Characterizing the Role of Moringa oleifera Lam (MO) Leaves and Root Extracts on Dictyostelium discoideum Cell Behavior
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dictyostelium Discoideum Cell Lines and Cell Culture
2.2. Source of Moringa Oleifera Leaves and Roots
2.3. Determination of Total Phenolics, Tocopherols, Flavonoids, Rutin, and Gallic Acid Through the Use of High-Performance Liquid Chromatography (HPLC)
2.4. GC/MS Analysis of Moringa oleifera Leaves and Roots
2.5. Preparation of Moringa oleifera Leaf and Root Extracts
2.6. D. discoideum Growth Assay
2.7. Viability Assay
2.8. D. discoideum Development Experiment
2.9. Statistical Analysis
3. Results
3.1. HPLC Analysis for Moringa oleifera Roots and Leaves Extracts
3.2. GC/MS Analysis of the Leave and Roots of Moringa oleifera
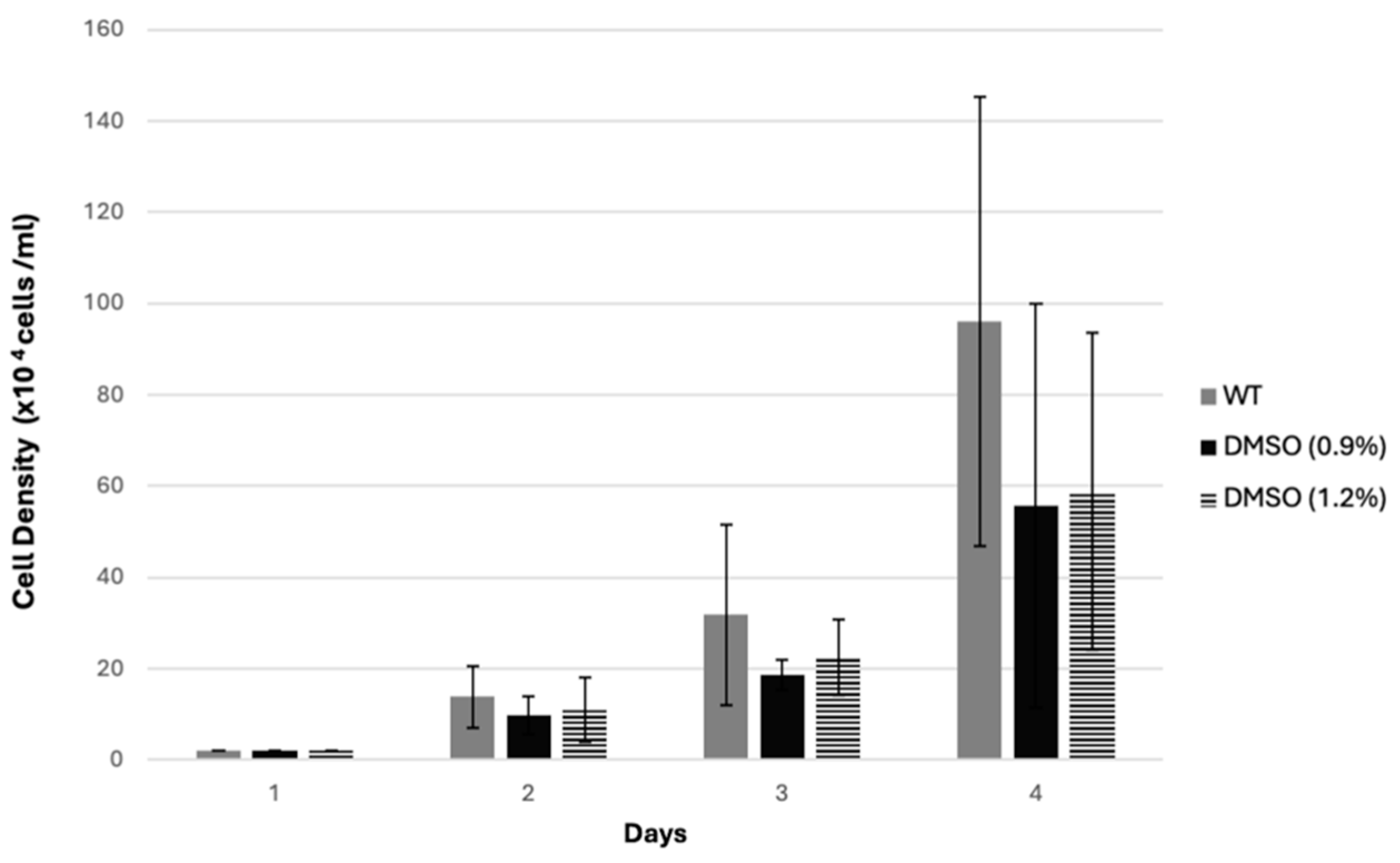
3.3. Effect of ML and MR Extracts at Different Concentrations on D. discoideum Cell Growth
3.4. Effect of ML and MR Extracts at Concentrations of 900 µg/mL and 1200 µg/mL on D. Discoideum’s Development Life Cycle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abd Rani, N.Z.; Husain, K.; Kumolosasi, E. Moringa Genus: A Review of Phytochemistry and Pharmacology. Front. Pharmacol. 2018, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Asante, F.A.; Oduro, I.; Ellis, W.O.; Saalia, F.K. Effect of Planting Period and Site on the Chemical Composition and Milk Acceptability of Tigernut (Cyperus Esculentus L.) Tubers in Ghana. Am. J. Food Nutr. 2014, 2, 49–54. [Google Scholar]
- Singh, A.; Navneet, X. Ethnomedicinal, pharmacological and antimicrobial aspects of Moringa oleifera lam.: A review. J. Phytopharmacol. 2018, 7, 45–50. [Google Scholar] [CrossRef]
- Parvathy, M.V.S.; Umamaheshwari, A. Cytotoxic Effect of Moringa oleifera Leaf Extracts on Human Multiple Myeloma Cell Lines. Trends Med. Res. 2007, 2, 44–50. [Google Scholar]
- Tiloke, C.; Phulukdaree, A.; Chuturgoon, A.A. The antiproliferative effect of Moringa oleifera crude aqueous leaf extract on cancerous human alveolar epithelial cells. BMC Complement. Altern. Med. 2013, 13, 226. [Google Scholar] [CrossRef]
- Jung, I.L.; Lee, J.H.; Kang, S.C. A potential oral anticancer drug candidate, Moringa oleifera leaf extract, induces the apoptosis of human hepatocellular carcinoma cells. Oncol. Lett. 2015, 10, 1597–1604. [Google Scholar] [CrossRef]
- Leelawat, S.; Leelawat, K. Moringa oleifera Extracts Induce Cholangiocarcinoma Cell Apoptosis by Induction of Reactive Oxygen Species Production. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 183–189. [Google Scholar]
- Vongsak, B.; Mangomool, S.; Gritsanapan, W. Antioxidant Activity and Induction of mRNA Expressions of Antioxidant Enzymes in HEK-293 Cells of Moringa oleifera Leaf Extract. Planta Med. 2015, 81, 1084–1089. [Google Scholar] [CrossRef]
