Simple Summary
Capture fisheries are reaching their limit, so the increasing demand for fish protein can only be met through aquaculture. One attractive sector within this industry is the culture of salmonids, which are a) uniquely under pressure due to overfishing and b) the most valuable finfish per unit of weight. The culture of these animals is threatened by many diseases, some caused by bacteria, which can result in large financial losses for fish farmers. Unfortunately, the current methods for the control of aquatic bacterial diseases are either unsustainable (antibiotics) or not very effective (vaccines). This is primarily due to a lack of knowledge surrounding the successful immune function of fish. To improve vaccine design and other methods of control, a deeper understanding of fish immunology is essential. This review highlights the current understanding of fish antibacterial immunity in the context of salmonid culture. Additionally, the successes and shortcomings of current methods used to combat bacterial diseases in salmonid aquaculture will be addressed. Improving our understanding of the salmonid immune system will help to reduce aquaculture losses in the future.
Abstract
The aquaculture industry is continuously threatened by infectious diseases, including those of bacterial origin. Regardless of the disease burden, aquaculture is already the main method for producing fish protein, having displaced capture fisheries. One attractive sector within this industry is the culture of salmonids, which are (a) uniquely under pressure due to overfishing and (b) the most valuable finfish per unit of weight. There are still knowledge gaps in the understanding of fish immunity, leading to vaccines that are not as effective as in terrestrial species, thus a common method to combat bacterial disease outbreaks is the use of antibiotics. Though effective, this method increases both the prevalence and risk of generating antibiotic-resistant bacteria. To facilitate vaccine design and/or alternative treatment efforts, a deeper understanding of the teleost immune system is essential. This review highlights the current state of teleost antibacterial immunity in the context of salmonid aquaculture. Additionally, the success of current techniques/methods used to combat bacterial diseases in salmonid aquaculture will be addressed. Filling the immunology knowledge gaps highlighted here will assist in reducing aquaculture losses in the future.
1. The Impact of Global Aquaculture
Given that both fresh- and saltwater account for 72% of Earth’s surface area, it was only a matter of time before aquatic environments became the new frontier for agriculture. Because the majority of food animals are currently raised on land, it is unsurprising that insights or advancements in aquatic animal husbandry have lagged behind those of terrestrial species. As the global population increases, and given the limited availability of productive land, the necessity of utilizing aquatic habitats for animal food production is clear. Additionally, due to their high polyunsaturated fatty acid content [1], many aquatic species provide an alternative and heart healthy protein source in an age when cardiovascular disease is the leading cause of death worldwide [2]. For these reasons and more, global interest for fish protein is high. It is so high, in fact, that fisheries cannot meet the global demand while also adhering to the harvesting restrictions that are currently in place [3]. This places a burden on wild populations because effective enforcement of these restrictions is logistically difficult.
The culture of aquatic species, or aquaculture, can provide an alternative to alleviate some of the pressure on wild populations. For many aquatic species, this culture production is in its nascent form, meaning that time will be required to understand and optimize these industrial practises. One manifestation of the issues faced by aquaculture is the increased prevalence of infectious disease, including bacterial pathogens. Bacteria are able to take advantage of novel, high density farm environments and thrive. This results in many of these prevalent microorganisms becoming opportunistic pathogens in aquaculture settings. Obtaining a deeper understanding of bacterial diseases that impact aquaculture, as well as what constitutes an effective immune response in relevant hosts, is invaluable for the improvement of this industry. For the purposes of this review, the term “aquaculture” will be refer to the culture of finfish species.
The utilization of aquatic environments means that some of the difficulties confronted by fish farmers are very different when compared to their terrestrial counterparts. Common sources of financial losses include environmental/husbandry (algal blooms, temperature oscillations, hypoxia, supersaturation, etc.), chemical (nitrogen fluctuations, pH variation, etc.), predation, escapees and infectious disease [4]. Many of these problems can result in devastating financial losses, but few compare to the consistent annual losses derived from infectious disease. In 2014, of the $70 billion dollars of aquaculture product that was destined for human consumption, 10% of this was lost due to infectious disease [5,6]. Though there are many different types of infectious agents that contribute to the significant financial losses experienced in aquaculture, this review will focus on bacterial diseases of salmonid culture, the most valuable finfish species per unit of weight within this global industry [7].
2. Common Bacterial Diseases in Salmonid Culture
Cultured salmonids are susceptible to many bacterial pathogens (Table 1). The stress induced by conditions such as overcrowding, temperature fluctuations and excessive handling can result in normally benign microorganisms becoming opportunistic pathogens [8]. The aquatic environments in which these animals reside are known to support the growth of bacteria for long periods of time. Though not immediately causing infection, these opportunistic bacterial pathogens can survive independently of their hosts [9,10,11]. When animals are stressed, these microorganisms are well situated to become major impediments for aquaculture. The ectothermic nature of fish means that these animals have no control over their body temperature as it is simply a representation of their surrounding environment [12]. As a result, there are different opportunistic bacterial pathogens that have taken advantage of the variety of temperature niches. For fish farmers, this has made management and prevention strategies difficult as there is a large degree of variability in the route of entry, virulence factors, disease presentations and pathologic cycle between the various bacterial pathogens (reviewed in [13,14]). The significance of bacterial pathogens in salmonid aquaculture, combined with the extensive knowledge gaps regarding host immunity, means that further investigation of the salmonid immune system could prove invaluable.

Table 1.
Common bacterial pathogens in salmonid aquaculture. A list of bacterial pathogens that affect the aquaculture production of salmonids, their Gram reaction and the diseases/conditions that they are associated with. Several of these conditions will be discussed throughout this review in order to describe various methods used in aquaculture to prevent and/or treat. The “−” symbol denotes a negative Gram reaction while the “+” symbol denotes a positive Gram reaction.
3. Teleostean Immunity: Our Current Understanding of the Antibacterial Response
Despite their divergence occurring 320–350 million years ago (reviewed in [15]), the teleost immune system contains many components comparable to what is known in mammals. Bony fishes are divided into the Sarcopterygii (the lobe-finned fish) and the Actinopterygii (ray-finned fish) to which Teleostei (Greek for “complete bone”) belongs (reviewed in [16]). Salmonids, which will be heavily emphasized in this review, are members of Teleostei. The teleosts comprise 95% of surviving fish species, which represents approximately half of all extant vertebrate species [17]. The remarkable success of this class, along with their ability to thrive in a wide range of environments, suggests that teleosts developed an impressive immune arsenal to counter pathogen challenge. Much like the highly studied mammalian model, the immune system of teleosts can be separated into two main branches: the innate and adaptive immune systems. The following information represents a brief summary of teleostean immune defenses that are common responses to bacterial pathogens. For a more in-depth overview of known teleostean immune mechanisms, please see [18,19,20,21,22,23].
3.1. Innate Immunity of Fish
Due to the limitations of teleost adaptive immunity (i.e., slow initiation, limited antibody repertoire, etc.), the burden of preventing and combatting infectious agents falls heavily to the innate immune system. Fish have been shown to have all of the mammalian aspects generally associated with innate immunity including physical barriers (skin and mucous membranes), humoral parameters (complement, natural antibody, toll-like receptors, etc.) and cellular components (phagocytosis, NK cells, etc.). As the first line of defense, it is not surprising that the majority of the broad-spectrum parameters of innate immunity are highly conserved across species and taxa. In all jawed vertebrates, the innate immune system features a rapid defensive response towards invading pathogens and tissue damage. However, it cannot provide well-directed, specific protection from individual pathogens or long-term immunological memory.
3.1.1. Cells of Innate Immunity
All of the innate immune cells that are observed in mammalian blood are also present in the blood of teleosts (monocytes, neutrophils, basophils and eosinophils), albeit at very different circulating concentrations. Neutrophils, basophils and eosinophils are referred to together as granulocytes and are aptly named due to the presence of cytoplasmic granules. These are filled with enzymes and host defense peptides that can support immune responses during infections and/or allergic reactions (reviewed in [24,25,26,27]). Monocytes patrol the blood, contributing to inflammation, immune defenses and homeostasis by clearing pathogens and cellular debris. Additionally, monocytes can enter tissues and differentiate into macrophages or dendritic cells (DCs) to replenish these important immune cells (reviewed in [28]). Of these peripheral blood leukocytes, only monocytes, eosinophils and neutrophils are phagocytic. When it comes to cell numbers, the granulocytes are the most prevalent circulating WBC in mammals and represent 45–65% of this population, 92% of which are neutrophils. Meanwhile, monocytes make up only 8% of total mammalian WBCs (reviewed in [29,30]). In comparison, granulocytes comprise just 2–3% of teleostean WBCs, while monocytes are merely 0.1% [31,32]. Despite differences in circulating concentrations, the function of these WBCs appears to be conserved between the two taxa.
As the principal phagocytic cells in fish, macrophages are considered one of the most important contributors to the innate immune defenses of these animals. Though macrophages can derive from monocytes (reviewed in [28]), this happens relatively infrequently [33]. Instead, recent evidence in mammals has shown that these cells are present in embryonic tissues (yolk sac and fetal liver) prior to hematopoiesis and can then persist as self-maintaining populations to perform organ specific functions (reviewed in [34]). Although further investigation is required, recent work with zebrafish has shown that tissue macrophages are present throughout adulthood even when adult hematopoiesis is absent [35]. This indicates that fish may also have tissue resident, self-maintaining macrophages. Functionally, macrophages are armed with many pattern recognition receptors (PRRs) enabling these cells to detect a multitude of pathogen associated molecular patterns (PAMPs) wherein strong binding will initiate phagocytosis of the foreign entity (reviewed in [36]). Once ingested, macrophages can rapidly kill foreign invaders through the production of toxic reactive intermediates and phagolysosomal acidification (reviewed in [19]). Besides their antimicrobial function, these cells are also able to present antigens to T cells and, depending on the surrounding stimuli, can orchestrate the appropriate immune response via cytokine secretion (reviewed in [19]). Finally, once an immune reaction has ceased, the phagocytic function of macrophages is critical for maintaining tissue homeostasis by clearing cellular debris (reviewed in [37]). The dynamic and heterogeneous nature of macrophages means that these cells can be distinguished depending on the source of activation and the resultant differences in cellular function, referred to as polarization (reviewed in [38,39]). In fish, the M1 macrophage polarization state is characterized the best and appears to serve a vital role in host protection against bacterial pathogens (reviewed in [18,19]). As a result, vaccination efforts for aquatic bacterial pathogens should focus on tailoring the teleostean immune response to stimulate the polarization of the M1 macrophage phenotype.
3.1.2. Pattern Recognition Receptors (PRRs)
A hallmark of innate immunity is the recognition of conserved, nonspecific molecules that are associated with infectious agents and/or cellular damage. These molecules make patterns that the host can recognize. This is accomplished by germline-encoded receptors known as pattern recognition receptors (PRRs) that are found on/within many different cell types. These receptors are collectively capable of binding to many different PAMPs, ensuring that the immune system will be notified when host barriers are breached. Upon binding to their associated ligands, a signalling cascade is initiated to stimulate transcription factors (such as NF-κΒ, AP-1, and NFAT), leading to the upregulation of genes involved in inflammatory responses so that the origin of the PAMP can be effectively opposed (reviewed in [40,41,42]). To date, there are six classes of PRRs which are categorized based on shared structural elements: toll-like receptors (TLRs), C-type lectin receptors (CLRs), NOD-like receptors (NLRs), AIM2-like receptors (ALRs), RIG-I-like receptors (RLRs) and cGAS/STING receptors (reviewed in [41]). Though not as well understood as their mammalian counterparts, receptors from all but the ALRs have been identified in teleosts [43,44,45,46,47]. Although there are some similarities between mammalian and teleostean PRRs, some discrepancies do exist. The substantial differences in habitat, life cycle, genome structure and reproductive strategies between these two taxa may provide some explanations. Perhaps further study of teleostean PRRs would yield insight regarding which specific facets can influence the evolutionary conservation of these molecules.
Of the different PRR classes, TLRs were the first discovered and are also considered to be the best characterized in both mammalian and teleostean models. At least 20 different TLRs have been found in more than a dozen fish species [48,49], but direct evidence of ligand specificity has only been shown in TLR2, TLR3, TLR5M, TLR5S, TLR9, TLR21, and TLR22 (reviewed in [48]). Five of these specifically bind and recognize PAMPs derived from bacteria such as peptidoglycan (TLR2, [50,51,52]), lipoteichoic acid (TLR2, [50,52]), flagellin (TLR5M and TLR5S, [53,54,55]) and CpG DNA (TLR9 and TLR21, [56,57,58,59]). Additionally, there are convincing results suggesting that some of the nonmammalian TLRs, such as TLR14, TLR18, and TLR25, may also be associated with sensing bacterial PAMPs [60,61,62,63]. Considering that these receptors are likely to target bacterial pathogens specific to the aquatic environment, they may represent effective targets for novel vaccine formulations in aquaculture.
3.1.3. Antimicrobial Peptides
Aside from notifying the immune system to the presence of a foreign entity, some aspects of innate immunity can act to directly destroy the infectious agent, as observed with antimicrobial peptides (AMPs). AMPs are a diverse class of highly conserved molecules that are produced as a first line of defense in all multicellular organisms, including teleosts. These small peptides (12–50 amino acids) are essential components of innate immunity capable of antimicrobial activity against a broad range of microbial pathogens (reviewed in [64]). Importantly, this also includes multi-drug resistant isolates [65,66] which have become a major concern in aquaculture. AMPs are often produced constitutively, but they can also be induced upon exposure to pathogens and/or other trauma [67,68]. Most AMPs are cationic amphipathic peptides that function by attacking the negatively charged membranes of microorganisms (reviewed in [69]). AMPs are characterized based on their secondary structures as one of four types: β-sheet, α-helix, extended or loop. Of these four types, β-sheet and α-helix are the most prevalent (reviewed in [70]). Functionally, they can be described as either membrane disruptive AMPs, inducing membrane permeabilization, or nonmembrane disruptive AMPs, which directly passage into cells and act on intracellular targets (reviewed in [71]). Besides the direct destruction of pathogens, AMPS can perform immunomodulatory functions in higher vertebrates (reviewed in [72]) and as a result are also called “host defense peptides” (HDPs). The potential immunomodulatory effects are diverse including stimulation of chemotaxis, immune cell differentiation, initiation of adaptive immunity and stimulation of both pro- and anti- inflammatory cytokines [73,74,75,76]. As many AMPs have multiple functions that can be both bactericidal and immunostimulatory in nature, there has been growing interest regarding their use in aquaculture as an alternative for antibiotics and/or as adjuvants (reviewed in [77,78,79]).
3.1.4. Respiratory Burst Activity
An essential immunological response for eliminating bacterial pathogens is the respiratory burst activity (RBA) of phagocytes. Following ingestion of foreign particles, these immune cells can kill most bacteria by producing reactive oxygen intermediates (ROI). The production of ROI requires NADPH oxidase (NOX), which catalyzes the conversion of molecular oxygen into superoxide anions (reviewed in [80,81]). Upon formation, the superoxide anion will then transform into further ROIs such as hydrogen peroxide, hydroxyl radical, and hypochlorous acid, all of which efficiently kill the phagocytosed microorganisms [82]. Inactive NOX consists of six subunits wherein gp91phox and p22phox are membrane proteins (Figure 1A) that together are known as flavocytochrome b558 (cyt b558, [83]). The remaining four regulatory subunits, (p40phox, p47phox, p67phox and Rac2) normally exist in the cytosol (Figure 1A) but upon the activation of leukocytes by particulate stimuli, will translocate to the membrane and associate with cyt b558 (Figure 1B) to form the active oxidase (reviewed in [80,83]). It is well established that fish phagocytes possess all of these NADPH oxidase components as well as an RBA response comparable to that of mammals [84,85,86]. Additionally, this immune defense has been extensively studied in relation to bacterial pathogens of fish [87,88,89,90]. Given that the RBA in fish is not markedly influenced by temperature (reviewed in [91,92,93,94]), this innate immune response is an essential antibacterial mechanism for these poikilothermic organisms.
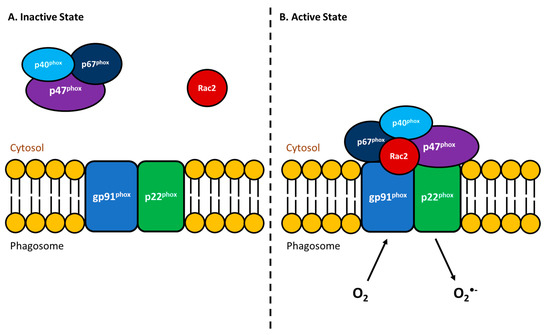
Figure 1.
Schematic depiction of NADPH oxidase activation to enable the respiratory burst activity of phagocytes. The NADPH oxidase enzyme consists of six subunits. (A) When the phagocyte is in an inactive state, two of the subunits (gp91phox and p22phox) are transmembrane components, while the remaining four components are cytosolic (p40phox, p47phox, p67phox and Rac2). (B) Upon the phagocyte being activated by external stimuli, the four cytosolic components complex with gp91phox and p22phox to form the active NADPH oxidase. When in the active form, the now functional enzyme can convert molecular oxygen into superoxide anions to aid in the degradation or killing of that which was phagocytosed.
3.2. Adaptive Immunity of Fish
All organisms have innate immune mechanisms, and while there are indications of adaptive immunity in invertebrates, this branch of the immune system appears to be an advancement that is specific to gnathostomes (jawed vertebrates). The adaptive immune system is remarkably flexible, capable of recognizing and initiating protective responses against specific foreign agents. Upon subsequent exposures, the adaptive immune system will remember antigens from a foreign invader, making it possible to mount a stronger and more efficient immune response (reviewed in [95,96]). Though delayed when compared to mammalian counterparts, the specificity of the teleostean adaptive immune system is essential for long-lasting immunological memory. As such, this branch of the immune system is critical for vaccine design, an enterprise in need of improvement for the aquaculture industry.
3.2.1. Cells of Adaptive Immunity
Much like in mammalian models, lymphocytes are considered the adaptive immune cells of fish. The two types of lymphocytes, T and B lymphocytes, represent the only cells capable of recognizing and responding specifically to an antigenic epitope. This antigen detection is based on compatibility with either the surface T cell receptor (TCR) or B cell receptor (BCR) depending on the lymphocyte. The genes that encode for these receptors undergo a series of DNA recombination events, providing them with an immense phenotypic diversity to improve the likelihood of antigen recognition (reviewed in [97]). Depending on the type of activation, T lymphocytes can produce cytokines to direct immune responses (CD4+ T cell, reviewed in [98]) or induce programmed cell death in virally infected cells (CD8+ T cell, reviewed in [99]). In comparison, B cells will transform into plasma cells following activation to produce antigen specific antibodies (reviewed in [100]). Though participating in a variety of different activities, the proficient function of both T and B lymphocytes is crucial for the success of the adaptive immune system.
In mammals, lymphocytes represent the largest blood cell in diameter (8-10 µm) and account for approximately 20–40% of WBCs (reviewed in [29,30]). This is quite different from teleost species where lymphocytes are smaller in size (5–8 µm) and represent the dominant circulating leukocyte at 83–90% of total WBCs [31,32,101]. Regardless of the model system used, T and B lymphocytes appear identical when observed under a microscope, making it impossible to discern between the two without identifying cell surface markers. Though studied in detail for mammalian models, this was not possible in teleosts due to the absence of appropriate antibodies. Fortunately, with the recent development of antibodies specific to some of the cell surface markers on salmonid lymphocytes [102,103,104], comparative immunologists are able to finally start understanding adaptive immune functions in teleosts.
