---|---|---|---|
| Petaurista mishmiensis | GLM | Train | 0.987 | 0.013 | 94.5 | 0.889 | 0.889 | 0.946 | 0.943 |
| CV | 0.974 | 94.4 | 0.887 | 0.887 | 0.946 | 0.942 | |||
| MARS | Train | 0.994 | 0.040 | 96.7 | 0.934 | 0.934 | 0.968 | 0.966 | |
| CV | 0.954 | 91.6 | 0.829 | 0.83 | 0.936 | 0.893 | |||
| MaxEnt | Train | 0.974 | 0.032 | 95 | 0.9 | 0.9 | 0.946 | 0.955 | |
| CV | 0.942 | 92.7 | 0.854 | 0.853 | 0.924 | 0.929 | |||
| RF | Train | 0.98 | 0.003 | 93.9 | 0.879 | 0.878 | 0.935 | 0.943 | |
| CV | 0.977 | 92.7 | 0.854 | 0.853 | 0.924 | 0.929 | |||
| Petaurista mechukaensis | GLM | Train | 0.976 | 0.076 | 92 | 0.842 | 0.833 | 0.917 | 0.925 |
| CV | 0.9 | 85.2 | 0.708 | 0.693 | 0.844 | 0.864 | |||
| MARS | Train | 0.963 | 0.035 | 89.7 | 0.794 | 0.785 | 0.898 | 0.896 | |
| CV | 0.928 | 84.1 | 0.671 | 0.664 | 0.862 | 0.81 | |||
| MaxEnt | Train | 0.894 | 0.035 | 74.7 | 0.463 | 0.463 | 0.796 | 0.667 | |
| CV | 0.859 | 76.3 | 0.536 | 0.516 | 0.75 | 786 | |||
| RF | Train | 0.92 | 0.012 | 83.4 | 0.669 | 0.656 | 0.833 | 0.836 | |
| CV | 0.932 | 85.2 | 0.698 | 0.686 | 0.872 | 0.826 |
| Species | Variables | GLM | MARS | MAXENT | RF | μ (Mean) | μ (Mean) % |
|---|---|---|---|---|---|---|---|
| Petaurista mishmiensis | aspect | 0 | 0 | 0.00194 | 0 | 0.000485 | 0.09 |
| bio_15 | 0 | 0.1965 | 0.10238 | 0 | 0.07472 | 13.18 | |
| bio_19 | 0 | 0.43604 | 0.04734 | 0.00048 | 0.120965 | 21.34 | |
| bio_2 | 0 | 0.04954 | 0 | 0 | 0.012385 | 2.19 | |
| bio_3 | 0.18854 | 0 | 0 | 0 | 0.047135 | 8.32 | |
| bio_7 | 0.20828 | 0 | 0.10346 | 0.01328 | 0.081255 | 14.34 | |
| elevation | 0 | 0.23594 | 0.03232 | 0 | 0.067065 | 11.83 | |
| euc_river | 0.1634 | 0.09014 | 0.08374 | 0.00398 | 0.085315 | 15.05 | |
| hum_foot | 0 | 0 | 0.00584 | 0 | 0.00146 | 0.26 | |
| montane | 0.15948 | 0 | 0.02264 | 0 | 0.04553 | 8.03 | |
| slope | 0.1142 | 0 | 0.0074 | 0 | 0.0304 | 5.36 | |
| Petaurista mechukaensis | aspect | 0 | 0 | 0.001 | 0.001 | 0.0005 | 0.07 |
| bio_15 | 0.30726 | 0.02008 | 0.02498 | 0.0142 | 0.09163 | 13.46 | |
| bio_19 | 0.34306 | 0.49132 | 0.32502 | 0.0004 | 0.28995 | 42.58 | |
| bio_2 | 0 | 0.01118 | 0 | 0.00016 | 0.002835 | 0.42 | |
| bio_3 | 0 | 0 | 0 | 0.00028 | 0.00007 | 0.01 | |
| bio_7 | 0.29926 | 0.06036 | 0.10358 | 0.00008 | 0.11582 | 17.01 | |
| elevation | 0.15122 | 0.15754 | 0.10714 | 0.00012 | 0.104005 | 15.27 | |
| euc_river | 0.1771 | 0.0568 | 0.00352 | 0.0021 | 0.05988 | 8.79 | |
| hum_foot | 0 | 0 | 0.002 | 0.001 | 0.00075 | 0.11 | |
| montane | 0 | 0 | 0.05358 | 0.001 | 0.013645 | 2.00 | |