- Pareek, A.; Jain, V.; Pareek, A.; Pant, M.; Gupta, M.M.; Chuturgoon, A.A. Moringa oleifera: An Updated Comprehensive Review of Its Pharmacological Activities, Ethnomedicinal, Phytopharmaceutical Formulation, Clinical, Phytochemical, and Toxicological Aspects. Int. J. Mol. Sci. 2023, 24, 2098. [Google Scholar] [CrossRef]
- Kessin, R.H. Dictyostelium: Evolution, Cell Biology, and the Development of Multicellularity; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Devreotes, P.; Horwitz, A.R. Signaling Networks that Regulate Cell Migration. Cold Spring Harb. Perspect. Biol. 2015, 7, a005959. [Google Scholar] [CrossRef]
- Singer, G.; Araki, T.; Weijer, C.J. Oscillatory CAMP Cell-Cell Signalling Persists during Multicellular Dictyostelium Development. Commun. Biol. 2019, 2, 139. [Google Scholar] [CrossRef] [PubMed]
- Draye, L.L.; Van Haastert, P.J.M. Transmembrane signalling in eukaryotes: A comparison between higher and lower eukaryotes. Plant Mol. Biol. 2015, 26, 1239–1270. [Google Scholar] [CrossRef] [PubMed]
- Schaf, J.; Damstra-Oddy, J.; Williams, R.S.B. Dictyostelium discoideum as a pharmacological model system to study the mechanisms of medicinal drugs and natural products. Int. J. Dev. Biol. 2019, 63, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Tiwari, P.; Sahu, P.K.; Kumar, S.A. Review of the Phytochemical and Pharmacological Characteristics of Moringa oleifera. J. Pharm. Bioallied. Sci. 2018, 10, 181–191. [Google Scholar]
- Garcia, G.L.; Parent, C.A. Signal relay during chemotaxis. J. Microsc. 2008, 231, 529–534. [Google Scholar] [CrossRef]
- Öztürk, N.; Tunçel, M.; Tunçel, N.B. Determination of Phenolic Acids by a Modified HPLC: Its Application to Various Plant Materials. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 587–596. [Google Scholar] [CrossRef]
- Carpenter, A.P. Determination of tocopherols in vegetable oils. J. Am. Oil Chem. Soc. 1979, 56, 668–671. [Google Scholar] [CrossRef]
- Rajanandh, M.G.; Kavitha, J. Quantitative estimation of β-sitosterol, total phenolic and flavonoid compounds in the leaves of Moringa oleifera. J. PharmTech Res. 2010, 2, 1409–1414. [Google Scholar]
- AlDayel, M.F.; El-Sherif, F. Evaluation of the effects of Chlorella vulgaris, Nannochloropsis salina, and Enterobacter cloacae on growth, yield and active compound compositions of Moringa oleifera under salinity. Saudi J. Biol. Sci. 2021, 28, 1687–1696. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Becker, K. Nutrional value and antinutritional components ofwhole and ethanol extracted Moringa oleifera leaves. Anim. Feed Sci. Tech. 1996, 63, 211–228. [Google Scholar] [CrossRef]