3.2.2. Major Histocompatibility (MH) Genes
To effectively combat the inevitable interaction with foreign entities, vertebrates have evolved two distinct antigen presentation pathways that, when paired with effective innate immune system activation, can stimulate long-term immunological memory. The major histocompatibility complex (MHC) molecules are critical in this important immune process yet the MHC gene equivalents in teleosts are not clustered on a single chromosome as they are in mammals so they are not considered to be a “complex”. Instead these genes can be found on more than one chromosome and as a result are simply referred to as Major Histocompatibility (MH) genes (reviewed in [105]). In the mammalian model, the endogenous antigen presentation pathway includes MHC class I molecules which are found within all nucleated cells. This pathway involves the processing of antigens from intracellular pathogens and their presentation via MHC class I to CD8+ cytotoxic T lymphocytes (reviewed in [106]). In comparison, the exogenous antigen presentation pathway uses MHC class II dimers which are generally only found on specific cell types, namely antigen presenting cells (APCs) that are capable of phagocytosis (reviewed in [107]). Once phagocytosed and processed, the antigens from extracellular sources are then loaded onto the MHC class II dimer and presented at the cell surface to CD4+ T lymphocytes (reviewed in [108]). In fish, the MHC equivalents function in an identical manner to what has been observed in mammals. Because this review focuses on bacterial pathogens, which are typically extracellular in nature, more emphasis will be placed on MH class II (Figure 2).
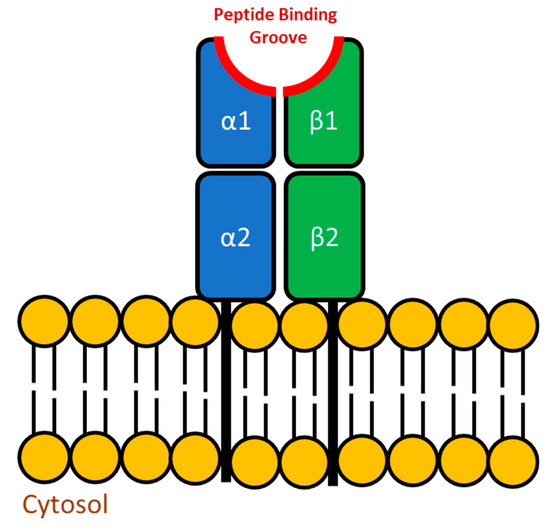
Figure 2.
Structure of the MH class II molecule. The structure and function of the teleostean MH class II dimer is very similar to mammalian MHC class II. The molecule is a dimer, consisting of an alpha chain and beta chain, both of which have transmembrane regions. As outlined in red, both the α1 and β1 domains of this molecule have a hypervariable region that is part of the peptide binding groove. It is this region that is capable of binding to compatible antigens and presenting it to T cells.
Per individual, the MH molecules play an important role by binding to and presenting well-matched peptides to appropriate T lymphocytes. The compatibility of these pathogen-derived antigens to MH molecules is controlled by allelic variation at the peptide binding groove. There are many possible alleles for the peptide binding region, and every individual within a species has a limited repertoire inherited from their parents in a Mendelian fashion (reviewed in [109,110]). The genetic polymorphism at the MH loci can provide more or less protection to pathogens, and thus these genes are believed to be under a strong selection pressure that is often governed by the surrounding habitat (reviewed in [111]). Regardless of the species, individuals that are heterozygous at MH loci are believed to be better protected because the resulting molecules should be able to bind to and present a more extensive collection of antigens [112,113]. In fish, this is supported by heterozygous individuals presenting less infection and/or mortality when challenged with an infectious agent [114,115]. Unlike what has been observed in some terrestrial species [116,117,118], specific MH alleles have not yet been shown to consistently predict resistance or susceptibility towards specific pathogens in fish, but perhaps more research is required.
3.2.3. Antibody Development
Antibody development and production is of paramount importance in the humoral immune response of all jawed vertebrates, including bony fishes. This defense is particularly important when dealing with extracellular threats, as is the case with most bacterial pathogens. Antibodies prevent the growth and colonization of bacterial pathogens by neutralization, complement activation and/or opsonization to enhance phagocytosis (reviewed in [119]). To date, there are three known antibody classes in teleosts based on differences in their constant region: IgM, IgD, and IgT (reviewed in [120]). IgM was the first isotype discovered in teleosts and can be found on B cells as well as secreted in the serum or mucus as a tetramer (reviewed in [121]). The secreted form of teleost IgM is by far the most prevalent immunoglobulin in the serum and is responsible for systemic immunity in bony fishes (reviewed in [122]). At much lower concentrations, IgM is also present in the gut and skin mucosa. Similar to mammals, all mature IgM B cells in teleosts also express IgD, a class of antibody whose function is still not fully understood regardless of the model system used (reviewed in [120]). However, there have recently been slight improvements in elucidating mammalian IgD function (reviewed in [123]). Lastly, IgT is a recently discovered antibody isotype exclusive to bony fishes [124]. IgT is present within the serum as monomers, while forming tetramers in the gut mucosa. With concentrations of IgT in the gut mucosa being double those observed in the serum [102], it was believed that this Ig class likely had a vital role in mucosal immunity. This has since been heavily supported with numerous studies demonstrating the role of IgT in teleostean mucosal immunity [125,126,127]. When considering adaptive immune defenses that are important for combatting extracellular bacterial pathogens, effective antibody production and development is invaluable.
Because teleosts do not have IgG as an antibody isotype (reviewed in [120]), any secondary antibody response observed is slight and uses a different approach than the canonical mammalian definition of immunological memory. This means that fish depend only on the low affinity but high avidity of IgM for repeated exposure to antigens, and thus a less intense secondary serum antibody response is often observed in these animals [128,129]. Additionally, fish do not appear to go through class switch recombination (CSR), despite having all of the necessary components and enzymes (i.e. AID, RAG1/2, Ikaros, TdT, etc.) required to complete this process (reviewed in [130]), due to the structure of their heavy chain genes. Interestingly, even though the catalytic domain of activation-induced (Cytidine) deaminase (AID) differs from tetrapods, when this enzyme is transfected into murine B cells it is still able to catalyze CSR [131]. Since fish do not appear capable of CSR, this finding revealed that the actual process of CSR must have evolved separately from the AID enzyme itself [131,132]. Protective responses have been observed in aquatic species for years following their initial exposure to antigens [133]. However, the requirements to consistently stimulate immunological memory/protection to selected pathogens must still be scientifically confirmed.
Rather than developing memory B cells, it has been proposed that the most mature stage of B lymphocytes in teleosts are long-lived plasma cells (LLPCs). These cells reside within the head kidney, a primary immune organ of fish that is considered to be analogous to mammalian bone marrow. These LLPCs have been observed to secrete high amounts of antibody and appear to be the only detectable source of prolonged, high-titered antibodies [134]. However, the idea of ‘immunological memory’ is called into question because the antibody response in fish shows a poor affinity maturation and slow development of the secondary immune response, taking 3–4 weeks to initiate in fish [135]. This is a stark contrast to the rapid development observed within the mammalian paradigm of immunological memory, which likely influences the lack of efficacious vaccines for aquaculture production
3.3. Cytokines
As described above, the immune system of vertebrates is a complex network connecting numerous cell types, barriers and specialized systems to prevent the entry and/or colonization of foreign entities within the host. The successful function of this multifaceted system depends on the ability of immune cells to migrate to and communicate with one another, a role fulfilled by extracellular mediators known as cytokines. Cytokines are a large family of small glycoproteins that are capable of acting in an autocrine, paracrine or endocrine fashion (reviewed in [136]). These soluble proteins play crucial roles in regulating inflammation, haematopoiesis, cellular movements and immune cell activation (reviewed in [137,138,139,140]). As such, cytokines act as an essential link between the innate and adaptive arms of the immune system. These small peptides ensure that vertebrates carry out an appropriate immune response to combat the specific threat. As a result, proper stimulation of cytokines could help increase the efficacy of vaccines and alternative treatment options for bacterial pathogens in aquaculture.
3.3.1. Chemokines
Chemotactic cytokines, or chemokines, are a large family of small proteins responsible for controlling the migratory patterns and positioning of immune cells (reviewed in [141]). Since the discovery of the very first teleostean chemokine in rainbow trout [142] several chemokines have been identified in other fish species, including Atlantic salmon, and zebrafish [143,144,145,146]. In both teleostean and mammalian models, interleukin (IL)-8, or CXCL8, is a highly studied chemokine due to its essential role in inflammation. Following its release by injured or infected tissue, IL-8 acts to recruit neutrophils to the site of injury. Upon arrival to these locations, neutrophils will then trigger proinflammatory responses, thereby attracting other important immune cells so that the threat can be eliminated [144,147]. IL-8 is an excellent indicator of immune activation as it shows not only the presence of these important immune cells, but also that they are actively engaged in clearing infections. IL-8 is an excellent indicator of immune system activation for research purposes. Additionally, effective manipulation of this cytokine could direct immune-based interventions, enabling a more accurate/efficient targeting of therapeutics.
3.3.2. Proinflammatory Cytokines
Inflammation is an essential response in combatting tissue damage of any type, including harm associated with bacterial infection and/or associated toxins. Though there are many important components involved in an inflammatory response (reviewed in [148]), the initiation and perpetuation of inflammation is governed primarily by proinflammatory cytokines produced by damaged cells or responding immune cells. In mammalian models, the three classical proinflammatory cytokines are IL-1β, IL-6 and tumour necrosis factor (TNFα). Upon tissue damage, keratinocytes and fibroblasts release IL-1β to induce fever, T cell proliferation and increasing vascular permeability (reviewed in [149]). While this is occurring, resident mast cells (MCs) also degranulate in response to any mechanical trauma, releasing a wide variety of inflammatory mediators, including TNFα and IL-6 (reviewed in [150,151]). The released cache of mediators further aids in vascular permeability and the activation/recruitment of circulating immune cells which produce more proinflammatory cytokines. Leukocytes that are normally restricted to blood vessels will then gain access to the site of tissue injury and attempt to eliminate any invading targets (reviewed in [152]). If the inflammatory response is successful in clearing the threat, it is followed by a resolution and repair phase mediated mainly by tissue-resident and recruited macrophages that shift the response from a proinflammatory to anti-inflammatory one (reviewed in [153]). While fish immune systems are not as well characterized as mammalian equivalents, they possess all of the major proinflammatory cell types and cytokines (reviewed in [138,154]).
3.3.3. Anti-Inflammatory Cytokines
Though necessary for homeostatic maintenance, inflammatory responses can be quite damaging to surrounding tissues. This makes the control of such reactions essential for the day-to-day welfare of the host. Regulation and control of inflammatory responses requires a constant and ever-changing balance between proinflammatory and anti-inflammatory cytokines. Under normal circumstances, as the source of tissue damage is cleared, there are fewer stimuli available to induce a strong inflammatory response. This enables the constitutively produced anti-inflammatory cytokines to skew the reaction towards tissue repair mechanisms. Though there are several cytokines that are considered to have anti-inflammatory properties (reviewed in [136]), IL-10 is on that is believed to function solely as a potent anti-inflammatory cytokine. Produced by almost all leukocyte subsets, including T cells, B cells, macrophages and mast cells (reviewed in [155]), IL-10 plays a vital role in ensuring that both innate and adaptive immune responses cannot have a strong reaction unless a true threat is present. The importance of controlling inflammation through endogenous levels of IL-10 was made clear by Kühn et al. [156] when they generated IL-10 knockout mice. The IL-10 deficient mice spontaneously developed inflammatory enteritis, indicating that the mice were unable to prevent the inflammatory response towards their own commensal gut-associated bacteria [156]. Although the technology for producing knockouts in salmonid species is not yet available, the importance of IL-10 in the resolution of inflammation has been made clear in grass carp, rainbow trout and Atlantic salmon [157,158,159]. As with most components of the immune system, inflammatory responses must be tightly regulated.
4. The Benefit of Understanding Bony Fish Immunology
Throughout history, human beings have relied on fish populations for sustenance. With regard to the environment, fish play a vital role in natural food webs by supporting the growth and survival of numerous species [160,161]. From an economic perspective, fish represent a valuable commercial product providing many career options while also ensuring that people have access to healthy protein sources worldwide. Aquaculture production also has the added benefit of ensuring that wild populations will not be dangerously overfished in order to meet the rising demand for this food source. As the intensive culture of fish is still in its infancy when compared to terrestrial agriculture, many developments still need to be made. One way to help enhance the productivity of aquaculture efforts is to obtain a deeper understanding of teleostean immunity. Advances here will lead to improved vaccine design and therapeutic options, thereby augmenting yields and strengthening fish health in these facilities.
Although there are many similarities between the immune system of fish and mammals, there are also notable differences. Despite the highly studied mammalian model providing a solid baseline, comparative immunologists must experimentally confirm whether mammalian immune responses and tissues are analogous in fish. As an example, the bone marrow of mammals is the site of haematopoiesis and where B cells develop (reviewed in [162]). However, fish do not have bone marrow, so instead this essential process occurs in the anterior portion of their kidney (reviewed in [163]) but research is still being conducted to fully understand this process in teleosts. When it comes to specific immune receptors, fish have many similarities to those found in mammals (reviewed in [164]). Nevertheless, teleostean equivalents quite often vary in number and ligand specificity. This can be seen when comparing TLRs between these two taxa. One mammalian example, human, is known to have 10 different TLRs, while at least 20 have been found in more than a dozen fish species (reviewed in [48,49,165]). Some of these human TLRs can be found in fish, while others have been lost in many bony fish species, such as TLR6 and TLR10 (reviewed in [165]). Despite these interesting differences between fish and mammalian TLRs, direct evidence of ligand specificity has only been shown in seven of the twenty TLRs found in fish [52,55,166,167]. Given the large differences in environment, combined with the fact that fish have undergone at least one, if not two, whole genome duplication (WGD) events not experienced by mammalian species (reviewed in [15]), it is not surprising that there are significant differences both functionally and genetically between fish and mammals. This emphasizes the importance of validating immune paradigms in teleosts before basing therapies on concepts that have only been confirmed in mammalian models.
Teleosts represent approximately half of all surviving vertebrate species, making them the largest and most diverse group of this subphylum [168]. Just as there are differences between species within the taxa Mammalia (reviewed in [169,170]), there are also distinct differences between species of fish, and indeed these differences are bigger given that fish emerged and diversified close to 400 million years ago (reviewed in [171]). As an example, the majority of studied fish have genes for both MH class I and MH class II molecules but the Atlantic cod (Gadus morhua) has lost the genes for MH class II and its accessory molecules [172]. Furthermore, the cod-like fish, broadnosed pipefish (Syngnathus typhle), has lost MH class II function while still maintaining some of the genetic information [173]. When the whole genome of cod was compared to other sequenced fish species, it was observed that cod has large gene expansions and several gene losses in the TLR repertoire [174]. It is possible that the loss of such a large component of adaptive immunity resulted in a greater dependence on the innate immune system in this particular species. Understanding interspecies differences in immunity such as these will help increase the efficacy of future therapies and vaccine initiatives.
5. Current Methods of Bacterial Disease Prevention in Aquaculture
5.1. Heritable Differences in Selectively Bred Fish
There are a number of ways in which the breeding of fish differs from other livestock, and this is due to the often-high fecundity of aquatic species. This allows for a strong selection intensity and results in large families, which can facilitate the extensive collection of the phenotypic records of close relatives for selection candidates within breeding programs (reviewed in [175]). Since it relates directly to the economic potential of an aquaculture facility, growth is often the initial focus of selective breeding endeavors. Heritability of growth has been observed in several fish species including tilapia (Oreochromis niloticus), rainbow trout (Oncorhynchus mykiss), Atlantic salmon (Salmo salar), Asian seabass (Lates calcarifer), as well as many others [176,177,178,179]. Though now well established, the first breeding programs for aquatic species used mass selection and were generally unsuccessful [180,181]. It is now understood that this was likely due to the accumulated effects of inbreeding as they are known to influence several economic traits including growth [178,182,183,184]. Fortunately, with the development of creative breeding programs, a reduction in inbreeding can be consistently predicted for some aquatic species [185]. However, because the effects of heterosis tend to vary between species and individual stocks, confirmation of genetic improvements should be analysed on a case-by-case basis.
Despite being extremely valuable, growth is certainly not the only factor that is selected for in aquaculture. Given the large losses due to infectious disease [5], many breeding programs have been adopted to maintain improved growth while also selecting for resistance to problematic pathogens (reviewed in [186]). In some cases, heritability of disease resistance is observed without negatively influencing animal growth [187], such as has been observed in rainbow trout with resistance towards F. psychrophilum [188] or F. columnare [189]. However, it has also been shown that selecting for resistance to a single pathogen can sometimes result in increased susceptibility to another or in decreased growth [190,191,192,193,194]. In these studies, the gold standard for determining disease resistance is survival comparisons during live infection challenges (reviewed in [195]). Unfortunately, the subsequent results of these challenges may not be representative as experimental infection models are often not comparable to what would be observed in a natural infection. For some aquatic pathogens, experimental models of infection that appropriately mimic live infection have still not been established. This has led to several alternative approaches for mimicking the outbreaks that are observed in aquaculture facilities, such as co-habitation [196,197], waterborne [198,199,200] and stress induction challenges [201,202]. The major issue with these approaches is that they are often not repeatable if they are successful in producing disease symptoms. Intraperitoneal injection is often used and will ensure a much more consistent infection status for disease challenge trials. However, this method is not representative of a natural outbreak as the integument and mucous barriers are bypassed. The lack of appropriate challenge models has been a confounding factor for determining whether disease resistance observed in a lab setting will translate to disease resistance in an intensive culture situation. Understandably, this has also made it difficult to determine the true efficacy of vaccines and other therapeutics prior to their use in aquaculture facilities.
In an attempt to make salmonid stocks that are more robust and/or resilient in the face of bacterial disease, several breeding strategies have been pursued. A typical first attempt is selective breeding or artificial selection, wherein plants/animals presenting desirable traits, such as resistance to infectious disease, are used in breeding regimes so that the next generation will present these traits at a higher frequency [12]. One example of this can be seen when trying to select for resistance in rainbow trout against F. psychrophilum, the causative agent of bacterial coldwater disease (BCWD). Because this condition primarily affects animals at a young age, different families initially present markedly variable survival to BCWD. However, as the fish grow larger in size this variability in survival appears to be lost when receiving i.p. injections with F. psychrophilum [203]. This phenomenon of age-related disease susceptibility has been reported with other aquatic diseases including F. branchiophilum [204] and infectious pancreatic necrosis virus (IPNV, [205]). However, several long-term breeding studies have shown that resistance to BCWD is heritable and also that breeding over multiple generations can achieve consistently high resistance [206,207,208]. Though promising, this is a large and costly undertaking that would be difficult to perform for every bacterial disease of interest in aquaculture and also only offers potential protection against a single pathogen. The functional immune components responsible for the observed resistance to BCWD have not yet been identified, though there is some evidence to support the hypotheses that MH class IB [209], spleen size [210], IgT+ B cells [211] and the microbiome of mucosal tissues [212] could be implicated. However, because correlation is not always causation, some of these examples have already been debunked [213], emphasizing the importance of trial repetition and design in future experiments.
5.2. Vaccinations and Their Efficacy
Although there are several different types of vaccines produced for aquaculture (reviewed in [214]), the majority that are used for bacterial pathogens are killed whole-cell preparations. These do provide some protection but when compared to the successes of terrestrial vaccine formulations, are limited at best. This lower efficacy is likely due to fundamental differences between the teleostean and mammalian adaptive immune response and how it translates into immunological memory, as described above in Section 3.2.3. Aside from differences in the functional immune response, which is still not fully understood, the environment of teleosts likely plays a significant role. Temperature has in particular been shown to drastically influence the binding kinetics of monoclonal antibodies produced from terrestrial organisms in vitro [215,216,217]. Indeed, temperature has been explored in vivo in various teleost species and has been shown to influence antibody development to a wide array of antigens, with lower temperatures generally associated with a decrease in antibody production [218,219]. It is well known that higher temperatures provide more energy, often leading to an accelerated interaction between ligands and targets. Because the majority of salmonid species are cold water fish, and therefore also have lower body temperatures, it would stand to reason that this would repress antibody production when compared to mammalian counterparts. However, this does not explain the comparably slow antibody development also observed in warmwater teleosts [219,220,221,222,223]. Though still slower than what is observed in the majority of terrestrial organisms, higher temperatures are generally associated with faster development and/or a higher antibody titer in salmonids [224,225,226,227]. Curiously, the opposite trend has been reported in some warmwater teleosts, as was seen in catfish (Ictalurus punctatus) following vaccination to Edwardsiella ictaluri bacterin. When held at a higher temperature (25 °C) for 60 days, the catfish presented lower antibody titers when compared to their counterparts held for 30 days at 25 °C, followed by 30 days at 12 °C [222]. If antibody production is directly related to physiological temperature, the opposite trend would have been anticipated. Thus, although temperature likely does play a role, it is not the sole explanation for the delayed immune response associated with teleostean antibody development and immunological memory.