| slope | 0 | 0.00718 | 0.00002 | 0 | 0.0018 | 0.26 |
| Species | Scenario | NP | PD | LPI | TE | LSI | AI |
|---|---|---|---|---|---|---|---|
| Petaurista mishmiensis | Present | 253 | 37,376,848.5 | 7.5356 | 73.728 | 20.9234 | 81.7262 |
| SSP 245 (2041–2060) | 243 | 35,899,502.7 | 5.4357 | 53.24 | 21.0629 | 78.9056 | |
| SSP 245 (2061–2080) | 219 | 32,353,872.8 | 2.8195 | 45.408 | 23.3214 | 71.9573 | |
| SSP 585 (2041–2060) | 238 | 32,513,416.7 | 3.1599 | 52.6 | 24.1074 | 70.92 | |
| SSP 585 (2061–2080) | 210 | 29,297,398 | 1.527 | 45.208 | 25.1094 | 66.2419 | |
| Petaurista mechukaensis | Present | 76 | 11,227,828 | 4.5431 | 43.088 | 16.3576 | 81.0088 |
| SSP 245 (2041–2060) | 98 | 14,477,988.7 | 4.2557 | 45.496 | 18.634 | 76.6144 | |
| SSP 245 (2061–2080) | 139 | 20,535,106.5 | 1.5591 | 32.776 | 17.2689 | 71.9054 | |
| SSP 585 (2041–2060) | 153 | 22,603,390.6 | 1.5828 | 48.928 | 20.0131 | 74.7021 | |
| SSP 585 (2061–2080) | 131 | 19,353,229.8 | 0.8339 | 30.936 | 17.6636 | 68.9848 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abedin, I.; Kamalakannan, M.; Mukherjee, T.; Choudhury, A.; Singha, H.; Abedin, J.; Banerjee, D.; Kim, H.-W.; Kundu, S. Fading into Obscurity: Impact of Climate Change on Suitable Habitats for Two Lesser-Known Giant Flying Squirrels (Sciuridae: Petaurista) in Northeastern India. Biology 2025, 14, 242. https://doi.org/10.3390/biology14030242
Abedin I, Kamalakannan M, Mukherjee T, Choudhury A, Singha H, Abedin J, Banerjee D, Kim H-W, Kundu S. Fading into Obscurity: Impact of Climate Change on Suitable Habitats for Two Lesser-Known Giant Flying Squirrels (Sciuridae: Petaurista) in Northeastern India. Biology. 2025; 14(3):242. https://doi.org/10.3390/biology14030242
Chicago/Turabian StyleAbedin, Imon, Manokaran Kamalakannan, Tanoy Mukherjee, Anwaruddin Choudhury, Hilloljyoti Singha, Joynal Abedin, Dhriti Banerjee, Hyun-Woo Kim, and Shantanu Kundu. 2025. "Fading into Obscurity: Impact of Climate Change on Suitable Habitats for Two Lesser-Known Giant Flying Squirrels (Sciuridae: Petaurista) in Northeastern India" Biology 14, no. 3: 242. https://doi.org/10.3390/biology14030242
APA StyleAbedin, I., Kamalakannan, M., Mukherjee, T., Choudhury, A., Singha, H., Abedin, J., Banerjee, D., Kim, H.-W., & Kundu, S. (2025). Fading into Obscurity: Impact of Climate Change on Suitable Habitats for Two Lesser-Known Giant Flying Squirrels (Sciuridae: Petaurista) in Northeastern India. Biology, 14(3), 242. https://doi.org/10.3390/biology14030242