- El Sherif, F.; Albotnoor, N.; Yap, Y.-K.; Meligy, A.; Khattab, S. Enhanced bioactive compounds composition of Lavandula officinalis in-vitro plantlets using NaCl and Moringa oleifera, Aloe vera and Spirulina platensis extracts. Ind. Crops Prod. 2020, 157, 112890. [Google Scholar] [CrossRef]
- Lee, K.B.; Choi, J.; Ahn, S.K.; Na, J.-K.; Shrestha, K.K.; Nguon, S.; Park, S.U.; Choi, S.; Kim, J.K. Quantification of Arbutin in Plant Extracts by Stable Isotope Dilution Gas Chromatography–Mass Spectrometry. Chromatographia 2018, 81, 533–538. [Google Scholar] [CrossRef]
- El Sherif, F.; Yap, Y.; Alamer, S.; Althumairy, D.; Khattab, S. Laser Seed Pretreatment Alters the Silybin Content and Anti-Dictyostelium discoideum Cell Growth Activity of Silybum marianum (L.) Fruit. Appl. Sci. 2023, 13, 3546. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa Oleifera: A food Plant With Multiple Medicinal Uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef]
- Bhadresha, K.; Thakore, V.; Brahmbhatt, J.; Upadhyay, V.; Nayan, J.; Rawal, R. Anticancer effect of Moringa oleifera leaves extract against lung cancer cell line via induction of apoptosis. Adv. Cancer Biol. Metastasis 2022, 6, 100072. [Google Scholar] [CrossRef]
- Cirmi, S.; Ferlazzo, N.; Gugliandolo, A.; Musumerci, L.; Mazzon, E.; Bramanti, A.; Navarra, M. Moringin from Moringa oleifera Seeds Inhibits Growth, Arrests Cell-Cycle, and Induces Apoptosis of SH-SY5Y Human Neuroblastoma Cells through the Modulation of NF- B and Apoptotic Related Factors. Int. J. Mol. Sci. 2019, 20, 1930. [Google Scholar] [CrossRef]
- Stohs, S.J.; Hartman, M.J. Review of the Safety and Efficacy of Moringa oleifera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef]
- Terbach, N.; Shah, R.; Kelemen, R.; Klein, P.S.; Gordienko, D.; Brown, N.A.; Wilkinson, C.J.; Williams, R.S.B. Identifying an uptake mechanism for the antiepileptic and bipolar disorder treatment valproic acid using the simple biomedical model Dictyostelium. J. Cell Sci. 2011, 124, 2267–2276. [Google Scholar] [CrossRef]
- Chang, P.; Orabi, B.; Deranieh, R.M.; Dham, M.; Hoeller, O.; Shimshoni, J.A.; Yagen, B.; Bialer, M.; Greenberg, M.L.; Walker, M.C.; et al. The antiepileptic drug valproic acid and other medium-chain fatty acids acutely reduce phosphoinositide levels independently of inositol in Dictyostelium. Dis. Models Mech. 2012, 5, 115–124. [Google Scholar] [CrossRef]
- Haver, H.N.; Scaglione, K.M. Dictyostelium Discoideum as a model for investigating neurodegenerative diseases. Front. Cell. Neurosci. 2021, 15, 759532. [Google Scholar] [CrossRef]