Rather than the rapid isotype switching following an initial exposure to a foreign entity, fish rely heavily on both IgM and IgT, antibody responses (reviewed in [120]). This reveals the importance of stimulating mucosal immunity for future vaccine design regimes, something that was not a central focus historically (reviewed in [228]). For certain bacterial pathogens, an immersion vaccination of fish has been found to be effective in inducing a mucosal response, as has been observed for V. anguillarum and F. psychrophilum [229,230]. However, in many of these cases boosters are required and application routines including those have not been developed for cultured fish (reviewed in [231]). In mammals, high antibody levels can be observed as early as two weeks following antigen exposure in what is known as the primary antibody response [41]. Yet based on recent data, even after four weeks and/or four months following live infection, the reported variability in teleost antibody production is high, with many individuals presenting low to negligible antibody production [203,232] and with a large variation in response even within the same family of fish [233]. This low level of antibody production is consistent with what has been observed previously in other teleostean models where antibody titers can require 12 weeks or longer to reach peak levels in rainbow trout [234,235]. Given that delayed antibody production is a consistent finding in teleosts, perhaps alternative approaches must be made to successfully stimulate protective responses towards bacterial pathogens. Importantly, this would require a deeper understanding of adaptive immunity in relevant teleostean species.
5.3. Understanding Bacterial Pathogens and the Resulting Disease State
Surprisingly, despite the negative impact that bacterial pathogens have on aquaculture facilities, there is limited research devoted to understanding the pathogenesis of these organisms. Elucidating the pathologic cycle has been instrumental in developing effective vaccines for several terrestrial pathogens. The vaccines for both tetanus (Clostridium tetani) and diphtheria (Corynebacterium diphtheriae) are toxoids as it is the toxin produced by the bacterium that causes fatalities, not the organism itself [236]. The influence of extracellular products on disease state has been observed with aquatic pathogens in vitro as has been reported with numerous bacteria including: F. psychrophilum [237], Pasteurella piscicida [238], A. salmonicida [239,240,241], and Moritella viscosa [242,243]. Furthermore, these observations have been translated to in vivo studies as was observed when infecting Atlantic salmon with M. viscosa [244] or when exposing rainbow trout to the extracellular products of A. salmonicida [245]. Interestingly, the conditioned media of F. psychrophilum alone has been shown to stimulate cytokine proinflammatory transcript levels in rainbow trout immune cells and to significantly inhibit their early phagocytic activity [237]. Indeed, the extracellular products of some aquatic bacteria have historically been a focus regarding their pathogenesis. Ostland and colleagues previously demonstrated that when a crude extracellular preparation of F. psychrophilum was injected directly into the muscle of rainbow trout, muscle necrosis was observed [246]. This led to further studies attempting to determine virulence factors of F. psychrophilum, including the identification of extracellular proteases and mutation analyses using the identified secreted proteases [247,248,249,250]. Unfortunately, identification of individual virulence factors has had limited success, but loss of virulence has been observed in some cases [247,251]. Removal of predicted virulence factors can result in a decrease in virulence for aquatic bacterial pathogens in vitro [252,253,254], but this is not always the case [255,256]. The majority of these experiments focus solely on the resulting virulence of the pathogens of interest. Some analyses have observed the immune function of the host, but these represent a small number in the field due to the high associated financial costs and time commitments. Therefore, studies that explore the interaction of bacterial pathogenesis with the host immune system are required to develop improved and efficient treatment options.
Like many terrestrial pathogens, it appears that some aquatic bacterial pathogens have both intracellular and extracellular components to their pathologic cycles [257,258,259,260,261,262,263,264]. This may further impede the design of some antibacterial therapies as vaccines for well-studied intracellular terrestrial pathogens, such as Mycobacterium tuberculosis, have shown variable efficacy (reviewed in [265,266]). In these situations, a typical humoral response will not successfully eliminate the pathogen of interest, so alternative methods of immune stimulation must be developed (reviewed in [267,268]). This mirrors the difficulties and variable success observed when attempting to develop a fish vaccine for R. salmoninarum, a known aquatic intracellular bacterial pathogen [269,270]. Similarly, it is believed that F. psychrophilum has both intracellular and extracellular aspects of its pathologic cycle, making vaccine development difficult [263,271]. Despite all that is unknown regarding F. psychrophilum, some promising vaccine candidates have been developed recently for BCWD [272,273,274], but these studies must be repeated and tested during natural outbreak/exposure conditions. For many bacterial pathogens, developing an effective vaccine or improving current formulations has required an understanding of the bacterial pathogenesis. This is precisely the information that is lacking with regard to many salmonid pathogens afflicting aquaculture and is also why studies should aim to understand the pathologic cycle of these pervasive organisms.
5.4. Adjuvants/Immunostimulants for Aquaculture
As seen in successful mammalian vaccine preparations, the presence of the antigen itself is important driving the specificity of immunological memory, but appropriate adjuvants are key to ensure that the resulting immune response is protective. If a vaccine contained purified protein antigens alone, this would result in only a slight antibody response with little to no T cell activation. Thus, multiple immunizations would likely be required to stimulate a sufficient antibody memory response (reviewed in [275]). For terrestrial animals, there are a variety of different and effective adjuvants used for vaccination programs (reviewed in [276]). Presently for fish, commercial vaccines consist mainly of purified antigens for the pathogen of interest as well as an emulsifying agent (reviewed in [277]). However, there has been significant research regarding the use of PAMPs, cytokines and other immunostimulants to enhance vaccine efficacy. For many of these studies, the protective response has been improved during experimental challenge with the pathogen of interest. The addition of flagellin along with Hsp60 and Hsp70 chaperonins to a subunit vaccine for P. salmonis resulted in a high protective response in Atlantic salmon [278]. One study used aluminum sulphate (alum), a common adjuvant of mammalian vaccines, in conjunction with an Escherichia coli mutant, to vaccinate for E. ictaluri in catfish. Following the disease challenge, the alum adjuvant resulted in 92% survival when compared to the 54% survival observed with the no adjuvant control [279]. Adjuvants have been an essential component in the successes of mammalian vaccines. Their addition to vaccine candidates for fish have had promising results, but further study is required to determine whether these results are reliable and/or reproducible.
To help promote an appropriate type of immune response, recombinant cytokines can also be added to vaccine preparations to act as adjuvants. With vaccines for teleost species, this is a relatively new undertaking that has shown some promising results. When recombinant IL-8 was added to a vaccine for Streptococcus iniae, catfish showed a 20% greater survival four weeks following the challenge with the pathogen [280]. However, this did not appear to provide long-lasting immune protection as survival did not differ significantly from the control at week eight [280]. Cytokines as adjuvants can also be used to strategically direct an immune response in aquatic species. The addition of IL-12 to a Nocardia seriolae vaccine was able to suppress humoral immunity and stimulate a much more protective cell-mediated response in juvenile amberjack (Seriola dumerili), resulting in an increase in survival by an impressive 88% when challenged with the bacterial pathogen [281]. Although not in combination with an actual vaccine preparation, rainbow trout receiving intraperitoneal injections of recombinant IL-1β presented a significantly increased survival to A. salmonicida as well as total leukocyte number in peritoneal exudates [282]. Aside from the physical addition of adjuvants to vaccine preparations, the host machinery can be manipulated to produce recombinant cytokines. This is accomplished through the injection of expression plasmids that contain DNA sequences for the desired proteins. Using these DNA vaccines, host-made cytokines such as IL-8 and IL-1β have shown promise as adjuvants in flounder, sea bass and rainbow trout [283,284,285,286], but have yet to be tested in aquaculture outbreak situations. Despite these promising indications, the use of recombinant salmonid cytokines requires further exploration. Unfortunately, there remains a lack of assays specifically targeting teleost immune proteins [287]. Thus, our understanding of these important immune modulators is primarily limited to the genomic level.
The production of AMPs by fish species has been extensively studied as an alternative treatment for antibiotics (reviewed in [288,289]). Aside from their direct impact on pathogens, AMPs have been shown to significantly influence the immune system of fish, making them prime candidates as indirect therapeutic agents and/or adjuvants. In fact, co-administration of AMPs with antigens from pathogens has been shown to boost immunogenicity in tilapia [290]. In salmonids, there have been few studies involving the co-administration of AMPs with vaccine candidates, but exposure to AMPs alone has been consistently shown to stimulate proinflammatory responses in vitro and in vivo [291,292,293]. There have also been examples of AMPs protecting salmonids in vivo when challenged with live bacterial pathogens [294]. Similarly, when fish are challenged with bacterial pathogens or relevant antigens, a significant induction of antimicrobial peptides in response to these stimuli has been reliably observed [295,296,297,298], indicating the defensive nature of these peptides. Teleostean AMPs have also shown synergistic effects when used in conjunction with other AMPs [299] or when used with therapeutic drugs [300] in laboratory settings. An example of this has been noted with pituitary adenylate-cyclase activating polypeptide (PACAP). When PACAP was administered in the presence of a bacterial pathogen, the AMP significantly enhanced the stimulation of proinflammatory transcripts [301]. When used alone, PACAP was able to significantly stimulate proinflammatory transcripts of rainbow trout immune cells, indicating that this peptide has an immunostimulatory activity similar to other studied mammalian AMPs [302,303,304]. Interestingly, these stimulatory effects are contrary to what has been observed with PACAP in mammalian studies wherein anti-inflammatory responses have been primarily observed [305,306,307]. This emphasizes the importance of confirming the activity of these molecules in each model organism before assigning a canonical function. Additionally, as a member of the secretin/glucagon/growth hormone-releasing hormone/vasoactive intestinal peptide superfamily [308], PACAP has also been linked to improved growth in some fish species [309,310,311], thereby adding another potential benefit for farmers if used as an adjuvant and/or a therapeutic agent. Given the need for appropriate adjuvants in fish vaccines, the use of AMPs may provide an effective, environmentally friendly alternative that is naturally produced by the host species.
This review highlights the negative impacts of bacteria in aquaculture settings, but it is important to acknowledge that certain bacteria can actually be beneficial for cultured aquatic species. This can be seen in studies investigating the use of probiotics as potential vaccine adjuvants and/or immunostimulants in aquaculture (reviewed in [312]). Probiotics are non-pathogenic microorganisms that, when ingested, exert a positive influence on the health or physiology of the host [313]. In rainbow trout, there have been several studies revealing that probiotic bacteria can prevent the colonization and growth of bacterial pathogens and/or enhance the survival of the host to F. psychrophilum, V. anguillarum, Y. ruckeri, and A. salmonicida infections [314,315,316,317,318,319,320,321,322,323,324]. Similar results have been presented in other salmonid species including brown trout (Salmo trutta) [325] and Atlantic salmon [326,327]. Additionally, dietary supplementation with probiotics has been shown to improve the efficacy of vaccine candidates in rainbow trout [328,329,330], outlining their potential as adjuvants. Though the mechanisms responsible for these promising biological effects are not fully understood, there is significant evidence indicating that some probiotics can stimulate leukocyte phagocytic activity [316,331], IgT expression [332,333], and inflammatory cytokine expression [332,334,335]. Moreover, because probiotics are generally administered in feed, the use of these treatments can often provide protective effects on the gut epithelium and towards gut immunity [333,336,337,338]. This was observed in Atlantic salmon when infection with V. anguillarum and A. salmonicida resulted in extensive damage to the intestinal mucosal epithelium, yet the same exposure to a probiotic strain of bacteria resulted in histological presentations that were indistinguishable from control fish [337]. Furthermore, when these fish were co-inoculated with pathogenic and probiotic bacterial strains, the damage to the intestinal mucosal epithelium was reduced or completely abolished [337]. Interestingly, alternative administration routes of probiotics have also shown favorable results, such as when Atlantic salmon received a probiotic bath which resulted in enhanced growth and decreased mortality/morbidity during a natural outbreak of M. viscosa [339]. The use of probiotics in aquaculture as a potential adjuvant/immunomodulator shows promise but further research is necessary to fully understand the mechanisms of action, optimal administration routes and potential side-effects from long-term use.
5.5. Alternative Study Models—The Value of Fish Cell Lines for Immune Analyses
Considering the diversity of teleostean species and their utility all over the world, relatively few cell lines are available to represent the many members of this infraclass, especially when compared to mammals. The first permanent fish cell line, RTG-2, was established in 1962 from rainbow trout gonadal tissue [340]. Since then, more fish cell lines have been created with more than 300 being developed from approximately 110 species of teleosts (reviewed in [341,342]). This only accounts for 0.4% of the known 26,000 teleostean species. Many of these cell lines are derived from industrially relevant species but as aquaculture practises change to meet market demands/preferences, the establishment of appropriate eukaryotic cell lines is necessary. With the rising interest in global salmonid aquaculture, the development of cell lines from relevant tissues and species will ensure that researchers have the necessary tools to explore various aspects of cellular function, physiology and immunity in these valuable species.
Historically, the primary purpose of fish cell lines has been to isolate, propagate and study aquatic viruses. Though less common, these cultures can also provide a controlled, cost-effective environment to explore both the pathogenesis and host cellular immune response associated with bacterial pathogens. This has been observed with several aquatic bacterial pathogens including Y. ruckeri [343], Francisella noatunensis [344], F. psychrophilum [237], V. anguillarum [345] and Photobacterium damselae [346]. These and other similar studies were able to significantly improve the understanding of the associated pathogens and helped to improve future in vivo trials. An analysis of P. salmonis using the rainbow trout spleen macrophage/monocyte-like cell line, RTS11, revealed important information regarding the immunomodulatory effects of this bacterium. This provided a possible explanation for why previous vaccine attempts were not stimulating protective responses in salmonids. Infection with P. salmonis results in an upregulation of the anti-inflammatory cytokine, IL-10, as well as a reduced amount of the antimicrobial peptide, hepicidin [262]. Additionally, vesicles containing hepicidin were unable to merge with the bacterium, rendering the bactericidal peptide useless [262]. Such detailed analyses of cellular interactions with bacterial pathogens could potentially be overlooked without the use of relevant fish cell lines.
Many species of teleost fish, particularly salmonids, have complex life cycles involving an early freshwater phase followed by a transition to a saltwater environment. This transition entails not only dramatic physiological changes that the animals have to undergo, but also exposure to pathogens present in both salt- and freshwater habitats. As such, it is unsurprising that we observe important differences in immune performance between salmonids from different life-stages [347]. This emphasizes the importance of developing cell lines, not only from different species, but also from different life stages to accurately represent relative immune responses. Several in vitro studies have been conducted with the saltwater pathogen, V. anguillarum, in salmonid cell cultures [348,349,350,351,352], revealing important properties regarding the virulence of this organism. As a common bacterial pathogen on the Pacific coast of North America, there has been previous in vitro work with this organism using the embryonic Chinook salmon (Oncorhynchus tshawytscha) cell line, CHSE-214 [345,350,353], but these studies primarily looked at properties of the bacterium itself with little study of cellular host immunity. Until recently, CHSE-214 was the only cell line derived from Chinook salmon, a native salmonid of the Pacific coast. However, with the recent development of CHSS [354] and CHST [232], studies can now be conducted using Chinook cell lines from adult fish. Furthermore, it was shown that the adult CHSS cell line contained immune receptors that were found to be absent on the embryonic CHSE-214 cells [354]. As previously shown in multiple studies, the developmental age of the animal can alter the immune defenses observed, even in the resulting cell lines produced [347,354,355]. Because the most significant economic losses in aquaculture are experienced with market-sized fish, cell lines from adult tissue donors would be very applicable for this industry. However, great numbers of losses can also occur during juvenile stages, so cell lines representing all life stages could provide interesting insights for aquaculture production.
Eukaryotic cell lines are very useful for biological experimentation, but these tools should not be perceived as a replacement for whole animal research. In situations where in vivo studies are not possible or there are technological limitations, cell lines can provide preliminary results that are hypothesis generating. However, it is important to recognize that each cell line represents one cell type from a single individual, and thus these model systems cannot be expected to perfectly represent whole animals or processes that require the interactions of multiple cell types like immune responses, let alone entire populations. This is supported by numerous studies wherein mammalian cell lines have revealed that passage number and culture time can influence gene expression, enzyme activity, morphology and cell reactivity/responsiveness [356,357,358,359,360,361,362]. Although there has been little study cataloging this disconnect in teleost cell lines, it undoubtedly entails similar limitations.
6. Concluding Remarks
The vertebrate immune system is a complicated network, consisting of molecules with complimentary and antagonistic actions, interacting cells, and cell-forming tissues that all work together in an effort to protect the body from infectious agents and other foreign substances. The complexity of the immune system did not arise spontaneously but rather is the result of over 500 million years of evolution. Over this time, the immune system has continuously evolved between taxa, maintaining components that are valuable while removing others that are of little use. Aquatic and terrestrial organisms occupy a wide variety of different habitats and are therefore subject to different evolutionary pressures. As such, the composition of the immune system between species can vary widely, reflecting these environmental differences. Thus, it is no surprise that we have been able to use comparative models to (a) enhance our understanding of mammalian immunity, (b) improve animal husbandry in a species-specific way and (c) discover useful immunological tools for molecular biology applications. This review has attempted to catalogue some of the more recent findings within teleostean antibacterial immunity, and due to enhanced interest in the culture of aquatic species, this field is rapidly expanding.
With the demand for salmonid culture continually increasing, treatment options to combat infectious disease outbreaks are invaluable. Specifically, for contagions of bacteria, the current approach is the use of antibiotics, despite the tight regulations limiting their use to prevent the development of antibiotic resistance. As a result, antibiotics are not a sustainable method of choice despite their efficacy in containing bacterial disease outbreaks in real time. Gaining a deeper knowledge of salmonid immunity is essential for streamlining some of the promising alternative methods for promoting disease resistance, such as through breeding strategies to select/promote heritable markers. In situations where this is not possible, improving prophylactic treatments or optimizing alternative treatment options/potential adjuvants, such as AMPs or probiotic bacterial supplementation, could provide environmentally friendly options for bacterial disease containment. Additionally, by learning more about the pathologic cycle of problematic bacterial pathogens and also what constitutes an effective immune response in the host, researchers would be able to tailor treatment regimes, enabling the development of new and improved vaccines. This can be accomplished not only through large and costly in vivo trials but also through improvements and developments to applicable cell lines, so that the host cellular immune response can be further elucidated. All of this has the potential to significantly reduce a large portion of the $6 billion dollars of disease losses in the global aquaculture industry. Salmonid aquaculture is an important industry, and as such, research meant to provide alternative treatment options for bacterial disease outbreaks is invaluable for helping to ensure the sustainability of this expanding industry.
Author Contributions
Reviewed the literature and wrote the first draft of the manuscript, S.L.S.; Contributed to the revisions of the manuscript, S.L.S. and B.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported through a Canada Research Council Research Chair and Natural Sciences and Engineering Research Council of Canada Discovery Grant (NSERC DG; Grant # RGPIN-2018-04116) to BD.
