Modification of Tumor Necrosis Factor-α and C-C Motif Chemokine Ligand 18 Secretion by Monocytes Derived from Patients with Diabetic Foot Syndrome
Abstract
:1. Introduction
2. Materials and Methods
- Signed and dated informed consent,
- Male and female aged ≥ 35 years old, and
- Patients with newly diagnosed T2DM not receiving glucose-lowering therapy, or
- Patients suffering from T2DM without DFS, or
- Patients suffering from T2DM with DFS, receiving insulin therapy.
- Current inflammatory condition (odontogenic, pulmonary, pelvic, etc.) and/or chronic disease;
- Current abuse of alcohol;
- Current smoking;
- Stage ≥ 3b chronic kidney disease including dialysis for acute renal failure within 12 months prior to inclusion in the study;
- Acute Human Immunodeficiency Viruses (HIV) infection, hepatitis С/D virus, hepatic cirrhosis;
- History of myocardial infarction and/or stroke within 2 months prior inclusion in the study;
- Pregnancy or lactation;
- Decompensated hypothyroidism;
- Acute trauma, surgical or other condition;
- Receiving metformin within 6 months prior to inclusion in the study;
- Patients with foot ulcers with signs of a systemic inflammatory reaction.
3. Results
3.1. Pro- and Anti-Inflammatory Responce of Blood-Derived Monocytes from Patients with Different Durations of T2DM
3.2. The Status of Carbohydrate Metabolism and Pro- and Anti-Inflammatory Responce of Blood-Derived Monocytes
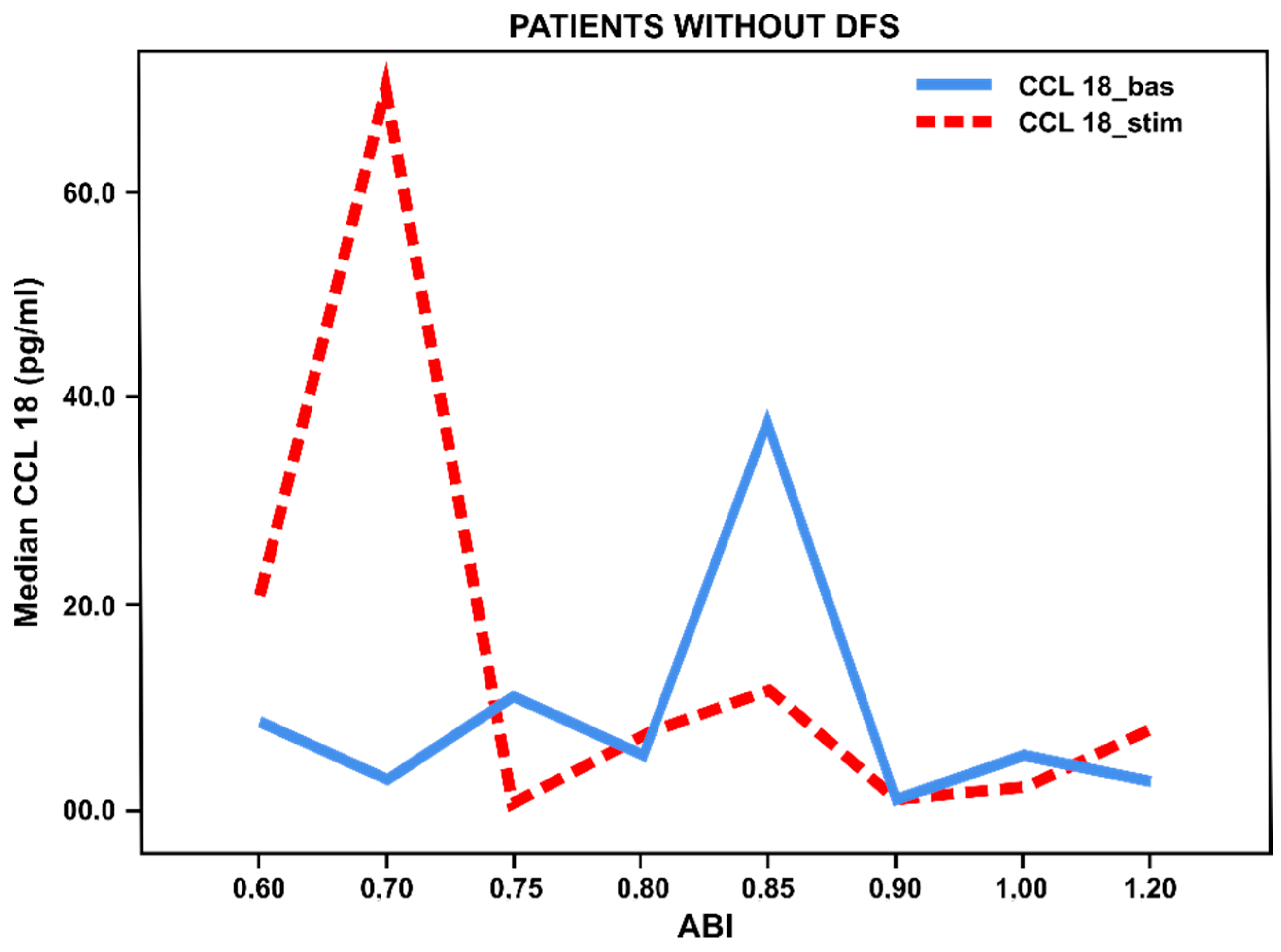
3.3. Pro- and Anti-Inflammatory Activation Status of Blood-Derived Monocytes from Patients with Lower Limbs Ischemia
3.4. Pro- and Anti-Inflammatory Activation of Blood-Derived Monocytes, Depending on the Duration of the Foot Ulcers
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dedov, I.I.; Kalashnikova, M.F.; Belousov, D.Y. Analysis of the cost of type 2 diabetes in Russia: the results of a Russian multicenter observational pharmacoepidemiological study of Forsyth SD2T. Diabetes Mellit. 2017, 20, 403–419. [Google Scholar] [CrossRef] [Green Version]
- Wass, J.; Owen, К. Oxford Handbook of Endocrinology and Diabetes; Oxford University Press: Oxford, UK, 2013; pp. 684–806. [Google Scholar] [CrossRef]
- Gurieva, I.V.; Svetlova, O.V.; Khlopin, H.M. Diabetic painful neuropathy: The effect of “hyperglycemic memory” on pathogenetic on pathogenetic approaches to treatment. Rus. Med. J. 2013, 27. Available online: https://www.rmj.ru/articles/bolevoy_sindrom/Bolevaya_diabeticheskaya_neyropatiya_vliyanie_giperglikemicheskoy_pamyati_na_patogeneticheskie_podhody_k_lecheniyu/ (accessed on 17 November 2019).
- Levin, O.S. Polyneuropathy. Сlinical Management; Medical Information Agency of Russia: Moscow, Russia, 2016; p. 156. [Google Scholar]