- Story, C.L.; Williams, R.S.B.; Fisher, P.R.; Annesley, S.J. Dictyostelium discoideum: A Model System for Neurological Disorders. Cells 2022, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- Asante, W.J.; Nasare, I.L.; Tom-Dery, D.; Ochire-Boadu, K.; Kentil, K.B. Nutrient composition of Moringa oleifera leaves from two agro ecological zones in Ghana. Acad. J. 2014, 8, 65–71. [Google Scholar]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kregiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed]
- Aydin, H.H.; Celik, H.A.; Deveci, R.; Karacali, S.; Saydam, G.; Omay, S.B.; Batur, Y. Induction of apoptosis by fatty acid ethyl esters in HepG2 cells. Food Chem. Toxicol. 2005, 43, 139–145. [Google Scholar] [CrossRef]
- Saeed, N.M.; El-Demerdash, E.; Abdel-Rahman, H.M.; Algandaby, M.M.; Al-Abbasi, F.A.; Abdel-Naim, A.B. Anti-inflammatory activity of methyl palmitate and ethyl palmitate in different experimental rat models. Toxicol. Appl. Pharmacol. 2012, 264, 84–93. [Google Scholar] [CrossRef]
- Martínez-Pérez, C.; Ward, C.; Turnbull, A.K.; Mullen, P.; Cook, G.; Meehan, J.; Jarman, E.J.; Thomson, P.I.T.; Campbell, C.J.; McPhail, D.; et al. Antitumour activity of the novel flavonoid Oncamex in preclinical breast cancer models. Br. J. Cancer 2016, 114, 905–916. [Google Scholar] [CrossRef]
- Waheed, A.; Ludtmann, M.H.R.; Pakes, N.; Robery, S.; Kuspa, A.; Dinh, C.; Baines, D.; Williams, R.S.B.; Carew, M.A. Naringenin inhibits the growth of Dictyostelium and MDCK-derived cysts in a TRPP2 (polycystin-2)-dependent manner. Br. J. Pharmacol. 2014, 171, 2659–2670. [Google Scholar] [CrossRef]
- Sanaye, P.M.; Mojaveri, M.R.; Ahmadian, R.; Jahromi, M.S.; Bahramsoltani, R. Apigenin and its dermatological applications: A comprehensive review. Phytochemistry 2022, 113390, 113390. [Google Scholar] [CrossRef]
- Malchow, D.; Schaloske, R.; Schlatterer, C. An increase in cytosolic Ca2+ delays cAMP oscillations in Dictyostelium cells. Biochem. J. 1996, 319, 323–327. [Google Scholar] [CrossRef]
- Aizawa, H.; Sutoh, K.; Yahara, I. Overexpression of cofilin stimulates bundling of actin filaments, membrane ruffling, and cell movement in Dictyostelium. J. Cell Biol. 1996, 132, 335–344. [Google Scholar] [CrossRef]
- Gloss, A.; Rivero, F.; Khaire, N.; Muller, R.; Loomis, W.F.; Schleicher, M.; Noegel, A.A. Villidin, a novel WD-repeat and Villin- related protein from Dictyostelium, is associated with membranes and the cytoskeleton. Mol. Biol. Cell 2003, 14, 2716–2727. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, F.; Snaidero, N.; Kleinberger, G.; Madore, C.; Daria, A.; Werner, G.; Krasemann, S.; Capell, A.; Trümbach, D.; Wurst, W.; et al. TREM2 deficiency impairs chemotaxis and microglial responses to neuronal injury. EMBO Rep. 2017, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Mathavarajah, S.; Mclaren, M.D.; Huber, R.J. Cln3 function is linked to osmoregulation in a Dictyostelium model of Batten disease. BBA—Mol. Basis Dis. 2018, 1864, 3559–3573. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Myre, M.A.; Cotman, S.L. Aberrant adhesion impacts early development in a Dictyostelium model for juvenile neuronal ceroid lipofuscinosis. Cell Adhes. Migr. 2017, 11, 399–418. [Google Scholar] [CrossRef]
- Ghimire, S.; Subedi, L.; Acharya, N.; Gaire, B.P. Moringa oleifera: A Tree of Life as a Promising Medicinal Plant for Neurodegenerative Diseases. J. Agric. Food Chem. 2021, 69, 12–56. [Google Scholar] [CrossRef]
- Amrutia, J.N.; Lala, M.; Srinivasa, U.; Shabaraya, A.R.; Semuel, M.R. Anticonvulsant activity of Moringa oleifera leaf. Int. Res. J. Pharm. 2011, 10, 160–162. [Google Scholar]
- Charrière, K.; Schneider, V.; Perrignon-Sommet, M.; Jacquin-Piques, A.; Vejux, A. Exploring the Role of Apigenin in Neuroinflammation: Insights and Implications. Int. J. Mol. Sci. 2024, 25, 5041. [Google Scholar] [CrossRef]



| Chemical | Amount | Unit | |
|---|---|---|---|
| Leaf | Root | ||
| Total apigenin | 10.5 | 2.65 | µmol/g |
| Total polyphenols | 28.1 | 29.3 | mg/g |
| Total flavonoids | 81 | 1.53 | mg/g |
| Total tocopherols | 5 | 5.36 | μg/g |
| Total rutin | 0.278 | Absent | mg/g |
| Total gallic acid | 0.128 | 0.09594 | mg/g |
| Essential Oil Compounds | Area % | |
|---|---|---|
| Root | Leaves | |
| Benzylamine | 29.28 | 0.02 |
| 2,3-Dihydro-2,5-dihydroxy-6-methyl-4H-pyran-4-one | 0.17 | 0.02 |
| 2-Methyl-1-butene | 1.55 | 0.09 |
| Lauric acid | 0.12 | 0.09 |
| Dibutyl phthalate | 0.1 | 0.05 |
| Palmitic acid | 7.8 | 1.23 |
| Ethyl stearate | 0.67 | 7.15 |
| Ethyl Linoleate | 1.03 | 4.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamer, S.A.; Sherif, F.E. Characterizing the Role of Moringa oleifera Lam (MO) Leaves and Root Extracts on Dictyostelium discoideum Cell Behavior. Biology 2025, 14, 284. https://doi.org/10.3390/biology14030284
Alamer SA, Sherif FE. Characterizing the Role of Moringa oleifera Lam (MO) Leaves and Root Extracts on Dictyostelium discoideum Cell Behavior. Biology. 2025; 14(3):284. https://doi.org/10.3390/biology14030284
Chicago/Turabian StyleAlamer, Sarah Abdulaziz, and Fadia El Sherif. 2025. "Characterizing the Role of Moringa oleifera Lam (MO) Leaves and Root Extracts on Dictyostelium discoideum Cell Behavior" Biology 14, no. 3: 284. https://doi.org/10.3390/biology14030284
APA StyleAlamer, S. A., & Sherif, F. E. (2025). Characterizing the Role of Moringa oleifera Lam (MO) Leaves and Root Extracts on Dictyostelium discoideum Cell Behavior. Biology, 14(3), 284. https://doi.org/10.3390/biology14030284