Acknowledgments
The authors would like to acknowledge George Heath for his editing assistance. The authors would also like to thank the reviewers for their time and suggestions that helped to improve the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Taşbozan, O.; Gökçe, M.A. Fatty Acids in Fish. In Fatty Acids; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- WHO. Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Kvamsdal, S.F.; Eide, A.; Ekerhovd, N.-A.; Enberg, K.; Gudmundsdottir, A.; Hoel, A.H.; Mills, K.E.; Mueter, F.J.; Ravn-Jonsen, L.; Sandal, L.K.; et al. Harvest Control Rules in Modern Fisheries Management. Elem. Sci. Anthr. 2016, 4, 000114. [Google Scholar] [CrossRef]
- Van Anrooy, R. Review of the Current State of World Aquaculture Insurance; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Brummett, R.E.; Alvial, A.; Kibenge, F.; Forster, J.; Burgos, J.M.; Ibarra, R.; St-Hilaire, S.; Chamberlain, G.C.; Lightner, D.V.; van Khoa, L.; et al. Reducing Disease Risk in Aquaculture; Agriculture and Environmental Services Discussion Paper; No. 9; World Bank Group: Washington, DC, USA, 2014. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Lucas, J.S.; Southgate, P.C. Aquaculture: Farming Aquatic Animals and Plants, 2nd ed.; John Wiley & Sons: West Sussex, UK, 2012. [Google Scholar]
- Meyer, F.P. Aquaculture Disease and Health Management. J. Anim. Sci. 1991, 69, 4201–4208. [Google Scholar] [CrossRef]
- Wedemeyer, G.A.; Nelson, N.C. Survival of Two Bacterial Fish Pathogens (Aeromonas salmonicida and the Enteric Redmouth Bacterium) in Ozonated, Chlorinated, and Untreated Waters. J. Fish. Res. Board Can. 1977, 34, 429–432. [Google Scholar] [CrossRef]
- Hoff, K.A. Survival of Vibrio anguillarum and Vibrio salmonicida at different salinities. Appl. Environ. Microbiol. 1989, 55, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Madetoja, J.; Nystedt, S.; Wiklund, T. Survival and Virulence of Flavobacterium psychrophilum in Water Microcosms. FEMS Microbiol. Ecol. 2003, 43, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, E. Henderson’s Dictionary of Biology; Pearson Benjamin Cummings Prentice Hall: London, UK, 2008. [Google Scholar]
- Ringø, E.; Myklebust, R.; Mayhew, T.M.; Olsen, R.E. Bacterial Translocation and Pathogenesis in the Digestive Tract of Larvae and Fry. Aquaculture 2007, 268, 251–264. [Google Scholar] [CrossRef]
- Defoirdt, T. Virulence Mechanisms of Bacterial Aquaculture Pathogens and Antivirulence Therapy for Aquaculture. Rev. Aquac. 2014, 6, 100–114. [Google Scholar] [CrossRef]
- Glasauer, S.M.K.; Neuhauss, S.C.F. Whole-Genome Duplication in Teleost Fishes and Its Evolutionary Consequences. Mol. Genet. Genom. 2014, 289, 1045–1060. [Google Scholar] [CrossRef]
- Ravi, V.; Venkatesh, B. The Divergent Genomes of Teleosts. Annu. Rev. Anim. Biosci. 2018, 6, 47–68. [Google Scholar] [CrossRef]
- Helfman, G.; Collette, B.B.; Facey, D.E.; Bowen, B.W. The Diversity of Fishes: Biology, Evolution, and Ecology, 2nd ed.; Helfman, G., Collette, B.B., Facey, D.E., Bowen, B.W., Eds.; Wiley & Sons Ltd.: West Sussex, UK, 2009. [Google Scholar]
- Hodgkinson, J.W.; Grayfer, L.; Belosevic, M. Biology of Bony Fish Macrophages. Biology 2015, 4, 881–906. [Google Scholar] [CrossRef]
- Grayfer, L.; Kerimoglu, B.; Yaparla, A.; Hodgkinson, J.W.; Xie, J.; Belosevic, M. Mechanisms of Fish Macrophage Antimicrobial Immunity. Front. Immunol. 2018, 9, 1105. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Qin, Z.; Liu, H.; Lin, L.; Ye, J.; Li, J. Recent Advances on Phagocytic B Cells in Teleost Fish. Front. Immunol. 2020, 824. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.; Brown, M.L. A Review of Immune System Components, Cytokines, and Immunostimulants in Cultured Finfish Species. Open J. Anim. Sci. 2017, 7, 267–288. [Google Scholar] [CrossRef]
- Zou, J.; Secombes, C.J. The Function of Fish Cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef]
- Reyes-Cerpa, S.; Maisey, K.; Reyes-Lpez, F.; Toro-Ascuy, D.; Mara, A.; Imarai, M. Fish Cytokines and Immune Response. In New Advances and Contributions to Fish Biology; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Shamri, R.; Xenakis, J.J.; Spencer, L.A. Eosinophils in Innate Immunity: An Evolving Story. Cell Tissue Res. 2011, 343, 57–83. [Google Scholar] [CrossRef]
- Varricchi, G.; Raap, U.; Rivellese, F.; Marone, G.; Gibbs, B.F. Human Mast Cells and Basophils—How Are They Similar How Are They Different? Immunol. Rev. 2018, 282, 8–34. [Google Scholar] [CrossRef]
- Voehringer, D. Recent Advances in Understanding Basophil Functions in vivo. F1000Research 2017, 6, 1464. [Google Scholar] [CrossRef]
- Sheshachalam, A.; Srivastava, N.; Mitchell, T.; Lacy, P.; Eitzen, G. Granule Protein Processing and Regulated Secretion in Neutrophils. Front. Immunol. 2014, 5, 448. [Google Scholar] [CrossRef]
- Jakubzick, C.V.; Randolph, G.J.; Henson, P.M. Monocyte Differentiation and Antigen-Presenting Functions. Nat. Rev. Immunol. 2017, 17, 349–362. [Google Scholar] [CrossRef]
- Mathur, A.; Tripathi, A.; Kuse, M. Scalable System for Classification of White Blood Cells from Leishman Stained Blood Stain Images. J. Pathol. Inform. 2013, 4, 15. [Google Scholar] [CrossRef]
- Houwen, B. The Differential Cell Count. Lab Hematol. 2001, 7, 89–100. [Google Scholar]
- Niimi, A.J.; Lowe-Jinde, L. Differential Blood Cell Ratios of Rainbow Trout (Salmo gairdneri) Exposed to Methylmercury and Chlorobenzenes. Arch. Environ. Contam. Toxicol. 1984, 13, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Blaxhall, P.C.; Daisley, K.W. Routine Haematological Methods for Use with Fish Blood. J. Fish Biol. 1973, 5, 771–781. [Google Scholar] [CrossRef]
- Hashimoto, D.; Chow, A.; Noizat, C.; Teo, P.; Beasley, M.B.; Leboeuf, M.; Becker, C.D.; See, P.; Price, J.; Lucas, D.; et al. Tissue-Resident Macrophages Self-Maintain Locally throughout Adult Life with Minimal Contribution from Circulating Monocytes. Immunity 2013, 38, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Plüddemann, A. Tissue Macrophages: Heterogeneity and Functions. BMC Biol. 2017, 15, 53. [Google Scholar] [CrossRef]
- Soza-Ried, C.; Hess, I.; Netuschil, N.; Schorpp, M.; Boehm, T. Essential Role of C-myb in Definitive Hematopoiesis Is Evolutionarily Conserved. Proc. Natl. Acad. Sci. USA 2010, 107, 17304–17308. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage Biology in Development, Homeostasis and Disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, C.; Lin, Z.; Zhan, S.; Kong, L.; Fang, C.; Li, J. Macrophage Polarization and Function with Emphasis on the Evolving Roles of Coordinated Regulation of Cellular Signaling Pathways. Cell Signal. 2014, 26, 192–197. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Punt, J.; Stranford, S.; Jones, P.; Owen, J. Kuby Immunology, 8th ed.; W.H. Freeman: New York, NY, USA, 2019. [Google Scholar]
- Taguchi, T.; Mukai, K. Innate Immunity Signalling and Membrane Trafficking. Curr. Opin. Cell Biol. 2019, 59, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.J.; Lu, X.J.; Chen, Q.; Chen, J. Molecular Characterization and Functional Analysis of a Novel C-Type Lectin Receptor-like Gene from a Teleost Fish, Plecoglossus altivelis. Fish Shellfish Immunol. 2015, 44, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, Z.; Yu, L.; Zhang, B.; Wang, J.; Zhou, J. Nucleotide-Binding and Oligomerization Domain (NOD)-like Receptors in Teleost Fish: Current Knowledge and Future Perspectives. J. Fish Dis. 2018, 41, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Zou, P.F.; Nie, P. Retinoic Acid-Inducible Gene I (RIG-I)-like Receptors (RLRs) in fish: Current Knowledge and Future Perspectives. Immunology 2017, 151, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Poynter, S.; Lisser, G.; Monjo, A.; DeWitte-Orr, S. Sensors of Infection: Viral Nucleic Acid PRRs in Fish. Biology 2015, 4, 460–493. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ji, J.; Jiang, X.; Shao, T.; Fan, D.; Jiang, X.; Lin, A.; Xiang, L.; Shao, J. Characterization of CGAS Homologs in Innate and Adaptive Mucosal Immunities in Zebrafish Gives Evolutionary Insights into CGAS-STING Pathway. FASEB J. 2020, 34, 7786–7809. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, X.; Zhou, C.; Li, L.; Nie, G.; Li, X. Toll-like Receptor Recognition of Bacteria in Fish: Ligand Specificity and Signal. Pathways. Fish Shellfish Immunol. 2014, 41, 380–388. [Google Scholar] [CrossRef]
- Nie, L.; Cai, S.-Y.; Shao, J.-Z.; Chen, J. Toll-Like Receptors, Associated Biological Roles, and Signaling Networks in Non-Mammals. Front. Immunol. 2018, 9, 1523. [Google Scholar] [CrossRef]
- Samanta, M.; Swain, B.; Basu, M.; Panda, P.; Mohapatra, G.B.; Sahoo, B.R.; Maiti, N.K. Molecular Characterization of Toll-like Receptor 2 (TLR2), Analysis of Its Inductive Expression and Associated down-Stream Signaling Molecules Following Ligands Exposure and Bacterial Infection in the Indian Major Carp, Rohu (Labeo rohita). Fish Shellfish Immunol. 2012, 32, 411–425. [Google Scholar] [CrossRef]
- Basu, M.; Swain, B.; Sahoo, B.R.; Maiti, N.K.; Samanta, M. Induction of Toll-like Receptor (TLR) 2, and MyD88-Dependent TLR-Signaling in Response to Ligand Stimulation and Bacterial Infections in the Indian Major Carp, Mrigal (Cirrhinus mrigala). Mol. Biol. Rep. 2012, 39, 6015–6028. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.M.S.; Hermsen, T.; Taverne-Thiele, A.J.; Savelkoul, H.F.J.; Wiegertjes, G.F. Evolution of Recognition of Ligands from Gram-Positive Bacteria: Similarities and Differences in the TLR2-Mediated Response between Mammalian Vertebrates and Teleost Fish. J. Immunol. 2010, 184, 2355–2368. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.D.; Asahi, T.; Kondo, H.; Hirono, I.; Aoki, T. Molecular Cloning and Expression Study on Toll-like Receptor 5 Paralogs in Japanese Flounder, Paralichthys olivaceus. Fish Shellfish Immunol. 2010, 29, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, I.; Sepulcre, M.P.; Meseguer, J.; Mulero, V. Molecular Cloning, Phylogenetic Analysis and Functional Characterization of Soluble Toll-like Receptor 5 in Gilthead Seabream, Sparus aurata. Fish Shellfish Immunol. 2013, 35, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Tsujita, T.; Tsukada, H.; Nakao, M.; Oshiumi, H.; Matsumoto, M.; Seya, T. Sensing Bacterial Flagellin by Membrane and Soluble Orthologs of Toll-like Receptor 5 in Rainbow Trout (Onchorhynchus mykiss). J. Biol. Chem. 2004, 279, 48588–48597. [Google Scholar] [CrossRef] [PubMed]
- Iliev, D.B.; Skjæveland, I.; Jørgensen, J.B. CpG Oligonucleotides Bind TLR9 and RRM-Containing Proteins in Atlantic Salmon (Salmo salar). BMC Immunol. 2013, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Franch, R.; Cardazzo, B.; Antonello, J.; Castagnaro, M.; Patarnello, T.; Bargelloni, L. Full-Length Sequence and Expression Analysis of Toll-like Receptor 9 in the Gilthead Seabream (Sparus aurata L.). Gene 2006, 378, 42–51. [Google Scholar] [CrossRef]
- Gao, H.; Wu, L.; Sun, J.S.; Geng, X.Y.; Pan, B.P. Molecular Characterization and Expression Analysis of Toll-like Receptor 21 CDNA from Paralichthys olivaceus. Fish Shellfish Immunol. 2013, 35, 1138–1145. [Google Scholar] [CrossRef]
- Wang, W.; Shen, Y.; Pandit, N.P.; Li, J. Molecular Cloning, Characterization and Immunological Response Analysis of Toll-like Receptor 21 (TLR21) Gene in Grass Carp, Ctenopharyngodon idella. Dev. Comp. Immunol. 2013, 40, 227–231. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Li, D.; Huang, A.; Bu, G.; Meng, F.; Kong, F.; Cao, X.; Han, X.; Pan, X.; et al. Teleost-Specific TLR25 Identified from Schizothorax prenanti May Recognize Bacterial/Viral Components and Activate NF-ΚB and Type I IFNs Signaling Pathways. Fish Shellfish Immunol. 2018, 82, 361–370. [Google Scholar] [CrossRef]
- Shan, S.; Liu, D.; Liu, R.; Zhu, Y.; Li, T.; Zhang, F.; An, L.; Yang, G.; Li, H. Non-Mammalian Toll-like Receptor 18 (Tlr18) Recognizes Bacterial Pathogens in Common Carp (Cyprinus carpio L.): Indications for a Role of Participation in the NF-ΚB Signaling Pathway. Fish Shellfish Immunol. 2018, 72, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.D.; Kondo, H.; Hirono, I.; Aoki, T. Molecular Cloning and Characterization of Toll-like Receptor 14 in Japanese Flounder, Paralichthys olivaceus. Fish Shellfish Immunol. 2011, 30, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Chu, Q.; Xu, T. MiR-122 Involved in the Regulation of Toll-like Receptor Signaling Pathway after Vibrio anguillarum Infection by Targeting TLR14 in Miiuy Croaker. Fish Shellfish Immunol. 2016, 58, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Gallo, R.L. Antimicrobial Peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef]
- Lai, Y.; Gallo, R.L. AMPed up Immunity: How Antimicrobial Peptides Have Multiple Roles in Immune Defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef]
- Lee, J.K.; Luchian, T.; Park, Y. New Antimicrobial Peptide Kills Drug-Resistant Pathogens without Detectable Resistance. Oncotarget 2018, 9, 15616–15634. [Google Scholar] [CrossRef]
- O’Neill, H.C.; Griffiths, K.L.; Periasamy, P.; Hinton, R.A.; Hey, Y.-Y.; Petvises, S.; Tan, J.K.H. Spleen as a Site for Hematopoiesis of a Distinct Antigen Presenting Cell Type. Stem Cells Int. 2011, 2011, 954275. [Google Scholar] [CrossRef]
- Schittek, B.; Hipfel, R.; Sauer, B.; Bauer, J.; Kalbacher, H.; Stevanovic, S.; Schirle, M.; Schroeder, K.; Blin, N.; Meier, F.; et al. Dermcidin: A Novel Human Antibiotic Peptide Secreted by Sweat Glands. Nat. Immunol. 2001, 2, 1133–1137. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial Peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef]
- Kang, H.-K.; Kim, C.; Seo, C.H.; Park, Y. The Therapeutic Applications of Antimicrobial Peptides (AMPs): A Patent Review. J. Microbiol. 2017, 55, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Otvos, L.; Cudic, M. Broth Microdilution Antibacterial Assay of Peptides. Methods Mol. Biol. 2007, 386, 309–320. [Google Scholar] [CrossRef]
- Elssner, A.; Duncan, M.; Gavrilin, M.; Wewers, M.D. A Novel P2X 7 Receptor Activator, the Human Cathelicidin-Derived Peptide LL37, Induces IL-1β Processing and Release. J. Immunol. 2004, 172, 4987–4994. [Google Scholar] [CrossRef]
- Yu, J.; Mookherjee, N.; Wee, K.; Bowdish, D.M.E.; Pistolic, J.; Li, Y.; Rehaume, L.; Hancock, R.E.W. Host Defense Peptide LL-37, in Synergy with Inflammatory Mediator IL-1beta, Augments Immune Responses by Multiple Pathways. J. Immunol. 2007, 179, 7684–7691. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Brown, K.L.; Bowdish, D.M.E.; Doria, S.; Falsafi, R.; Hokamp, K.; Roche, F.M.; Mu, R.; Doho, G.H.; Pistolic, J.; et al. Modulation of the TLR-Mediated Inflammatory Response by the Endogenous Human Host Defense Peptide LL-37. J. Immunol. 2006, 176, 2455–2464. [Google Scholar] [CrossRef] [PubMed]
- di Nardo, A.; Braff, M.H.; Taylor, K.R.; Na, C.; Granstein, R.D.; McInturff, J.E.; Krutzik, S.; Modlin, R.L.; Gallo, R.L. Cathelicidin Antimicrobial Peptides Block Dendritic Cell TLR4 Activation and Allergic Contact Sensitization. J. Immunol. 2007, 178, 1829–1834. [Google Scholar] [CrossRef]
- Falco, A.; Martinez-Lopez, A.; Coll, J.P.; Estepa, A. The Potential for Antimicrobial Peptides to Improve Fish Health in Aquaculture. In Infectious Disease in Aquaculture, Brian Austin; Woodhead Publishing Limited: Philadelphia, PA, USA, 2012; pp. 457–479. [Google Scholar] [CrossRef]
- Rajanbabu, V.; Chen, J.Y. Applications of Antimicrobial Peptides from Fish and Perspectives for the Future. Peptides 2011, 32, 415–420. [Google Scholar] [CrossRef]
- Valero, Y.; Saraiva-Fraga, M.; Costas, B.; Guardiola, F.A. Antimicrobial Peptides from Fish: Beyond the Fight against Pathogens. Rev. Aquac. 2020, 12, 224–253. [Google Scholar] [CrossRef]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH Oxidases: An Overview from Structure to Innate Immunity-Associated Pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef]
- Nguyen, G.T.; Green, E.R.; Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front. Cell. Infect. Microbiol. 2017, 7, 373. [Google Scholar] [CrossRef]
- DeLeo, F.R.; Quinn, M.T. Assembly of the Phagocyte NADPH Oxidase: Molecular Interaction of Oxidase Proteins. J. Leukoc. Biol. 1996, 60, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, W.M. The Phagocyte NOX2 NADPH Oxidase in Microbial Killing and Cell Signaling. Curr. Opin. Immunol. 2019, 60, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Stafford, J.L.; Galvez, F.; Goss, G.G.; Belosevic, M. Induction of Nitric Oxide and Respiratory Burst Response in Activated Goldfish Macrophages Requires Potassium Channel Activity. Dev. Comp. Immunol. 2002, 26, 445–459. [Google Scholar] [CrossRef]