- Lotfy, M.; Adeghate, J.; Kalasz, H.; Singh, J.; Adeghate, E. Chronic complications of diabetes mellitus: A mini review. Curr. Diabetes Rev. 2017, 13, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Nedosugova, L.V. Pathogenesis, clinical manifestations, approaches to the treatment of diabetic polyneuropathy. Med. Counc. 2013, 12, 43–49. [Google Scholar] [CrossRef]
- Lankin, V.Z.; Tikhaze, A.K.; Belenkov, I. Free radical processes in diseases of the cardiovascular system. Cardiology 2000, 40, 48–61. [Google Scholar]
- Lankin, V.Z.; Tikhaze, A.K.; Belenkov, I. Antioxidants in complex therapy of atherosclerosis: pro et contra. Kardiologiia 2004, 44, 72–81. [Google Scholar]
- Sobenin, I.A. Principles of Pathogenetic Therapy of Atherosclerosis. Use of Cellular Models. Doctoral Dissertation, Research Institute for Pathology and Pathophysiology, Moscow, Russia, 2010. [Google Scholar]
- Titov, V.N. General features of atherosclerosis and inflammation: specificity of atherosclerosis as an inflammatory process. Clin. Lab. Diagn. 2000, 4, 3–10. [Google Scholar]
- Tousoulis, D.; Davies, G.; Stefanadis, С. Inflammatory and thrombotic mechanisms in coronary atherosclerosis. Heart 2003, 89, 993–997. [Google Scholar] [CrossRef]
- Ley, K.; Miller, Y.I.; Hedrick, C.C. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb. Vasc. Biol. 2011, 31, 1506–1516. [Google Scholar] [CrossRef] [Green Version]
- Shin, R.H.; Wang, C.Y.; Yang, C.M. NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A. Oxidative Stress and Stress-Activated Signaling Pathways: А Unifying Hypothesis of Type 2 Diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, S.; Martinez, F.O. Alternative Activation of Macrophages: Mechanism and Functions. Immunity 2010, 28, 593–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, N.; Moeller, J.; Vogel, V. Mechanobiology of Macrophages: How Physical Factors Coregulate Macrophage Plasticity and Phagocytosis. Ann. Rev. Biomed. Eng. 2019, 21, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F.J. Biomaterial based modulation of macrophage activation: a review and suggested design principles. Materialstoday 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Lyamina, S.V.; Malyshev, I. Activation of macrophages in the modern concept of the formation of the immune response. J. Fundam. Res. 2014, 10, 930–935. [Google Scholar]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [Green Version]
- Mirza, R.E.; Koh, T.J. Dysregulation of monocyte/macrophage response in wounds of diabetic mice. Cytokine 2011, 56, 256–264. [Google Scholar] [CrossRef]
- Miao, M.; Niu, Y.; Xie, T.; Yuan, B.; Qing, C.; Lu, S. Diabetes impaired wound healing and altered macrophage activation: a possible pathophysiologic correlation. Wound Repair Regen. 2012, 20, 203–213. [Google Scholar] [CrossRef]