- Sepulcre, M.P.; López-Castejón, G.; Meseguer, J.; Mulero, V. The Activation of Gilthead Seabream Professional Phagocytes by Different PAMPs Underlines the Behavioural Diversity of the Main Innate Immune Cells of Bony Fish. Mol. Immunol. 2007, 44, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Boltaña, S.; Doñate, C.; Goetz, F.W.; MacKenzie, S.; Balasch, J.C. Characterization and Expression of NADPH Oxidase in LPS-, Poly(I:C)- and Zymosan-Stimulated Trout (Oncorhynchus mykiss W.) Macrophages. Fish Shellfish Immunol. 2009, 26, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Sharp, G.J.E.; Secombes, C.J. The Role of Reactive Oxygen Species in the Killing of the Bacterial Fish Pathogen Aeromonas salmonicida by Rainbow Trout Macrophages. Fish Shellfish Immunol. 1993, 3, 119–129. [Google Scholar] [CrossRef]
- Ardó, L.; Jeney, Z.; Adams, A.; Jeney, G. Immune Responses of Resistant and Sensitive Common Carp Families Following Experimental Challenge with Aeromonas hydrophila. Fish Shellfish Immunol. 2010, 29, 111–116. [Google Scholar] [CrossRef]
- Hodgkinson, J.W.; Ge, J.Q.; Grayfer, L.; Stafford, J.; Belosevic, M. Analysis of the Immune Response in Infections of the Goldfish (Carassius auratus L.) with Mycobacterium marinum. Dev. Comp. Immunol. 2012, 38, 456–465. [Google Scholar] [CrossRef]
- Havixbeck, J.J.; Rieger, A.M.; Churchill, L.J.; Barreda, D.R. Neutrophils Exert Protection in Early Aeromonas veronii Infections through the Clearance of Both Bacteria and Dying Macrophages. Fish Shellfish Immunol. 2017, 63, 18–30. [Google Scholar] [CrossRef]
- Collazos, M.E.; Ortega, E.; Barriga, C. Effect of Temperature on the Immune System of a Cyprinid Fish (Tinca tinca, L). Blood Phagocyte Function at Low Temperature. Fish Shellfish Immunol. 1994, 4, 231–238. [Google Scholar] [CrossRef]
- Collazos, M.E.; Barriga, C.; Ortega, E. Enhanced Granulocyte Phagocytosis at Low Winter Temperature and High Summer Temperature in the Tench (Tinca tinca L.). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1994, 109, 643–648. [Google Scholar] [CrossRef]
- Nikoskelainen, S.; Bylund, G.; Lilius, E.M. Effect of Environmental Temperature on Rainbow Trout (Oncorhynchus mykiss) Innate Immunity. Dev. Comp. Immunol. 2004, 28, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Le Morvan, C.; Troutaud, D.; Deschaux, P. Differential Effects of Temperature on Specific and Nonspecific Immune Defences in Fish. J. Exp. Biol. 1998, 201, 165–168. [Google Scholar] [PubMed]
- Cooper, M.D.; Alder, M.N. The Evolution of Adaptive Immune Systems. Cell 2006, 124, 815–822. [Google Scholar] [CrossRef]
- Flajnik, M.F.; Kasahara, M. Origin and Evolution of the Adaptive Immune System: Genetic Events and Selective Pressures. Nat. Rev. Genet. 2010, 11, 47–59. [Google Scholar] [CrossRef]
- Nemazee, D. Role of B Cell Antigen Receptor in Regulation of V(D)J Recombination and Cell Survival. Immunol. Res. 2000, 21, 259–263. [Google Scholar] [CrossRef]
- Abbas, A.K.; Murphy, K.M.; Sher, A. Functional Diversity of Helper T Lymphocytes. Nature 1996, 383, 787–793. [Google Scholar] [CrossRef]
- Barry, M.; Bleackley, R.C. Cytotoxic T Lymphocytes: All Roads Lead to Death. Nat. Rev. Immunol. 2002, 2, 401–409. [Google Scholar] [CrossRef]
- Lebien, T.W.; Tedder, T.F. B Lymphocytes: How They Develop and Function. Blood 2008, 112, 1570–1580. [Google Scholar] [CrossRef]
- Da Silva Pitombeira, M.; Martins, J.M.; Martins, J.M. Hematology of the Spanish Mackerel, Scomberomorus maculatus. Copeia 1970, 1970, 182. [Google Scholar] [CrossRef]
- Zhang, Y.-A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; Lapatra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a Primitive Immunoglobulin Class Specialized in Mucosal Immunity. Nat. Immunol. 2012, 11, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Maisey, K.; Toro-Ascuy, D.; Montero, R.; Reyes-López, F.E.; Imarai, M. Identification of CD3ε, CD4, CD8β Splice Variants of Atlantic Salmon. Fish Shellfish Immunol. 2011, 31, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, F.; Dijkstra, J.M.; Kotterba, P.; Korytář, T.; Kock, H.; Köllner, B.; Jaureguiberry, B.; Nakanishi, T.; Fischer, U. The Expression of CD8α Discriminates Distinct T Cell Subsets in Teleost Fish. Dev. Comp. Immunol. 2011, 35, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.; Stet, R.J.M. The Relationship between Major Histocompatibility Receptors and Innate Immunity in Teleost Fish. Dev. Comp. Immunol. 2001, 25, 683–699. [Google Scholar] [CrossRef]
- Neefjes, J.; Jongsma, M.L.M.; Paul, P.; Bakke, O. Towards a Systems Understanding of MHC Class I and MHC Class II Antigen Presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef]
- Landsverk, O.J.B.; Bakke, O.; Gregers, T.F. MHC II and the Endocytic Pathway: Regulation by Invariant Chain. Scand. J. Immunol. 2009, 70, 184–193. [Google Scholar] [CrossRef]
- Blum, J.S.; Wearsch, P.A.; Cresswell, P. Pathways of Antigen Processing. Annu. Rev. Immunol. 2013, 31, 443–473. [Google Scholar] [CrossRef]
- Choo, S.Y. The HLA System: Genetics, Immunology, Clinical Testing, and Clinical Implications. Yonsei Med. J. 2007, 48, 11–23. [Google Scholar] [CrossRef]
- Garcia, M.A.A.; Yebra, B.G.; Flores, A.L.L.; Guerra, E.G. The Major Histocompatibility Complex in Transplantation. J. Transplant. 2012, 2012, 842141. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Dijkstra, J.M. Major Histocompatibility Complex (MHC) Genes and Disease Resistance in Fish. Cells 2019, 8, 378. [Google Scholar] [CrossRef]
- Lenz, T.L. Computational Prediction of MHC II-Antigen Binding Supports Divergent Allele Advantage and Explains Trans-Species Polymorphism. Evolution 2011, 65, 2380–2390. [Google Scholar] [CrossRef]
- Pierini, F.; Lenz, T.L. Divergent Allele Advantage at Human MHC Genes: Signatures of Past and Ongoing Selection. Mol. Biol. Evol. 2018, 35, 2145–2158. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.L.; Neff, B.D. Major Histocompatibility Complex Heterozygote Advantage and Widespread Bacterial Infections in Populations of Chinook Salmon (Oncorhynchus tshawytscha). Mol. Ecol. 2009, 18, 4716–4729. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.A.; Kirkland, M.; Heath, J.W.; Heath, D.D.; Dixon, B. Breeding Strategy and Rearing Environment Effects on the Disease Resistance of Cultured Chinook Salmon (Oncorhynchus tshawytscha). Aquaculture 2014, 422–423, 160–166. [Google Scholar] [CrossRef]
- Pazderka, F.; Longenecker, B.M.; Law, G.R.J.; Stone, H.A.; Ruth, R.F. Histocompatibility of Chicken Populations Selected for Resistance to Marek’s Disease. Immunogenetics 1975, 2, 93–100. [Google Scholar] [CrossRef]
- Hill, A.V.S.; Elvin, J.; Willis, A.C.; Aidoo, M.; Allsopp, C.E.M.; Gotch, F.M.; Ming Gao, X.; Takiguchis, M.; Greenwood, B.M.; Townsend, A.R.M.; et al. Molecular Analysis of the Association of HLA-B53 and Resistance to Severe Malaria. Nature 1992, 360, 434–439. [Google Scholar] [CrossRef]
- Shen, H.; Han, G.; Jia, B.; Jiang, S.; Du, Y. MHC-DRB1/DQB1 Gene Polymorphism and Its Association with Resistance/Susceptibility to Cystic echinococcosis in Chinese Merino Sheep. J. Parasitol. Res. 2014, 2014, 272601. [Google Scholar] [CrossRef]
- Forthal, D.N. Functions of Antibodies. Microbiol. Spectr. 2014, 2, 23–48. [Google Scholar]
- Sunyer, J.O. Fishing for Mammalian Paradigms in the Teleost Immune System. Nat. Immunol. 2013, 14, 320–326. [Google Scholar] [CrossRef]
- Mashoof, S.; Criscitiello, M.F. Fish Immunoglobulins. Biology 2016, 5, 45. [Google Scholar] [CrossRef]
- Parra, D.; Reyes-Lopez, F.E.; Tort, L. Mucosal Immunity and B Cells in Teleosts: Effect of Vaccination and Stress. Front. Immunol. 2015, 6, 354. [Google Scholar] [CrossRef] [PubMed]
- Gutzeit, C.; Chen, K.; Cerutti, A. The Enigmatic Function of IgD: Some Answers at Last. Eur. J. Immunol. 2018, 48, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.D.; Landis, E.D.; Phillips, R.B. Discovery of a Unique Ig Heavy-Chain (IgT) in Rainbow Trout: Implications for a Distinctive B Cell Developmental Pathway in Teleost Fish. Proc. Natl. Acad. Sci. USA 2005, 102, 6919–6924. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Parra, D.; Gómez, D.; Salinas, I.; Zhang, Y.A.; von Gersdorff Jørgensen, L.; Heinecke, R.D.; Buchmann, K.; LaPatra, S.; Oriol Sunyer, J. Teleost Skin, an Ancient Mucosal Surface That Elicits Gut-like Immune Responses. Proc. Natl. Acad. Sci. USA 2013, 110, 13097–13102. [Google Scholar] [CrossRef]
- Piazzon, M.C.; Galindo-Villegas, J.; Pereiro, P.; Estensoro, I.; Calduch-Giner, J.A.; Gómez-Casado, E.; Novoa, B.; Mulero, V.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Differential Modulation of IgT and IgM upon Parasitic, Bacterial, Viral, and Dietary Challenges in a Perciform Fish. Front. Immunol. 2016, 7, 637. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Kong, W.; Yin, Y.X.; Dong, F.; Huang, Z.Y.; Yin, G.M.; Dong, S.; Salinas, I.; Zhang, Y.A.; Xu, Z. Mucosal Immunoglobulins Protect the Olfactory Organ of Teleost Fish against Parasitic Infection. PLoS Pathog. 2018, 14, e1007251. [Google Scholar] [CrossRef]
- Solem, S.T.; Stenvik, J. Antibody Repertoire Development in—A Review with Emphasis on Salmonids and Gadus morhua L. Dev. Comp. Immunol. 2006, 30, 57–76. [Google Scholar] [CrossRef]
- Kaattari, S.L.; Zhang, H.L.; Khor, I.W.; Kaattari, I.M.; Shapiro, D.A. Affinity Maturation in Trout: Clonal Dominance of High Affinity Antibodies Late in the Immune Response. Dev. Comp. Immunol. 2002, 26, 191–200. [Google Scholar] [CrossRef]
- Dickerson, H.W.; Findly, R.C. Vertebrate Adaptive Immunity-Comparative Insights from a Teleost Model. Front. Immunol. 2017, 8, 1379. [Google Scholar] [CrossRef]
- Barreto, V.M.; Pan-Hammarstrom, Q.; Zhao, Y.; Hammarstrom, L.; Misulovin, Z.; Nussenzweig, M.C. AID from Bony Fish Catalyzes Class Switch Recombination. J. Exp. 2005, 202, 733–738. [Google Scholar] [CrossRef]
- Wakae, K.; Magor, B.G.; Saunders, H.; Nagaoka, H.; Kawamura, A.; Kinoshita, K.; Honjo, T.; Muramatsu, M. Evolution of Class Switch Recombination Function in Fish Activation-Induced Cytidine Deaminase, AID. Int. Immunol. 2006, 18, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Craig Findly, R.; Zhao, X.; Noe, J.; Camus, A.C.; Dickerson, H.W. B Cell Memory Following Infection and Challenge of Channel Catfish with Ichthyophthirius multifiliis. Dev. Comp. Immunol. 2013, 39, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Bromage, E.S.; Kaattari, I.M.; Zwollo, P.; Kaattari, S.L. Plasmablast and Plasma Cell Production and Distribution in Trout Immune Tissues. J. Immunol. 2004, 173, 7317–7323. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Kaattari, I.; Kaattari, S. Plasmablasts and Plasma Cells: Reconsidering Teleost Immune System Organization. Dev. Comp. Immunol. 2011, 35, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, V.; Brennan, S.; Chambers, G.; George, P. The Albumins of Chinook Salmon (Oncorhynchus tshawytscha) and Brown Trout (Salmo trutta) Appear to Lack a Propeptide. Arch. Biochem. Biophys. 1998, 350, 239–244. [Google Scholar] [CrossRef]
- Grayfer, L.; Belosevic, M. Cytokine Regulation of Teleost Inflammatory Responses. In New Advances and Contributions to Fish Biology; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V.M. Signaling in Innate Immunity and Inflammation. CSH Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A Guide to Chemokines and Their Receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and Chemokine Receptors: Positioning Cells for Host Defense and Immunity. Annu. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef]
- Dixon, B.; Shum, B.; Adams, E.J.; Magor, K.; Hedrick, R.P.; Muir, D.G.; Parham, P. CK-1, a Putative Chemokine of Rainbow Trout (Oncorhynchus mykiss). Immunol. Rev. 1998, 166, 341–348. [Google Scholar] [CrossRef]
- Alejo, A.; Tafalla, C. Chemokines in Teleost Fish Species. Dev. Comp. Immunol. 2011, 35, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.; Tafalla, C. Teleost Chemokines and Their Receptors. Biology 2015, 4, 756–784. [Google Scholar] [CrossRef] [PubMed]
- Nomiyama, H.; Hieshima, K.; Osada, N.; Kato-Unoki, Y.; Otsuka-Ono, K.; Takegawa, S.; Izawa, T.; Yoshizawa, A.; Kikuchi, Y.; Tanase, S.; et al. Extensive Expansion and Diversification of the Chemokine Gene Family in Zebrafish: Identification of a Novel Chemokine Subfamily CX. BMC Genom. 2008, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- Peatman, E.; Liu, Z. Evolution of CC Chemokines in Teleost Fish: A Case Study in Gene Duplication and Implications for Immune Diversity. Immunogenetics 2007, 59, 613–623. [Google Scholar] [CrossRef]
- Kobayashi, Y. Neutrophil Infiltration and Chemokines. Crit. Rev. Immunol. 2006, 26, 307–315. [Google Scholar] [CrossRef]
- Martin, P.; Leibovich, S.J. Inflammatory Cells during Wound Repair: The Good, the Bad and the Ugly. Trends Cell Biol. 2005, 15, 599–607. [Google Scholar] [CrossRef]
- Feghali, C.A.; Wright, T.M. Cytokines in Acute and Chronic Inflammation. Front. Biosci. 1997, 2, d12–d26. [Google Scholar] [CrossRef]
- Wulff, B.C.; Wilgus, T.A. Mast Cell Activity in the Healing Wound: More than Meets the Eye? Exp. Dermatol. 2013, 22, 507–510. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast Cells and Inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 21–33. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of Inflammation: The Beginning Programs the End. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Sfacteria, A.; Brines, M.; Blank, U. The Mast Cell Plays a Central Role in the Immune System of Teleost Fish. Mol. Immunol. 2015, 63, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef] [PubMed]
- Kühn, R.; Löhler, J.; Rennick, D.; Rajewsky, K.; Müller, W. Interleukin-10-Deficient Mice Develop Chronic Enterocolitis. Cell 1993, 75, 263–274. [Google Scholar] [CrossRef]
- Reyes-Cerpa, S.; Reyes-López, F.E.; Toro-Ascuy, D.; Ibañez, J.; Maisey, K.; Sandino, A.M.; Imarai, M. IPNV Modulation of pro and anti-Inflammatory Cytokine Expression in Atlantic Salmon Might Help the Establishment of Infection and Persistence. Fish Shellfish Immunol. 2012, 32, 291–300. [Google Scholar] [CrossRef]
- Reyes-Cerpa, S.; Reyes-López, F.; Toro-Ascuy, D.; Montero, R.; Maisey, K.; Acuña-Castillo, C.; Sunyer, J.O.; Parra, D.; Sandino, A.M.; Imarai, M. Induction of Anti-Inflammatory Cytokine Expression by IPNV in Persistent Infection. Fish Shellfish Immunol. 2014, 41, 172–182. [Google Scholar] [CrossRef]
- Wei, H.; Yin, L.; Feng, S.; Wang, X.; Yang, K.; Zhang, A.; Zhou, H. Dual-Parallel Inhibition of IL-10 and TGF-Β1 Controls LPS-Induced Inflammatory Response via NF-ΚB Signaling in Grass Carp Monocytes/Macrophages. Fish Shellfish Immunol. 2015, 44, 445–452. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F. Fishing down Marine Food Webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef]
- Wipfli, M.S.; Baxter, C.V. Linking Ecosystems, Food Webs, and Fish Production: Subsidies in Salmonid Watersheds. Fisheries 2010, 35, 373–387. [Google Scholar] [CrossRef]
- Mercier, F.E.; Ragu, C.; Scadden, D.T. The Bone Marrow at the Crossroads of Blood and Immunity. Nat. Rev. Immunol. 2012, 12, 49–60. [Google Scholar] [CrossRef]
- Secombes, C.J.; Wang, T. The Innate and Adaptive Immune System of Fish. In Infectious Disease in Aquaculture, Brian Austin; Woodhead Publishing Limited: Philadelphia, PA, USA, 2012; pp. 3–68. [Google Scholar] [CrossRef]
- Uribe, C.; Folch, H.; Enriquez, R.; Moran, G. Innate and Adaptive Immunity in Teleost Fish: A Review. Vet. Med. 2011, 56, 486–503. [Google Scholar] [CrossRef]
- Palti, Y. Toll-like Receptors in Bony Fish: From Genomics to Function. Dev. Comp. Immunol. 2011, 35, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Phelan, P.E.; Mellon, M.T.; Kim, C.H. Functional Characterization of Full-Length TLR3, IRAK-4, and TRAF6 in Zebrafish (Danio rerio). Mol. Immunol. 2005, 42, 1057–1071. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, A.; Oshiumi, H.; Tsujita, T.; Mitani, H.; Kasai, H.; Yoshimizu, M.; Matsumoto, M.; Seya, T. Teleost TLR22 Recognizes RNA Duplex to Induce IFN and Protect Cells from Birnaviruses. J. Immunol. 2008, 181, 3474–3485. [Google Scholar] [CrossRef]
- Volff, J.N. Genome Evolution and Biodiversity in Teleost Fish. Heredity 2005, 94, 280–294. [Google Scholar] [CrossRef]
- Zschaler, J.; Schlorke, D.; Arnhold, J. Differences in Innate Immune Response between Man and Mouse. Crit. Rev. Immunol. 2014, 34, 433–454. [Google Scholar] [CrossRef]
- Tao, L.; Reese, T.A. Making Mouse Models That Reflect Human Immune Responses. Trends Immunol. 2017, 38, 181–193. [Google Scholar] [CrossRef]
- Brazeau, M.D.; Friedman, M. The Origin and Early Phylogenetic History of Jawed Vertebrates. Nature 2015, 520, 490–498. [Google Scholar] [CrossRef]
- Star, B.; Nederbragt, A.J.; Jentoft, S.; Grimholt, U.; Malmstrøm, M.; Gregers, T.F.; Rounge, T.B.; Paulsen, J.; Solbakken, M.H.; Sharma, A.; et al. The Genome Sequence of Atlantic Cod Reveals a Unique Immune System. Nature 2011, 477, 207–210. [Google Scholar] [CrossRef]
- Haase, D.; Roth, O.; Kalbe, M.; Schmiedeskamp, G.; Scharsack, J.P.; Rosenstiel, P.; Reusch, T.B.H. Absence of Major Histocompatibility Complex Class II Mediated Immunity in Pipefish, Syngnathus typhle: Evidence from Deep Transcriptome Sequencing. Biol. Lett. 2013, 9, 20130044. [Google Scholar] [CrossRef]
- Solbakken, M.H.; Tørresen, O.K.; Nederbragt, A.J.; Seppola, M.; Gregers, T.F.; Jakobsen, K.S.; Jentoft, S. Evolutionary Redesign of the Atlantic Cod (Gadus morhua L.) Toll-like Receptor Repertoire by Gene Losses and Expansions. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gjedrem, T.; Robinson, N. Advances by Selective Breeding for Aquatic Species: A Review. Agric. Sci. 2014, 5, 1152–1158. [Google Scholar] [CrossRef]
- Thodesen, J.; Grisdale-Helland, B.; Helland, S.J.; Gjerde, B. Feed Intake, Growth and Feed Utilization of Offspring from Wild and Selected Atlantic Salmon (Salmo salar). Aquaculture 1999, 180, 237–246. [Google Scholar] [CrossRef]
- Perry, G.M.L.; Martyniuk, C.M.; Ferguson, M.M.; Danzmann, R.G. Genetic Parameters for Upper Thermal Tolerance and Growth-Related Traits in Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2005, 250, 120–128. [Google Scholar] [CrossRef]
- Bentsen, H.B.; Gjerde, B.; Eknath, A.E.; de Vera, M.S.P.; Velasco, R.R.; Danting, J.C.; Dionisio, E.E.; Longalong, F.M.; Reyes, R.A.; Abella, T.A.; et al. Genetic Improvement of Farmed Tilapias: Response to Five Generations of Selection for Increased Body Weight at Harvest in Oreochromis niloticus and the Further Impact of the Project. Aquaculture 2017, 468, 206–217. [Google Scholar] [CrossRef]
- Ye, B.; Wan, Z.; Wang, L.; Pang, H.; Wen, Y.; Liu, H.; Liang, B.; Lim, H.S.; Jiang, J.; Yue, G. Heritability of Growth Traits in the Asian Seabass (Lates calcarifer). Aquac. Fish. 2017, 2, 112–118. [Google Scholar] [CrossRef]
- Moav, R.; Wohlfarth, G. Two-Way Selection for Growth Rate in the Common Carp (Cyprinus carpio L.). Genetics 1976, 82, 83–101. [Google Scholar]
- Hulata, G.; Wohlfarth, G.W.; Halevy, A. Mass Selection for Growth Rate in the Nile Tilapia (Oreochromis niloticus). Aquaculture 1986, 57, 177–184. [Google Scholar] [CrossRef]
- Bentsen, H.B.; Eknath, A.E.; Palada-de Vera, M.S.; Danting, J.C.; Bolivar, H.L.; Reyes, R.A.; Dionisio, E.E.; Longalong, F.M.; Circa, A.V.; Tayamen, M.M.; et al. Genetic Improvement of Farmed Tilapias: Growth Performance in a Complete Diallel Cross Experiment with Eight Strains of Oreochromis niloticus. Aquaculture 1998, 160, 145–173. [Google Scholar] [CrossRef]
- Bentsen, H.B.; Olesen, I. Designing Aquaculture Mass Selection Programs to Avoid High Inbreeding Rates. Aquaculture 2002, 204, 349–359. [Google Scholar] [CrossRef]
- Rodgveller, C.J.; Smoker, W.W.; Gray, A.K.; Joyce, J.E.; Gharrett, A.J. Effects of Inbreeding and Family Origin on Variation of Size of Chinook Salmon Oncorhynchus tshawytscha. Alsk. Fish. Res. Bull. 2005, 11, 73–81. [Google Scholar]
- D’Ambrosio, J.; Phocas, F.; Haffray, P.; Bestin, A.; Brard-Fudulea, S.; Poncet, C.; Quillet, E.; Dechamp, N.; Fraslin, C.; Charles, M.; et al. Genome-Wide Estimates of Genetic Diversity, Inbreeding and Effective Size of Experimental and Commercial Rainbow Trout Lines Undergoing Selective Breeding. Genet. Sel. Evol. 2019, 51, 26. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, J.M.; Houston, R.D.; Newman, S. Genetics and Genomics of Disease Resistance in Salmonid Species. Front. Genet. 2014, 5, 415. [Google Scholar] [CrossRef] [PubMed]
- Imsland, A.K.; Jonassen, T.M.; Langston, A.; Hoare, R.; Wergeland, H.; FitzGerald, R.; Mulcahy, M.; Stefansson, S.O. The Interrelation of Growth and Disease Resistance of Different Populations of Juvenile Atlantic Halibut (Hippoglossus hippoglossus L.). Aquaculture 2002, 204, 167–177. [Google Scholar] [CrossRef]
- Silverstein, J.T.; Vallejo, R.L.; Palti, Y.; Leeds, T.D.; Rexroad, C.E.; Welch, T.J.; Wiens, G.D.; Ducrocq, V. Rainbow Trout Resistance to Bacterial Cold-Water Disease Is Moderately Heritable and Is Not Adversely Correlated with Growth. J. Anim. Sci. 2009, 87, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Evenhuis, J.P.; Leeds, T.D.; Marancik, D.P.; Lapatra, S.E.; Wiens, G.D. Rainbow Trout (Oncorhynchus mykiss) Resistance to Columnaris Disease Is Heritable and Favorably Correlated with Bacterial Cold Water Disease Resistance. J. Anim. Sci. 2015, 93, 1546–1554. [Google Scholar] [CrossRef]
- Fevolden, S.E.; Refstie, T.; Røed, K.H. Disease Resistance in Rainbow Trout (Oncorhynchus mykiss) Selected for Stress Response. Aquaculture 1992, 104, 19–29. [Google Scholar] [CrossRef]
- Gjøen, H.M.; Refstie, T.; Ulla, O.; Gjerde, B. Genetic Correlations between Survival of Atlantic Salmon in Challenge and Field Tests. Aquaculture 1997, 158, 277–288. [Google Scholar] [CrossRef]
- Grimholt, U.; Larsen, S.; Nordmo, R.; Midtlyng, P.; Kjoeglum, S.; Storset, A.; Saebø, S.; Stet, R.J.M. MHC Polymorphism and Disease Resistance in Atlantic Salmon (Salmo salar); Facing Pathogens with Single Expressed Major Histocompatibility Class I and Class II Loci. Immunogenetics 2003, 55, 210–219. [Google Scholar] [CrossRef]
- Yáñez, J.M.; Bangera, R.; Lhorente, J.P.; Barría, A.; Oyarzún, M.; Neira, R.; Newman, S. Negative Genetic Correlation between Resistance against Piscirickettsia salmonis and Harvest Weight in Coho Salmon (Oncorhynchus kisutch). Aquaculture 2016, 459, 8–13. [Google Scholar] [CrossRef]
- Trang, T.T.; Hung, N.H.; Ninh, N.H.; Knibb, W.; Nguyen, N.H. Genetic Variation in Disease Resistance against White Spot Syndrome Virus (WSSV) in Liptopenaeus vannamei. Front. Genet. 2019, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Ødegård, J.; Baranski, M.; Gjerde, B.; Gjedrem, T. Methodology for Genetic Evaluation of Disease Resistance in Aquaculture Species: Challenges and Future Prospects. Aquac. Res. 2011, 42, 103–114. [Google Scholar] [CrossRef]
- Murray, C.B.; Evelyn, T.P.T.; Beacham, T.D.; Barner, L.W.; Ketcheson, J.E.; Prosperi-Porta, L. Experimental Induction of Bacterial Kidney Disease in Chinook Salmon by Immersion and Cohabitation Challenges. Dis. Aquat. Org. 1992, 12, 91–96. [Google Scholar] [CrossRef]
- Munang’andu, H.M.; Santi, N.; Fredriksen, B.N.; Løkling, K.E.; Evensen, Ø. A Systematic Approach towards Optimizing a Cohabitation Challenge Model for Infectious Pancreatic Necrosis Virus in Atlantic Salmon (Salmo salar L). PLoS ONE 2016, 11, e0148467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xu, D.H.; Shoemaker, C. Experimental Induction of Motile Aeromonas Septicemia in Channel Catfish (Ictalurus punctatus) by Waterborne Challenge with Virulent Aeromonas hydrophila. Aquac. Rep. 2016, 3, 18–23. [Google Scholar] [CrossRef]
- Long, A.; Fehringer, T.R.; Lafrentz, B.R.; Call, D.R.; Cain, K.D. Development of a Waterborne Challenge Model for Flavobacterium psychrophilum. FEMS Microbiol. Lett. 2014, 359, 154–160. [Google Scholar] [CrossRef]
- Frisch, K.; Småge, S.B.; Vallestad, C.; Duesund, H.; Brevik, Ø.J.; Klevan, A.; Olsen, R.H.; Sjaatil, S.T.; Gauthier, D.; Brudeseth, B.; et al. Experimental Induction of Mouthrot in Atlantic Salmon Smolts Using Tenacibaculum maritimum from Western Canada. J. Fish Dis. 2018, 41, 1247–1258. [Google Scholar] [CrossRef]
- Taksdal, T.; Ramstad, A.; Stangeland, K.; Dannevig, B.H. Induction of Infectious Pancreatic Necrosis (IPN) in Covertly Infected Atlantic Salmon, Salmo salar L., Post-Smolts by Stress Exposure, by Injection of IPN Virus (IPNV) and by Cohabitation. J. Fish Dis. 1998, 21, 193–204. [Google Scholar] [CrossRef]
- Henriksen, M.M.M.; Madsen, L.; Dalsgaard, I. Effect of Hydrogen Peroxide on Immersion Challenge of Rainbow Trout Fry with Flavobacterium psychrophilum. PLoS ONE 2013, 8, 62590. [Google Scholar] [CrossRef]
- Semple, S.L.; Kellendonk, C.J.; Al-Hussinee, L.; Macinnes, J.I.; Lumsden, J.S.; Dixon, B. Serum IgM, MH Class IIβ Genotype and Respiratory Burst Activity Do Not Differ between Rainbow Trout Families Displaying Resistance or Susceptibility to the Coldwater Pathogen, Flavobacterium psychrophilum. Aquaculture 2018, 483, 131–140. [Google Scholar] [CrossRef]
- Good, C.M.; Thorburn, M.A.; Stevenson, R.M.W. Factors Associated with the Incidence of Bacterial Gill Disease in Salmonid Lots Reared in Ontario, Canada Government Hatcheries. Prev. Vet. Med. 2008, 83, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.J.; Pearson, M.D. Infectious Pancreatic Necrosis in Atlantic Salmon, Salmo salar L. J. Fish Dis. 2005, 28, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Wiens, G.D.; Palti, Y.; Leeds, T.D. Three Generations of Selective Breeding Improved Rainbow Trout (Oncorhynchus mykiss) Disease Resistance against Natural Challenge with Flavobacterium psychrophilum during Early Life-Stage Rearing. Aquaculture 2018, 497, 414–421. [Google Scholar] [CrossRef]
- Marancik, D.; Gao, G.; Paneru, B.; Ma, H.; Hernandez, A.G.; Salem, M.; Yao, J.; Palti, Y.; Wiens, G.D. Whole-Body Transcriptome of Selectively Bred, Resistant-, Control-, and Susceptible-Line Rainbow Trout Following Experimental Challenge with Flavobacterium psychrophilum. Front. Genet. 2014, 5, 453. [Google Scholar] [CrossRef]
- Wiens, G.D.; Vallejo, R.L.; Leeds, T.D.; Palti, Y.; Hadidi, S.; Liu, S.; Evenhuis, J.P.; Welch, T.J.; Rexroad, C.E. Assessment of Genetic Correlation between Bacterial Cold Water Disease Resistance and Spleen Index in a Domesticated Population of Rainbow Trout: Identification of QTL on Chromosome Omy19. PLoS ONE 2013, 8, e75749. [Google Scholar] [CrossRef]
- Johnson, N.A.; Vallejo, R.L.; Silverstein, J.T.; Welch, T.J.; Wiens, G.D.; Hallerman, E.M.; Palti, Y. Suggestive Association of Major Histocompatibility IB Genetic Markers with Resistance to Bacterial Cold Water Disease in Rainbow Trout (Oncorhynchus mykiss). Mar. Biotechnol. 2008, 10, 429–437. [Google Scholar] [CrossRef]
- Hadidi, S.; Glenney, G.W.; Welch, T.J.; Silverstein, J.T.; Wiens, G.D. Spleen Size Predicts Resistance of Rainbow Trout to Flavobacterium psychrophilum Challenge. J. Immunol. 2008, 180, 4156–4165. [Google Scholar] [CrossRef]
- Zwollo, P.; Hennessey, E.; Moore, C.; Marancik, D.P.; Wiens, G.D.; Epp, L. A BCWD-Resistant Line of Rainbow Trout Exhibits Higher Abundance of IgT+ B Cells and Heavy Chain Tau Transcripts Compared to a Susceptible Line Following Challenge with Flavobacterium psychrophilum. Dev. Comp. Immunol. 2017, 74, 190–199. [Google Scholar] [CrossRef]
- Brown, R.M.; Wiens, G.D.; Salinas, I. Analysis of the Gut and Gill Microbiome of Resistant and Susceptible Lines of Rainbow Trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2019, 86, 497–506. [Google Scholar] [CrossRef]
- Wiens, G.D.; Marancik, D.P.; Zwollo, P.; Kaattari, S.L. Reduction of Rainbow Trout Spleen Size by Splenectomy Does Not Alter Resistance against Bacterial Cold Water Disease. Dev. Comp. Immunol. 2015, 49, 31–37. [Google Scholar] [CrossRef]
- Assefa, A.; Abunna, F. Maintenance of Fish Health in Aquaculture: Review of Epidemiological Approaches for Prevention and Control of Infectious Disease of Fish. Vet. Med. Int. 2018, 2018, 5432497. [Google Scholar] [CrossRef] [PubMed]
- Dejaegere, A.; Choulier, L.; Lafont, V.; de Genst, E.; Altschuh, D. Variations in Antigen-Antibody Association Kinetics as a Function of PH and Salt Concentration: A QSAR and Molecular Modeling Study. Biochemistry 2005, 44, 14409–14418. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.W.; Andrew, S.M.; Hogarth, M.P.; Pietersz, G.A.; McKenzie, I.F.C. The Effect of Temperature on the Binding Kinetics and Equilibrium Constants of Monoclonal Antibodies to Cell Surface Antigens. Mol. Immunol. 1990, 27, 327–333. [Google Scholar] [CrossRef]
- Encarnação, J.C.; Barta, P.; Fornstedt, T.; Andersson, K. Impact of Assay Temperature on Antibody Binding Characteristics in Living Cells: A Case Study. Biomed. Rep. 2017, 7, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Le Morvan, C.; Deschaux, P.; Troutaud, D. Effects and Mechanisms of Environmental Temperature on Carp (Cyprinus carpio) Anti-DNP Antibody Response and Non-Specific Cytotoxic Cell Activity: A Kinetic Study. Dev. Comp. Immunol. 1996, 20, 331–340. [Google Scholar] [CrossRef]
- Avtalion, R.R. Temperature Effect on Antibody Production and Immunological Memory, in Carp (Cyprinus carpio) Immunized against Bovine Serum Albumin (BSA). Immunology 1969, 17, 927–931. [Google Scholar]
- Grabowski, L.D.; LaPatra, S.E.; Cain, K.D. Systemic and Mucosal Antibody Response in Tilapia, Oreochromis niloticus (L.), Following Immunization with Flavobacterium columnare. J. Fish Dis. 2004, 27, 573–581. [Google Scholar] [CrossRef]
- Rijkers, G.T.; Frederix-Wolters, E.M.; van Muiswinkel, W.B. The Immune System of Cyprinid Fish. Kinetics and Temperature Dependence of Antibody-Producing Cells in Carp (Cyprinus carpio). Immunology 1980, 41, 91–97. [Google Scholar]
- Plumb, J.A.; Wise, M.L.; Rogers, W.A. Modulary Effects of Temperature on Antibody Response and Specific Resistance to Challenge of Channel Catfish, Ictalurus punctatus, Immunized against Edwardsiella ictaluri. Vet. Immunol. Immunopathol. 1986, 12, 297–304. [Google Scholar] [CrossRef]
- Lange, M.D.; Webster, C.D. The Effect of Temperature on the Mucosal IgM Antibody Response to DNP-KLH in Channel Catfish (Ictalurus punctatus). Fish Shellfish Immunol. 2017, 70, 493–497. [Google Scholar] [CrossRef]
- Mikkelsen, H.; Lindenstrøm, T.; Nielsen, M.E. Effects of Temperature on Production and Specificity of Antibodies in Rainbow Trout (Oncorhynchus mykiss). J. World Aquac. Soc. 2006, 37, 518–522. [Google Scholar] [CrossRef]
- Alcorn, S.W.; Murray, A.L.; Pascho, R.J. Effects of Rearing Temperature on Immune Functions in Sockeye Salmon (Oncorhynchus nerka). Fish Shellfish Immunol. 2002, 12, 303–334. [Google Scholar] [CrossRef] [PubMed]
- Paterson, W.D.; Fryer, J.L. Effect of Temperature and Antigen Dose on the Antibody Response of Juvenile Coho Salmon (Oncorhynchus kisutch) to Aeromonas salmonicida Endotoxin. J. Fish Res. Board Can. 1974, 31, 1743–1749. [Google Scholar] [CrossRef]
- Lillehaug, A.; Ramstad, A.; Bækken, K.; Reitan, L.J. Protective Immunity in Atlantic Salmon (Salmo salar L.) Vaccinated at Different Water Temperatures. Fish Shellfish Immunol. 1993, 3, 143–156. [Google Scholar] [CrossRef]
- Munang’andu, H.M.; Mutoloki, S.; Evensen, Ø. An Overview of Challenges Limiting the Design of Protective Mucosal Vaccines for Finfish. Front. Immunol. 2015, 6, 542. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, H.; Chang, X.; Tang, Y.; Liu, Q.; Zhang, Y. Notable Mucosal Immune Responses Induced in the Intestine of Zebrafish (Danio rerio) Bath-Vaccinated with a Live Attenuated Vibrio anguillarum Vaccine. Fish Shellfish Immunol. 2014, 40, 99–108. [Google Scholar] [CrossRef]
- Hoare, R.; Ngo, T.P.H.; Bartie, K.L.; Adams, A. Efficacy of a Polyvalent Immersion Vaccine against Flavobacterium psychrophilum and Evaluation of Immune Response to Vaccination in Rainbow Trout Fry (Onchorynchus mykiss L.). Vet. Res. 2017, 48. [Google Scholar] [CrossRef]
- Sudheesh, P.S.; Cain, K.D. Prospects and Challenges of Developing and Commercializing Immersion Vaccines for Aquaculture. Int. Biol. Rev. 2017, 1, 1–20. [Google Scholar] [CrossRef]
- Semple, S.L. Improving Aquaculture: The Impact of Bacterial Disease Treatments on Salmonid Immune Performance. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2019. [Google Scholar]
- Figueroa, C.; Veloso, P.; Espin, L.; Dixon, B.; Afonso, J.M.; Soto, C.; Conejeros, P.; Gallardo, J.A. Host Genetic Variation Explains Reduced Protection of Commercial Vaccines against Piscirickettsia salmonis in Atlantic Salmon. Sci. Rep. 2020, in press. [Google Scholar]
- Ye, J.; Kaattari, I.M.; Kaattari, S.L. The Differential Dynamics of Antibody Subpopulation Expression during Affinity Maturation in a Teleost. Fish Shellfish Immunol. 2011, 30, 372–377. [Google Scholar] [CrossRef]