- Nikiforov, N.G.; Galstyan, K.O.; Nedosugova, L.V.; Elizova, N.V.; Kolmychkova, K.I.; Ivanova, E.A. Proinflammatory monocyte polarization in type 2 diabetes mellitus and coronary heart disease. Vessel Plus 2017, 1, 192–195. [Google Scholar] [CrossRef] [Green Version]
- Orekhov, A.N.; Nikiforov, N.G.; Elizova, N.V.; Ivanova, E.A.; Makeev, V.J. Phenomenon of individual monocyteence in human monocyte activation. Exp. Mol. Pathol. 2015, 99, 151–154. [Google Scholar] [CrossRef]
- Barnes, P.J.; Karin, M. Nuclear factor-kB: A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Mirza, R.E.; Fang, M.M.; Weinheimer-Haus, E.M.; Ennis, W.J.; Koh, T.J. Sustained Inflammasome Activity in Macrophages Impairs Wound Healing in Type 2 Diabetic Humans and Mice. Diabetes 2014, 63, 1103–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacLeod, A.S.; Mansbridge, J.N. The Innate Immune System in Acute and Chronic Wounds. Adv. Wound Care 2016, 5, 65–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandara, A.A.; Mustoe, T.A. Oxygen in wound healing more than a nutrient. World J. Surg. 2004, 28, 294–300. [Google Scholar] [CrossRef]
- Morton, L.M.; Phillips, T.J. Wound healing and treating wounds: Monocyteential diagnosis and evaluation of chronic wounds. J. Am. Acad. Dermatol. 2016, 74, 589–605. [Google Scholar] [CrossRef]
- Qing, C. The molecular biology in wound healing & non-healing wound. Chin J. Traumatol. 2017, 20, 189–193. [Google Scholar] [CrossRef]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R. Macrophage plasticity and activation in tissue repair and remodeling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- Mallik, S.B.; Jayashree, B.S.; Shenoy, R.R. Epigenetic modulation of macrophage activation-perspectives in diabetic wounds. J. Diabetes Complicat. 2018, 32, 524–530. [Google Scholar] [CrossRef]
- Maruyama, K. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am. J. Pathol. 2007, 170, 1178–1191. [Google Scholar] [CrossRef] [Green Version]
- Brem, H. Tomic-Canic Cellular and moiecular basis of wound healing in diabetes. J. Clin. Investig. 2007, 117, 1219–1222. [Google Scholar] [CrossRef] [Green Version]





| Characteristics, Units | 1 | 2 | 3 |
|---|---|---|---|
| T2DM Newly Diagnosed (n = 28) | T2DM without DFS (n = 51) | T2DM with DFS (n = 42) | |
| Sex, male/female (%) | 11(39.3)/17(60.7) | 27(52.9)/24(47.1) | 29(69.0)/13(31.0) |
| Age, years | 59.1 ± 6.0 | 62.7 ± 8.2 | 62.0 ± 8.6 |
| BMI, kg/m2 | 31.5 ± 4.6 | 32.0 ± 5.0 | 30.8 ± 5.6 |
| HbA1c,% | 9.9 ± 2.3 | 9.0 ± 1.4 | 8.8 ± 1.8 |
| Total cholesterol, mmol/L | 5.0 ± 1.1 | 4.9 ± 1.6 | 5.1 ± 1.4 |
| Triglycerides, mmol/L | 2.1 ± 1.4 | 1.7 ± 0.9 | 2.1 ± 1.1 |
| HDL-C, mmol/L | 1.4 ± 0.6 | 1.3 ± 0.5 | 1.4 ± 0.9 |
| LDL-C, mmol/L | 3.0 ± 1.5 | 3.0 ± 1.2 | 2.9 ± 1.5 |
| Groups (n) | TNF-α pg/mL | CCL18 pg/mL | ||
|---|---|---|---|---|
| Basal | Stimulated | Basal | Stimulated | |
| (1) T2DM newly diagnosed (n = 28) | 650.0 (436.8; 922.3) | 1679.5 ** (1127.5; 2021.5) | 27.5 (14.5; 43.5) | 1123.0 ** (963.2; 1435.2) |
| (2) T2DM 10 (5; 15) years without DFS (n = 50) | 924.1 (133.8; 1610.0) | 1216.0 ** (279.0; 2309.9) | 3.0 (0; 9.6) | 6.7 ** (1.1; 28.6) |
| (3) T2DM 12 (6; 20) years with DFS (n = 42) | 674.3 (269.3; 1281.1) | 1271.5 ** (611.9; 2,857.5) | 9.4 (3.1; 40.1) | 12.8 * (4.1; 67.6) |
| (p 1vs2 = 0.050) * (p 1vs3 = 0.606) (p 2vs3 = 0.118) | (p 1vs2 = 0.930) (p 1vs3 = 0.624) (p 2vs3 = 0.640) | (p1vs2 < 0.001) ** (p1vs3 < 0.001) ** (p2vs3 = 0.645) | (p 1vs2 < 0.001) ** (p 1vs3 < 0.001) ** (p 2vs3 = 0.065) | |
| Parameter, pg/mL | T2DM w/o DFS | T2DM with DFS | p-Value | T2DM w/o DFS | T2DM with DFS | p-Value |
|---|---|---|---|---|---|---|
| HbA1c ≤ 7.5% | HbA1c > 7.5% | |||||
| TNF-α basal | 1610.0 (399.4; 1989.0) | 648.0 (261.3; 766.0) | 0.258 | 849.6 (90.2; 1490.5) | 828.0 (280.8; 1317.1) | 0.790 |
| TNF-α stimulated | 1683.0 (536.3; 2737.0) p = 0.051 | 755.0 (526.0; 1053.3) p = 0.011 * | 0.161 | 1069.6 (192.0; 2258.9) p < 0.001 ** | 1499.0 (598.9; 3117.5) p < 0.001 ** | 0.135 |
| CCL18 basal | 1.9 (0.1; 5.4) | 50.0 (2.9; 71.0) | 0.014 * | 3.5 (0.0; 10.9) | 8.0 (3.4; 23.3) | 0.013 * |
| CCL18 stimulated | 20.0 (2.7; 115.1) p = 0.017 * | 8.7 (2.3; 51.4) p = 0.594 | 0.489 | 2.9 (1.0; 18.4) p = 0.024 * | 13.1 (4.7; 74.5) p = 0.008 * | 0.033 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galstyan, K.O.; Nedosugova, L.V.; Martirosian, N.S.; Nikiforov, N.G.; Elizova, N.V.; Kolmychkova, K.I.; Sobenin, I.A.; Orekhov, A.N. Modification of Tumor Necrosis Factor-α and C-C Motif Chemokine Ligand 18 Secretion by Monocytes Derived from Patients with Diabetic Foot Syndrome. Biology 2020, 9, 3. https://doi.org/10.3390/biology9010003
Galstyan KO, Nedosugova LV, Martirosian NS, Nikiforov NG, Elizova NV, Kolmychkova KI, Sobenin IA, Orekhov AN. Modification of Tumor Necrosis Factor-α and C-C Motif Chemokine Ligand 18 Secretion by Monocytes Derived from Patients with Diabetic Foot Syndrome. Biology. 2020; 9(1):3. https://doi.org/10.3390/biology9010003
Chicago/Turabian StyleGalstyan, Karine O., Ludmila V. Nedosugova, Narine S. Martirosian, Nikita G. Nikiforov, Natalia V. Elizova, Kira I. Kolmychkova, Igor A. Sobenin, and Alexander N. Orekhov. 2020. "Modification of Tumor Necrosis Factor-α and C-C Motif Chemokine Ligand 18 Secretion by Monocytes Derived from Patients with Diabetic Foot Syndrome" Biology 9, no. 1: 3. https://doi.org/10.3390/biology9010003
APA StyleGalstyan, K. O., Nedosugova, L. V., Martirosian, N. S., Nikiforov, N. G., Elizova, N. V., Kolmychkova, K. I., Sobenin, I. A., & Orekhov, A. N. (2020). Modification of Tumor Necrosis Factor-α and C-C Motif Chemokine Ligand 18 Secretion by Monocytes Derived from Patients with Diabetic Foot Syndrome. Biology, 9(1), 3. https://doi.org/10.3390/biology9010003