- LaFrentz, B.R.; LaPatra, S.E.; Jones, G.R.; Congleton, J.L.; Sun, B.; Cain, K.D. Characterization of Serum and Mucosal Antibody Responses and Relative per Cent Survival in Rainbow Trout, Oncorhynchus mykiss (Walbaum), Following Immunization and Challenge with Flavobacterium psychrophilum. J. Fish Dis. 2002, 25, 703–713. [Google Scholar] [CrossRef]
- Liang, J.L.; Tiwari, T.; Moro, P.; Messonnier, N.E.; Reingold, A.; Sawyer, M.; Clark, T.A. Prevention of Pertussis, Tetanus, and Diphtheria with Vaccines in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2018, 67. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.L.; Bols, N.C.; Lumsden, J.S.; Dixon, B. Understanding the Pathogenesis of Flavobacterium psychrophilum Using the Rainbow Trout Monocyte/Macrophage-like Cell Line, RTS11, as an Infection Model. Microb. Pathog. 2020, 139, 103910. [Google Scholar] [CrossRef] [PubMed]
- Magarinos, B.; Santos, Y.; Romalde, J.L.; Rivas, C.; Barja, J.L.; Toranzo, A.E. Pathogenic Activities of Live Cells and Extracellular Products of the Fish Pathogen Pasteurella piscicida. J. Gen. Microbiol. 1992, 138, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, E.; Oshima, S.; Nomura, S. Toxicity and Immunogenicity of Aeromonas salmonicida Extracellular Products to Salmonids. J. Fish Dis. 1990, 13, 495–503. [Google Scholar] [CrossRef]
- Vanya Ewart, K.; Williams, J.; Richards, R.C.; Gallant, J.W.; Melville, K.; Douglas, S.E. The Early Response of Atlantic Salmon (Salmo salar) Macrophages Exposed in vitro to Aeromonas salmonicida Cultured in Broth and in Fish. Dev. Comp. Immunol. 2008, 32, 380–390. [Google Scholar] [CrossRef]
- Munro, A.L.S.; Hastings, T.S.; Ellis, A.E.; Liversidge, J. Studies on an Ichthyotoxic Material Produced Extracellularly by the Furunculosis Bacterium Aeromonas salmonicida. In Fish Diseases; Ahne, W., Ed.; Springer: Berlin/Heidelberg, Germany. [CrossRef]
- Bjornsdottir, B.; Gudmundsdottir, T.; Gudmundsdottir, B.K. Virulence Properties of Moritella viscosa Extracellular Products. J. Fish Dis. 2011, 34, 333–343. [Google Scholar] [CrossRef]
- Bjornsdottir, B.; Fast, M.D.; Sperker, S.A.; Brown, L.L.; Gudmundsdottir, B.K. Effects of Moritella viscosa Antigens on Pro-Inflammatory Gene Expression in an Atlantic Salmon (Salmo salar Linnaeus) Cell Line (SHK-1). Fish Shellfish Immunol. 2009, 26, 858–863. [Google Scholar] [CrossRef]
- MacKinnon, B.; Groman, D.; Fast, M.D.; Manning, A.J.; Jones, P.; St-Hilaire, S. Atlantic Salmon Challenged with Extracellular Products from Moritella viscosa. Dis. Aquat. Org. 2019, 133, 119–125. [Google Scholar] [CrossRef]
- Powell, M.D.; Briand, H.A.; Wright, G.M.; Burka, J.F. Rainbow Trout (Oncorhynchus mykiss Walbaum) Intestinal Eosinophilic Granule Cell (Egc) Response to Aeromonas salmonicida and Vibrio anguillarum Extracellular Products. Fish Shellfish Immunol. 1993, 3, 279–289. [Google Scholar] [CrossRef]
- Ostland, V.E.; Byrne, P.J.; Hoover, G.; Ferguson, H.W. Necrotic Myositis of Rainbow Trout, Oncorhynchus mykiss (Walbaum): Proteolytic Characteristics of a Crude Extracellular Preparation from Flavobacterium psychrophilum. J. Fish Dis. 2000, 23, 329–336. [Google Scholar] [CrossRef]
- Pérez-Pascual, D.; Rochat, T.; Kerouault, B.; Gómez, E.; Neulat-Ripoll, F.; Henry, C.; Quillet, E.; Guijarro, J.A.; Bernardet, J.F.; Duchaud, E. More Than Gliding: Involvement of GldD and GldG in the Virulence of Flavobacterium psychrophilum. Front. Microbiol. 2017, 8, 2168. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pascual, D.; Gómez, E.; Álvarez, B.; Méndez, J.; Reimundo, P.; Navais, R.; Duchaud, E.; Guijarro, J.A. Comparative Analysis and Mutation Effects of Fpp2-Fpp1 Tandem Genes Encoding Proteolytic Extracellular Enzymes of Flavobacterium psychrophilum. Microbiology 2011, 157, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Secades, P.; Alvarez, B.; Guijarro, J.A. Purification and Properties of a New Psychrophilic Metalloprotease (Fpp2) in the Fish Pathogen Flavobacterium psychrophilum. FEMS Microbiol. Lett. 2003, 226, 273–279. [Google Scholar] [CrossRef]
- Secades, P.; Alvarez, B.; Guijarro, J.A. Purification and Characterization of a Psychrophilic, Calcium-Induced, Growth-Phase-Dependent Metalloprotease from the Fish Pathogen Flavobacterium psychrophilum. Appl. Environ. Microbiol. 2001, 67, 2436–2444. [Google Scholar] [CrossRef]
- Pérez-Pascual, D.; Gómez, E.; Guijarro, J.A. Lack of a Type-2 Glycosyltransferase in the Fish Pathogen Flavobacterium psychrophilum Determines Pleiotropic Changes and Loss of Virulence. Vet. Res. 2015, 46, 1. [Google Scholar] [CrossRef]
- Lages, M.A.; Balado, M.; Lemos, M.L. The Expression of Virulence Factors in Vibrio anguillarum Is Dually Regulated by Iron Levels and Temperature. Front. Microbiol. 2019, 10, 2335. [Google Scholar] [CrossRef]
- Milton, D.L.; O’Toole, R.; Hörstedt, P.; Wolf-Watz, H. Flagellin A Is Essential for the Virulence of Vibrio anguillarum. J. Bacteriol. 1996, 178, 1310–1319. [Google Scholar] [CrossRef]
- Burr, S.; Wahli, T.; Segner, H.; Pugovkin, D.; Frey, J. Association of Type III Secretion Genes with Virulence of Aeromonas salmonicida Subsp. Salmonicida. Dis. Aquat. Org. 2003, 57, 167–171. [Google Scholar] [CrossRef]
- Bandin, I.; Santos, Y.; Bruno, D.W.; Raynard, R.S.; Toranzo, A.E.; Barja, J.L. Lack of Biological Activities in the Extracellular Products of Renibacterium salmoninarum. Can. J. Fish Aquat. Sci. 1991, 48, 421–425. [Google Scholar] [CrossRef]
- Ellis, A.E.; Burrows, A.S.; Stapleton, K.J. Lack of Relationship between Virulence of Aeromonas salmonicida and the Putative Virulence Factors: A-Layer, Extracellular Proteases and Extracellular Haemolysins. J. Fish Dis. 1988, 11, 309–323. [Google Scholar] [CrossRef]
- Ekman, E.; Norrgren, L. Pathology and Immunohistochemistry in Three Species of Salmonids after Experimental Infection with Flavobacterium psychrophilum. J. Fish Dis. 2003, 26, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Pujol, C.; Bliska, J.B. Turning Yersinia Pathogenesis Outside in: Subversion of Macrophage Function by Intracellular Yersiniae. Clin. Immunol. 2005, 114, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Gutenberger, S.; Duimstra, J.; Rohovec, J.; Fryer, J. Intracellular Survival of Renibacterium salmoninarum in Trout Mononuclear Phagocytes. Dis. Aquat. Org. 1997, 28, 93–106. [Google Scholar] [CrossRef]
- Grayson, T.H.; Cooper, L.F.; Wrathmell, A.B.; Roper, J.; Evenden, A.J.; Gilpin, M.L. Host Responses to Renibacterium salmoninarum and Specific Components of the Pathogen Reveal the Mechanisms of Immune Suppression and Activation. Immunology 2002, 106, 273–283. [Google Scholar] [CrossRef]
- Ramírez, R.; Ǵomez, F.A.; Marshall, S.H. The Infection Process of Piscirickettsia salmonis in Fish Macrophages Is Dependent upon Interaction with Host-Cell Clathrin and Actin. FEMS Microbiol. Lett. 2015, 362, 1–8. [Google Scholar] [CrossRef]
- Álvarez, C.A.; Gomez, F.A.; Mercado, L.; Ramírez, R.; Marshall, S.H. Piscirickettsia salmonis Imbalances the Innate Immune Response to Succeed in a Productive Infection in a Salmonid Cell Line Model. PLoS ONE 2016, 11, e0163943. [Google Scholar] [CrossRef]
- Nematollahi, A.; Pasmans, F.; Haesebrouck, F.; Decostere, A. Early Interactions of Flavobacterium psychrophilum with Macrophages of Rainbow Trout Oncorhynchus mykiss. Dis. Aquat. Org 2005, 64, 23–28. [Google Scholar] [CrossRef][Green Version]
- Nilsen, H.; Olsen, A.B.; Vaagnes, Ø.; Hellberg, H.; Bottolfsen, K.; Skjelstad, H.; Colquhoun, D.J. Systemic Flavobacterium psychrophilum Infection in Rainbow Trout, Oncorhynchus mykiss (Walbaum), Farmed in Fresh and Brackish Water in Norway. J. Fish Dis. 2011, 34, 403–408. [Google Scholar] [CrossRef]
- de la Maza, L.M.; Zhong, G.; Brunham, R.C. Update on Chlamydia Trachomatis Vaccinology. Am. Soc. Microbiol. 2017, 24, e00543-16. [Google Scholar] [CrossRef]
- Henao-Tamayo, M.; Ordway, D.J.; Orme, I.M. Memory T Cell Subsets in Tuberculosis: What Should We Be Targeting? Tuberculosis 2014, 94, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Titball, R.W. Vaccines against Intracellular Bacterial Pathogens. Drug Discov. Today 2008, 13, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.L.; Khader, S.A. Novel Vaccine Approaches for Protection against Intracellular Pathogens. Curr. Opin. Immunol. 2014, 28, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.G.; Wiens, G.D.; Hammell, K.L.; Rhodes, L.D. Vaccination against Bacterial Kidney Disease. In Fish Vaccination; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 255–272. ISBN 9780470674550. [Google Scholar] [CrossRef]
- Burnley, T.A.; Stryhn, H.; Burnley, H.J.; Hammell, K.L. Randomized Clinical Field Trial of a Bacterial Kidney Disease Vaccine in Atlantic Salmon, Salmo salar L. J. Fish Dis. 2010, 33, 545–557. [Google Scholar] [CrossRef]
- Decostere, A.; D’Haese, E.; Lammens, M.; Nelis, H.; Haesebrouck, F. In vivo Study of Phagocytosis, Intracellular Survival and Multiplication of Flavobacterium psychrophilum in Rainbow Trout, Oncorhynchus mykiss (Walbaum), Spleen Phagocytes. J. Fish Dis. 2001, 24, 481–487. [Google Scholar] [CrossRef]
- Hoare, R.; Jung, S.J.; Ngo, T.P.H.; Bartie, K.L.; Thompson, K.D.; Adams, A. Efficacy of a Polyvalent Injectable Vaccine against Flavobacterium psychrophilum Administered to Rainbow Trout (Oncorhynchus mykiss L.). J. Fish Dis. 2019, 42, 229–236. [Google Scholar] [CrossRef]
- Ma, J.; Bruce, T.J.; Sudheesh, P.S.; Knupp, C.; Loch, T.P.; Faisal, M.; Cain, K.D. Assessment of Cross-Protection to Heterologous Strains of Flavobacterium psychrophilum Following Vaccination with a Live-Attenuated Coldwater Disease Immersion Vaccine. J. Fish Dis. 2019, 42, 75–84. [Google Scholar] [CrossRef]
- Fredriksen, B.N.; Olsen, R.H.; Furevik, A.; Souhoka, R.A.; Gauthier, D.; Brudeseth, B. Efficacy of a Divalent and a Multivalent Water-in-Oil Formulated Vaccine against a Highly Virulent Strain of Flavobacterium psychrophilum after Intramuscular Challenge of Rainbow Trout (Oncorhynchus mykiss). Vaccine 2013, 31, 1994–1998. [Google Scholar] [CrossRef]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key Roles of Adjuvants in Modern Vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Di Pasquale, A.; Preiss, S.; da Silva, F.T.; Garçon, N. Vaccine Adjuvants: From 1920 to 2015 and Beyond. Vaccines 2015, 3, 320–343. [Google Scholar] [CrossRef]
- Tafalla, C.; Bøgwald, J.; Dalmo, R.A. Adjuvants and Immunostimulants in Fish Vaccines: Current Knowledge and Future Perspectives. Fish Shellfish Immunol. 2013, 35, 1740–1750. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, V.; Miquel, A.; Burzio, L.O.; Rosemblatt, M.; Engel, E.; Valenzuela, S.; Parada, G.; Valenzuela, P.D.T. A Vaccine against the Salmonid Pathogen Piscirickettsia salmonis Based on Recombinant Proteins. Vaccine 2006, 24, 5083–5091. [Google Scholar] [CrossRef] [PubMed]
- Tyler, J.W.; Klesius, P.H. Protection against Enteric Septicemia of Catfish (Ictalurus punctatus) by Immunization with the R-Mutant, Escherichia coli (J5). Am. J. Vet. Res. 1994, 55, 1256–1260. [Google Scholar] [PubMed]
- Wang, E.; Wang, J.; Long, B.; Wang, K.; He, Y.; Yang, Q.; Chen, D.; Geng, Y.; Huang, X.; Ouyang, P.; et al. Molecular Cloning, Expression and the Adjuvant Effects of Interleukin-8 of Channel Catfish (Ictalurus punctatus) against Streptococcus iniae. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Araki, K.; Hayashi, K.; Takeuchi, Y.; Shiozaki, K.; Suetake, H.; Yamamoto, A. Adjuvant Effect of Recombinant Interleukin-12 in the Nocardiosis Formalin-Killed Vaccine of the Amberjack Seriola dumerili. Fish Shellfish Immunol. 2017, 67, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Peddie, S.; Campos-Pérez, J.J.; Zou, J.; Secombes, C.J. The Effect of Intraperitoneally Administered Recombinant IL-1β on Immune Parameters and Resistance to Aeromonas salmonicida in the Rainbow Trout (Oncorhynchus mykiss). Dev. Comp. Immunol. 2003, 27, 801–812. [Google Scholar] [CrossRef]
- Buonocore, F.; Mazzini, M.; Forlenza, M.; Randelli, E.; Secombes, C.J.; Zou, J.; Scapigliati, G. Expression in Escherchia coli and Purification of Sea Bass (Dicentrarchus labrax) Interleukin 1β, a Possible Immunoadjuvant in Aquaculture. Mar. Biotechnol. 2004, 6, 53–59. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Q.; Xu, L.; Li, S.; Wang, D.; Zhao, J.; Liu, H.; Feng, J.; Lu, T. Effects of Different Cytokines on Immune Responses of Rainbow Trout in a Virus DNA Vaccination Model. Oncotarget 2017, 8, 112222–112235. [Google Scholar] [CrossRef]
- Guo, M.; Tang, X.; Sheng, X.; Xing, J.; Zhan, W. The Effects of IL-1β, IL-8, G-CSF and TNF-α as Molecular Adjuvant on the Immune Response to an E. Tarda Subunit Vaccine in Flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2018, 77, 374–384. [Google Scholar] [CrossRef]
- Wang, E.; Long, B.; Wang, K.; Wang, J.; He, Y.; Wang, X.; Yang, Q.; Liu, T.; Chen, D.; Geng, Y.; et al. Interleukin-8 Holds Promise to Serve as a Molecular Adjuvant in DNA Vaccination Model against Streptococcus iniae Infection in Fish. Oncotarget 2016, 7, 83938–83950. [Google Scholar] [CrossRef]
- Dixon, B.; Barreda, D.R.; Sunyer, J.O. Perspective on the Development and Validation of Ab Reagents to Fish Immune Proteins for the Correct Assessment of Immune Function. Front. Immunol. 2018, 9, 2957. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Bhat, R.A.H.; Pande, A. Antimicrobial Peptides of Fish: Innocuous Alternatives to Antibiotics. Rev. Aquac. 2020, 12, 85–106. [Google Scholar] [CrossRef]
- Brunner, S.R.; Varga, J.F.A.; Dixon, B. Antimicrobial Peptides of Salmonid Fish: From Form to Function. Biology 2020, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.; Carpio, Y.; Valdés, I.; Velázquez, J.; Zamora, Y.; Morales, R.; Morales, A.; Rodríguez, E.; Estrada, M.P. Co-Administration of Tilapia Alpha-Helical Antimicrobial Peptides with Subunit Antigens Boost Immunogenicity in Mice and Tilapia (Oreochromis niloticus). Vaccine 2014, 32, 223–229. [Google Scholar] [CrossRef]
- Zhang, X.J.; Wang, P.; Zhang, N.; Chen, D.D.; Nie, P.; Li, J.-L.; Zhang, Y.A. B Cell Functions Can Be Modulated by Antimicrobial Peptides in Rainbow Trout Oncorhynchus mykiss: Novel Insights into the Innate Nature of B Cells in Fish. Front. Immunol. 2017, 8, 388. [Google Scholar] [CrossRef]
- Bridle, A.; Nosworthy, E.; Polinski, M.; Nowak, B. Evidence of an Antimicrobial-Immunomodulatory Role of Atlantic Salmon Cathelicidins during Infection with Yersinia ruckeri. PLoS ONE 2011, 6, e23417. [Google Scholar] [CrossRef]
- Peter Chiou, P.; Khoo, J.; Bols, N.C.; Douglas, S.; Chen, T.T. Effects of Linear Cationic α-Helical Antimicrobial Peptides on Immune-Relevant Genes in Trout Macrophages. Dev. Comp. Immunol. 2006, 30, 797–806. [Google Scholar] [CrossRef]
- Jia, X.; Patrzykat, A.; Devlin, R.H.; Ackerman, P.A.; Iwama, G.K.; Hancock, R.E.W. Antimicrobial Peptides Protect Coho Salmon from Vibrio anguillarum Infections. Appl. Environ. Microbiol. 2000, 66, 1928–1932. [Google Scholar] [CrossRef]
- Furlan, M.; Rosani, U.; Gambato, S.; Irato, P.; Manfrin, A.; Mardirossian, M.; Venier, P.; Pallavicini, A.; Scocchi, M. Induced Expression of Cathelicidins in Trout (Oncorhynchus mykiss) Challenged with Four Different Bacterial Pathogens. J. Pept. Sci. 2018, 24, e3089. [Google Scholar] [CrossRef]
- Kitani, Y.; Fernandes, J.M.O.; Kiron, V. Identification of the Atlantic Cod L-Amino Acid Oxidase and Its Alterations Following Bacterial Exposure. Dev. Comp. Immunol. 2015, 50, 116–120. [Google Scholar] [CrossRef]
- Kitani, Y.; Hieu, D.Q.; Kiron, V. Cloning of Selected Body Surface Antimicrobial Peptide/Protein Genes of Atlantic Salmon and Their Responses to Aeromonas salmonicida. Fish Sci. 2019, 85, 847–858. [Google Scholar] [CrossRef]
- Ruangsri, J.; Kitani, Y.; Kiron, V.; Lokesh, J.; Brinchmann, M.F.; Karlsen, B.O.; Fernandes, J.M.O. A Novel Beta-Defensin Antimicrobial Peptide in Atlantic Cod with Stimulatory Effect on Phagocytic Activity. PLoS ONE 2013, 8, e62302. [Google Scholar] [CrossRef] [PubMed]
- Lauth, X.; Babon, J.J.; Stannard, J.A.; Singh, S.; Nizet, V.; Carlberg, J.M.; Ostland, V.E.; Pennington, M.W.; Norton, R.S.; Westerman, M.E. Bass Hepcidin Synthesis, Solution Structure, Antimicrobial Activities and Synergism, and in vivo Hepatic Response to Bacterial Infections. J. Biol. Chem. 2005, 280, 9272–9282. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.; Noga, E.J. Evidence for Synergism of the Antimicrobial Peptide Piscidin 2 with Antiparasitic and Antioomycete Drugs. J. Fish Dis. 2010, 33, 995–1003. [Google Scholar] [CrossRef]
- Semple, S.L.; Rodríguez-Ramos, T.; Carpio, Y.; Lumsden, J.S.; Estrada, M.P.; Dixon, B. PACAP Is Lethal to Flavobacterium psychrophilum Through Either Direct Membrane Permeabilization or Indirectly, by Priming the Immune Response in Rainbow Trout Macrophages. Front. Immunol. 2019, 10, 926. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Ushio, H.; Nakano, N.; Ng, W.; Sayama, K.; Hashimoto, K.; Nagaoka, I.; Okumura, K.; Ogawa, H. Antimicrobial Peptides Human β-Defensins Stimulate Epidermal Keratinocyte Migration, Proliferation and Production of Proinflammatory Cytokines and Chemokines. J. Investig. Dermatol. 2007, 127, 594–604. [Google Scholar] [CrossRef]
- Zhang, H.; Porro, G.; Orzech, N.; Müllen, B.; Liu, M.; Slutsky, A.S. Neutrophil Defensins Mediate Acute Inflammatory Response and Lung Dysfunction in Dose-Related Fashion. Am. J. Physiol. Cell. Mol. Physiol. 2001, 280, L947–L954. [Google Scholar] [CrossRef]
- Chaly, Y.V.; Paleolog, E.M.; Kolesnikova, T.S.; Tikhonov, I.I.; Petratchenko, E.V.; Voitenok, N.N. Neutrophil Alpha-Defensin Human Neutrophil Peptide Modulates Cytokine Production in Human Monocytes and Adhesion Molecule Expression in Endothelial Cells. Eur. Cytokine Netw. 2000, 11, 257–266. [Google Scholar]
- Martinez, C.; Abad, C.; Delgado, M.; Arranz, A.; Juarranz, M.G.; Rodriguez-Henche, N.; Brabet, P.; Leceta, J.; Gomariz, R.P. Anti-Inflammatory Role in Septic Shock of Pituitary Adenylate Cyclase-Activating Polypeptide Receptor. Proc. Natl. Acad. Sci. USA 2002, 99, 1053–1058. [Google Scholar] [CrossRef]
- Delgado, M.; Miller Jonakait, G.; Ganea, D. Vasoactive Intestinal Peptide and Pituitary Adenylate Cyclase-Activating Polypeptide Inhibit Chemokine Production in Activated Microglia. Glia 2002, 39, 148–161. [Google Scholar] [CrossRef]
- Delgado, M.; Munoz-Elias, E.J.; Gomariz, R.P.; Ganea, D. VIP and PACAP Inhibit IL-12 Production in LPS-Stimulated Macrophages. Subsequent Effect on IFNγ Synthesis by T Cells. J. Neuroimmunol. 1999, 96, 167–181. [Google Scholar] [CrossRef]
- Miyata, A.; Arimura, A.; Dahl, R.R.; Minamino, N.; Uehara, A.; Jiang, L.; Culler, M.D.; Coy, D.H. Isolation of a Novel 38 Residue-Hypothalamic Polypeptide Which Stimulates Adenylate Cyclase in Pituitary Cells. Biochem. Biophys. Res. Commun. 1989, 164, 567–574. [Google Scholar] [CrossRef]
- Lugo, J.M.; Rodriguez, A.; Helguera, Y.; Morales, R.; Gonzalez, O.; Acosta, J.; Besada, V.; Sanchez, A.; Estrada, M.P. Recombinant Novel Pituitary Adenylate Cyclase-Activating Polypeptide from African Catfish (Clarias gariepinus) Authenticates Its Biological Function as a Growth-Promoting Factor in Low Vertebrates. J. Endocrinol. 2008, 197, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.J.; Boulding, E.G. Associations between Single Nucleotide Polymorphisms in Candidate Genes and Growth Rate in Arctic Charr (Salvelinus alpinus L.). Heredity 2003, 91, 60–69. [Google Scholar] [CrossRef]
- Lugo, J.M.; Oliva, A.; Morales, A.; Reyes, O.; Garay, H.E.; Herrera, F.; Cabrales, A.; Pérez, E.; Estrada, M.P. The Biological Role of Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) in Growth and Feeding Behavior in Juvenile Fish. J. Pept. Sci. 2010, 16, 633–643. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Sun, Y.Z.; Wang, A.; Zhou, Z. Probiotics as Means of Diseases Control in Aquaculture, a Review of Current Knowledge and Future Perspectives. Front. Microbiol. 2018, 9, 2429. [Google Scholar] [CrossRef]
- Newaj-Fyzul, A.; Al-Harbi, A.H.; Austin, B. Review: Developments in the Use of Probiotics for Disease Control in Aquaculture. Aquaculture 2014, 431, 1–11. [Google Scholar] [CrossRef]
- Newaj-Fyzul, A.; Adesiyun, A.A.; Mutani, A.; Ramsubhag, A.; Brunt, J.; Austin, B. Bacillus Subtilis AB1 Controls Aeromonas Infection in Rainbow Trout (Oncorhynchus mykiss, Walbaum). J. Appl. Microbiol. 2007, 103, 1699–1706. [Google Scholar] [CrossRef]
- Sharifuzzaman, S.M.; Austin, B. Influence of Probiotic Feeding Duration on Disease Resistance and Immune Parameters in Rainbow Trout. Fish Shellfish Immunol. 2009, 27, 440–445. [Google Scholar] [CrossRef]
- Balcázar, J.L.; de Blas, I.; Ruiz-Zarzuela, I.; Vendrell, D.; Gironés, O.; Muzquiz, J.L. Enhancement of the Immune Response and Protection Induced by Probiotic Lactic Acid Bacteria against Furunculosis in Rainbow Trout (Oncorhynchus mykiss). FEMS Immunol. Med. Microbiol. 2007, 51, 185–193. [Google Scholar] [CrossRef]
- Brunt, J.; Newaj-Fyzul, A.; Austin, B. The Development of Probiotics for the Control of Multiple Bacterial Diseases of Rainbow Trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2007, 30, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Sharifuzzaman, S.M.; Austin, B. Development of Protection in Rainbow Trout (Oncorhynchus mykiss, Walbaum) to Vibrio anguillarum Following Use of the Probiotic Kocuria SM1. Fish Shellfish Immunol. 2010, 29, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Sharifuzzaman, S.M.; Abbass, A.; Tinsley, J.W.; Austin, B. Subcellular Components of Probiotics Kocuria SM1 and Rhodococcus SM2 Induce Protective Immunity in Rainbow Trout (Oncorhynchus mykiss, Walbaum) against Vibrio anguillarum. Fish Shellfish Immunol. 2011, 30, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Jöborn, A.; Olsson, J.C.; Westerdahl, A.; Conway, P.L.; Kjelleberg, S. Colonization in the Fish Intestinal Tract and Production of Inhibitory Substances in Intestinal Mucus and Faecal Extracts by Carnobacterium Sp. Strain K1. J. Fish Dis. 1997, 20, 383–392. [Google Scholar] [CrossRef]
- Tukmechi, A.; Rahmati Andani, H.R.; Manaffar, R.; Sheikhzadeh, N. Dietary Administration of Beta-Mercapto-Ethanol Treated Saccharomyces cerevisiae Enhanced the Growth, Innate Immune Response and Disease Resistance of the Rainbow Trout, Oncorhynchus mykiss. Fish Shellfish Immunol. 2011, 30, 923–928. [Google Scholar] [CrossRef]
- Burbank, D.R.; Shah, D.H.; LaPatra, S.E.; Fornshell, G.; Cain, K.D. Enhanced Resistance to Coldwater Disease Following Feeding of Probiotic Bacterial Strains to Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2011, 321, 185–190. [Google Scholar] [CrossRef]
- Abbass, A.; Sharifuzzaman, S.M.; Austin, B. Cellular Components of Probiotics Control Yersinia ruckeri Infection in Rainbow Trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2010, 33, 31–37. [Google Scholar] [CrossRef]
- Park, Y.; Lee, S.; Hong, J.; Kim, D.; Moniruzzaman, M.; Bai, S.C. Use of Probiotics to Enhance Growth, Stimulate Immunity and Confer Disease Resistance to Aeromonas salmonicida in Rainbow Trout (Oncorhynchus mykiss). Aquac. Res. 2017, 48, 2672–2682. [Google Scholar] [CrossRef]
- Balcázar, J.L.; de Blas, I.; Ruiz-Zarzuela, I.; Vendrell, D.; Calvo, A.C.; Márquez, I.; Gironés, O.; Muzquiz, J.L. Changes in Intestinal Microbiota and Humoral Immune Response Following Probiotic Administration in Brown Trout (Salmo trutta). Br. J. Nutr. 2007, 97, 522–527. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Sun, G.; Li, X.; Liu, Z. Growth, Immune Response, Antioxidant Capability, and Disease Resistance of Juvenile Atlantic Salmon (Salmo salar L.) Fed Bacillus velezensis V4 and Rhodotorula mucilaginosa Compound. Aquaculture 2019, 500, 65–74. [Google Scholar] [CrossRef]
- Robertson, P.A.W.; O’Dowd, C.; Burrells, C.; Williams, P.; Austin, B. Use of Carnobacterium Sp. as a Probiotic for Atlantic Salmon (Salmo salar L.) and Rainbow Trout (Oncorhynchus mykiss, Walbaum). Aquaculture 2000, 185, 235–243. [Google Scholar] [CrossRef]
- Soltani, M. Effect of the Probiotic, Lactobacillus plantarum on Growth Performance and Haematological Indices of Rainbow Trout (Oncorhynchus mykiss) Immunized with Bivalent Streptococcosis/Lactococcosis Vaccine. Iran. J. Fish Sci. 2019, 18, 283–295. [Google Scholar] [CrossRef]
- Soltani, M.; Pakzad, K.; Taheri-Mirghaed, A.; Mirzargar, S.; Shekarabi, S.P.H.; Yosefi, P.; Soleymani, N. Dietary Application of the Probiotic Lactobacillus plantarum 426951 Enhances Immune Status and Growth of Rainbow Trout (Oncorhynchus mykiss) Vaccinated Against Yersinia ruckeri. Probiotics Antimicrob. Proteins 2019, 11, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.M.; Soltani, M.; Ali Ebrahimzahe-Mousavi, H.; Pakzad, K. Influence of Probiotic, Lactobacillus plantarum on Serum Biochemical and Immune Parameters in Vaccinated Rainbow Trout (Oncorhynchus mykiss) against Streptococcosis/Lactococosis. Int. J. Aquat. Biol. 2016, 4, 285–294. [Google Scholar]
- Kim, D.H.; Austin, B. Innate Immune Responses in Rainbow Trout (Oncorhynchus mykiss, W.) Induced by Probiotics. Fish Shellfish Immunol. 2006, 21, 513–524. [Google Scholar] [CrossRef]
- Pérez-Sánchez, T.; Balcázar, J.L.; Merrifield, D.L.; Carnevali, O.; Gioacchini, G.; de Blas, I.; Ruiz-Zarzuela, I. Expression of Immune-Related Genes in Rainbow Trout (Oncorhynchus mykiss) Induced by Probiotic Bacteria during Lactococcus garvieae Infection. Fish Shellfish Immunol. 2011, 31, 196–201. [Google Scholar] [CrossRef]
- Al-Hisnawi, A.; Rodiles, A.; Rawling, M.D.; Castex, M.; Waines, P.; Gioacchini, G.; Carnevali, O.; Merrifield, D.L. Dietary Probiotic Pediococcus Acidilactici MA18/5M Modulates the Intestinal Microbiota and Stimulates Intestinal Immunity in Rainbow Trout (Oncorhynchus mykiss). J. World Aquac. Soc. 2019, 50, 1133–1151. [Google Scholar] [CrossRef]
- Vazirzadeh, A.; Roosta, H.; Masoumi, H.; Farhadi, A.; Jeffs, A. Long-Term Effects of Three Probiotics, Singular or Combined, on Serum Innate Immune Parameters and Expressions of Cytokine Genes in Rainbow Trout during Grow-Out. Fish Shellfish Immunol. 2020, 98, 748–757. [Google Scholar] [CrossRef]
- Panigrahi, A.; Viswanath, K.; Satoh, S. Real-Time Quantification of the Immune Gene Expression in Rainbow Trout Fed Different Forms of Probiotic Bacteria Lactobacillus rhamnosus. Aquac. Res. 2011, 42, 906–917. [Google Scholar] [CrossRef]
- Vasanth, G.K.; Kiron, V.; Kulkarni, A.; Dahle, D.; Lokesh, J.; Kitani, Y. A Microbial Feed Additive Abates Intestinal Inflammation in Atlantic Salmon. Front. Immunol. 2015, 6, 409. [Google Scholar] [CrossRef]
- Ringø, E.; Salinas, I.; Olsen, R.E.; Nyhaug, A.; Myklebust, R.; Mayhew, T.M. Histological Changes in Intestine of Atlantic Salmon (Salmo salar L.) Following in vitro Exposure to Pathogenic and Probiotic Bacterial Strains. Cell Tissue Res. 2007, 328, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Salinas, I.; Myklebust, R.; Esteban, M.A.; Olsen, R.E.; Meseguer, J.; Ringø, E. In vitro Studies of Lactobacillus delbrueckii Subsp. Lactis in Atlantic Salmon (Salmo salar L.) Foregut: Tissue Responses and Evidence of Protection against Aeromonas salmonicida Subsp. Salmonicida Epithelial Damage. Vet. Microbiol. 2008, 128, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Klakegg, Ø.; Salonius, K.; Nilsen, A.; Fülberth, M.; Sørum, H. Enhanced Growth and Decreased Mortality in Atlantic Salmon (Salmo salar) after Probiotic Bath. J. Appl. Microbiol. 2020, 129, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Quimby, M.C. Established Eurythermic Line of Fish Cells in vitro. Science 1962, 135, 1065–1066. [Google Scholar] [CrossRef] [PubMed]
- Fryer, J.L.; Lannan, C.N. Three Decades of Fish Cell Culture: A Current Listing of Cell Lines Derived from Fishes. J. Tissue Cult. Methods 1994, 16, 87–94. [Google Scholar] [CrossRef]
- Lakra, W.S.; Swaminathan, T.R.; Joy, K.P. Development, Characterization, Conservation and Storage of Fish Cell Lines: A Review. Fish Physiol. Biochem. 2011, 37, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Menanteau-Ledouble, S.; Lawrence, M.L.; El-Matbouli, M. Invasion and Replication of Yersinia ruckeri in Fish Cell Cultures. BMC Vet. Res. 2018, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Soto, E.; Yun, S.; Lewis, J.; Kearney, M.T.; Hansen, J. Interaction of Francisella noatunensis Subsp. Orientalis with Oreochromis mossambicus Bulbus Arteriosus Cell Line. Microb. Pathog. 2017, 105, 326–333. [Google Scholar] [CrossRef][Green Version]
- Ormonde, P.; Hörstedt, P.; O’Toole, R.; Milton, D.L. Role of Motility in Adherence to and Invasion of a Fish Cell Line by Vibrio anguillarum. J. Bacteriol. 2000, 182, 2326–2328. [Google Scholar] [CrossRef]
- López-Dóriga, M.V.; Barnes, A.C.; dos Santos, N.M.S.; Ellis, A.E. Invasion of Fish Epithelial Cells by Photobacterium damselae Subsp. Piscicida: Evidence for Receptor Specificity, and Effect of Capsule and Serum. Microbiology 2000, 146, 21–30. [Google Scholar] [CrossRef]
- Castro, R.; Jouneau, L.; Tacchi, L.; Macqueen, D.J.; Alzaid, A.; Secombes, C.J.; Martin, S.A.M.; Boudinot, P. Disparate Developmental Patterns of Immune Responses to Bacterial and Viral Infections in Fish. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Balebona, M.C.; Krovacek, K.; Moriñigo, M.A.; Mansson, I.; Faris, A.; Borrego, J.J. Neurotoxic Effect on Two Fish Species and a PC12 Cell Line of the Supernate of Vibrio alginolyticus and Vibrio anguillarum. Vet. Microbiol. 1998, 63, 61–69. [Google Scholar] [CrossRef]
- Toranzo, A.E.; Santos, Y.; Lemos, M.L.; Ledo, A.; Bolinches, J. Homology of Vibrio anguillarum Strains Causing Epizootics in Turbot, Salmon and Trout Reared on the Atlantic Coast of Spain. Aquaculture 1987, 67, 41–52. [Google Scholar] [CrossRef]
- Krovacek, K.; Faris, A.; Ahne, W.; MÃ¥nsson, I. Adhesion of Aeromonas hydrophila and Vibrio anguillarum to Fish Cells and to Mucus-Coated Glass Slides. FEMS Microbiol. Lett. 1987, 42, 85–89. [Google Scholar] [CrossRef]
- Chaves-Pozo, E.; Pelegrín, P.; García-Castillo, J.; García-Ayala, A.; Mulero, V.; Meseguer, J. Acidophilic Granulocytes of the Marine Fish Gilthead Seabream (Sparus aurata L.) Produce Interleukin-1β Following Infection with Vibrio anguillarum. Cell Tissue Res. 2004, 316, 189–195. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Yang, G.; Zhang, X.-H.; Li, Y. Mutational Analysis of the Zinc Metalloprotease EmpA of Vibrio anguillarum. FEMS Microbiol. Lett. 2007, 267, 56–63. [Google Scholar] [CrossRef]
- Sarmasik, A.; Chen, T.T. Bactericidal Activity of Cecropin B and Cecropin P1 Expressed in Fish Cells (CHSE-214): Application in Controlling Fish Bacterial Pathogens. Aquaculture 2003, 220, 183–194. [Google Scholar] [CrossRef]
- Semple, S.L.; Vo, N.T.K.; Poynter, S.J.; Li, M.; Heath, D.D.; DeWitte-Orr, S.J.; Dixon, B. Extracellular dsRNA Induces a Type I Interferon Response Mediated via Class A Scavenger Receptors in a Novel Chinook Salmon Derived Spleen Cell Line. Dev. Comp. Immunol. 2018. [Google Scholar] [CrossRef]
- Monjo, A.L.; Poynter, S.J.; DeWitte-Orr, S.J. CHSE-214: A Model for Studying Extracellular dsRNA Sensing in vitro. Fish Shellfish Immunol. 2017, 68, 266–271. [Google Scholar] [CrossRef]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, Ł.; Rygiel, T.; Król, M. Evaluation of Phenotypic and Functional Stability of RAW 264.7 Cell Line through Serial Passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef]
- Chung, C.Y.; Iida-Klein, A.; Wyatt, L.E.; Rudkin, G.H.; Ishida, K.; Yamaguchi, D.T.; Miller, T.A. Serial Passage of MC3T3-E1 Cells Alters Osteoblastic Function and Responsiveness to Transforming Growth Factor-Β1 and Bone Morphogenetic Protein-2. Biochem. Biophys. Res. Commun. 1999, 265, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Kwist, K.; Bridges, W.C.; Burg, K.J.L. The Effect of Cell Passage Number on Osteogenic and Adipogenic Characteristics of D1 Cells. Cytotechnology 2016, 68, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Jia, Q.; Di, K.Q.; Gao, S.M.; Wen, X.H.; Zhou, R.Y.; Wei, W.; Wang, L.Z. Passage Number Affects the Pluripotency of Mouse Embryonic Stem Cells as Judged by Tetraploid Embryo Aggregation. Cell Tissue Res. 2007, 327, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Langenbach, R.; Malick, L.; Kennedy, S. Ganglioside and Morphological Changes in Mouse Embryo Cells with Time. Cancer Lett. 1978, 4, 13–19. [Google Scholar] [CrossRef]
- Frattini, A.; Fabbri, M.; Valli, R.; de Paoli, E.; Montalbano, G.; Gribaldo, L.; Pasquali, F.; Maserati, E. High Variability of Genomic Instability and Gene Expression Profiling in Different HeLa Clones. Sci. Rep. 2015, 5, 15377. [Google Scholar] [CrossRef]
- Mohammadi Farsani, T.; Motevaseli, E.; Neyazi, N.; Khorramizadeh, M.R.; Zafarvahedian, E.; Ghahremani, M.H. Effect of Passage Number and Culture Time on the Expression and Activity of Insulin-Degrading Enzyme in Caco-2 Cells. Iran. Biomed. J. 2018, 22, 70–75. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).