The Immune Response of the Invasive Golden Apple Snail to a Nematode-Based Molluscicide Involves Different Organs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Nemaslug® Exposure
2.3. Animal Dissection and Organ Collection for RNA Extraction and Histological Analysis
2.4. Pomacea canaliculata BPI (Pc-BPI) Identification, Sequence Analysis, and Primer Identification
2.5. qPCR Protocol
2.6. Slide Preparation and Staining for Histological Analysis
2.7. Hemolymph Withdrawal and Cytocentrifugation
2.8. Riboprobe Synthesis
2.9. FISH Protocol
2.10. Histology and FISH Imaging
2.11. Statistical Analysis
3. Results
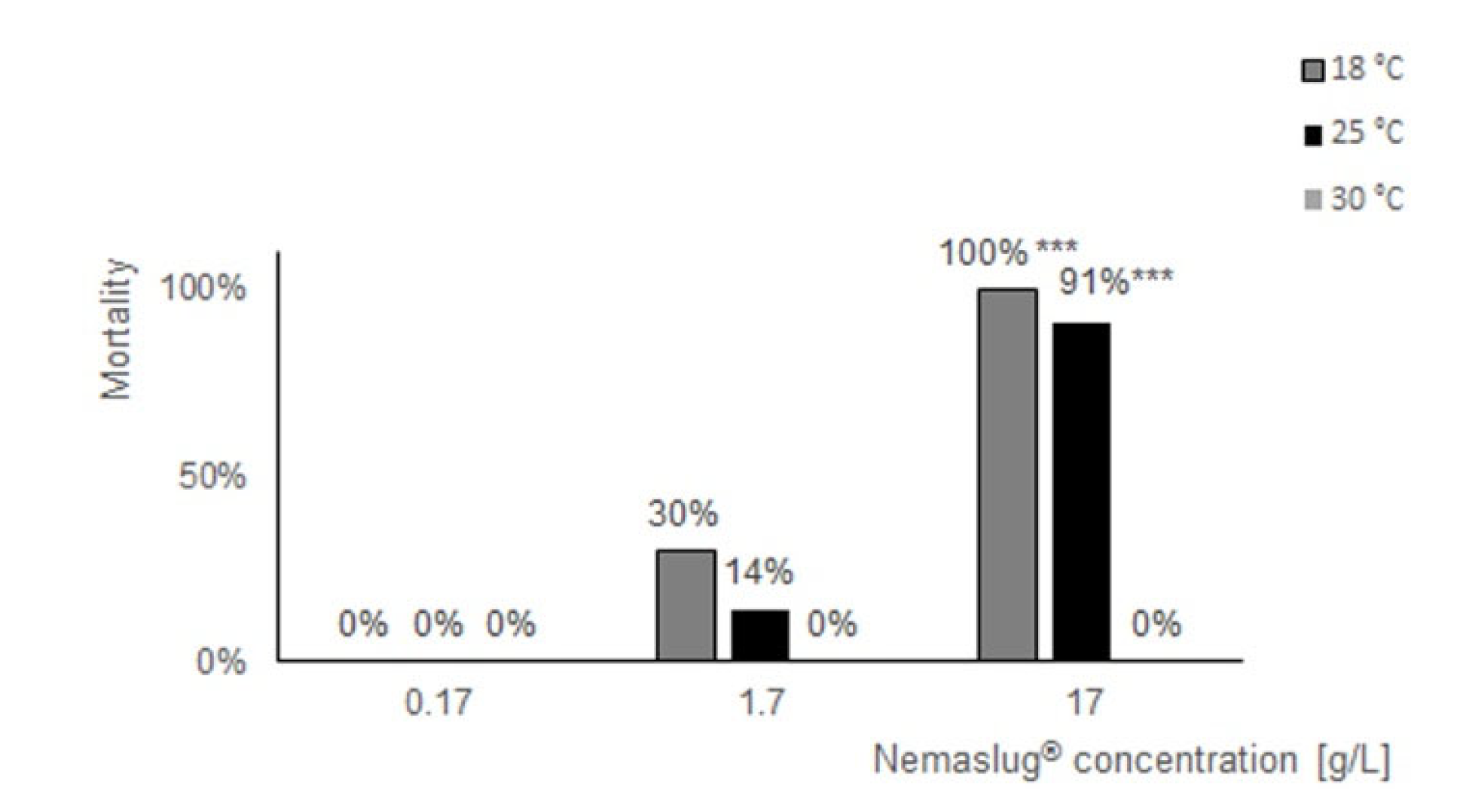
3.1. Effect of Nemaslug® on P. canaliculata Survival and Feeding
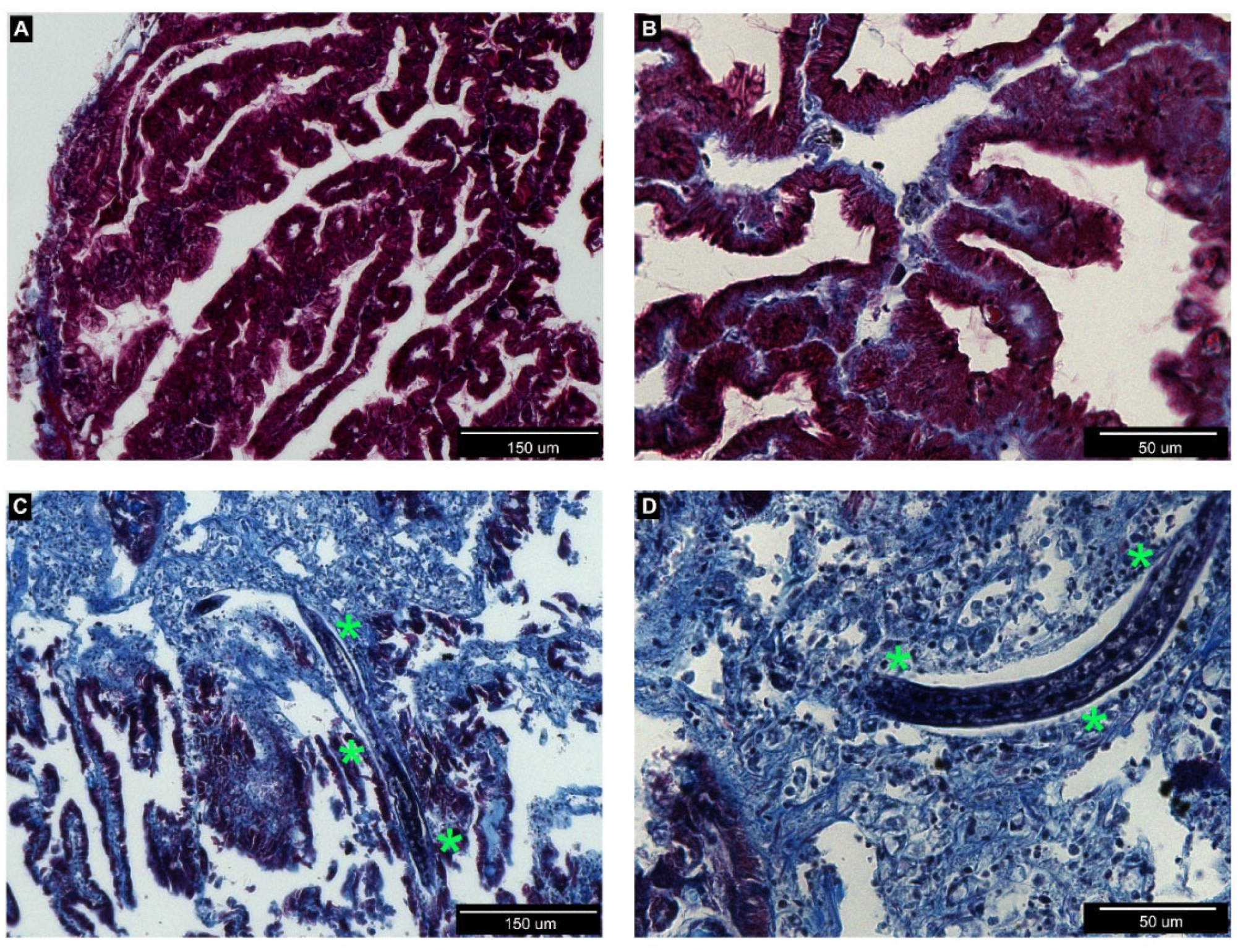
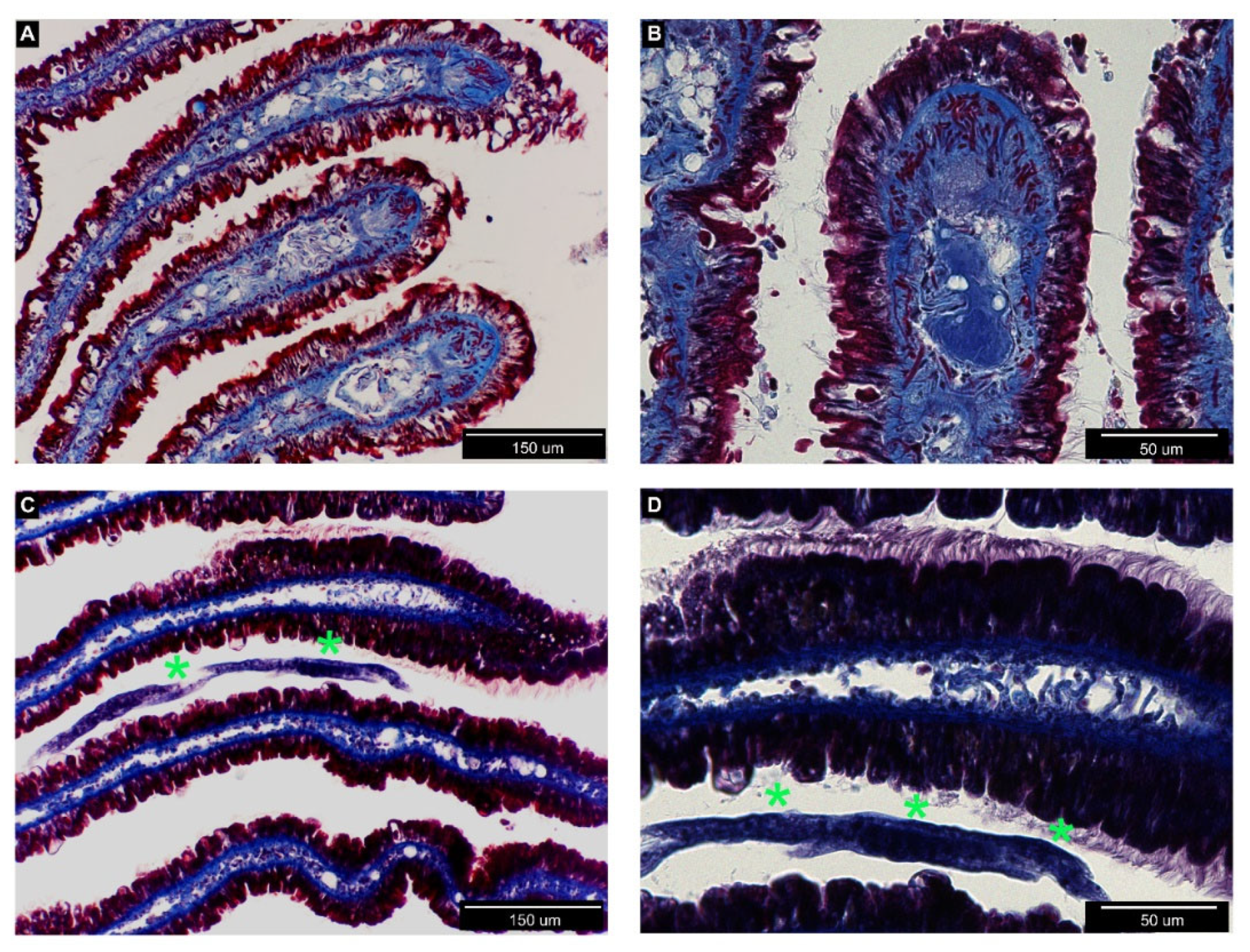
3.2. Histological Analysis of Nemaslug®-Treated Apple Snails
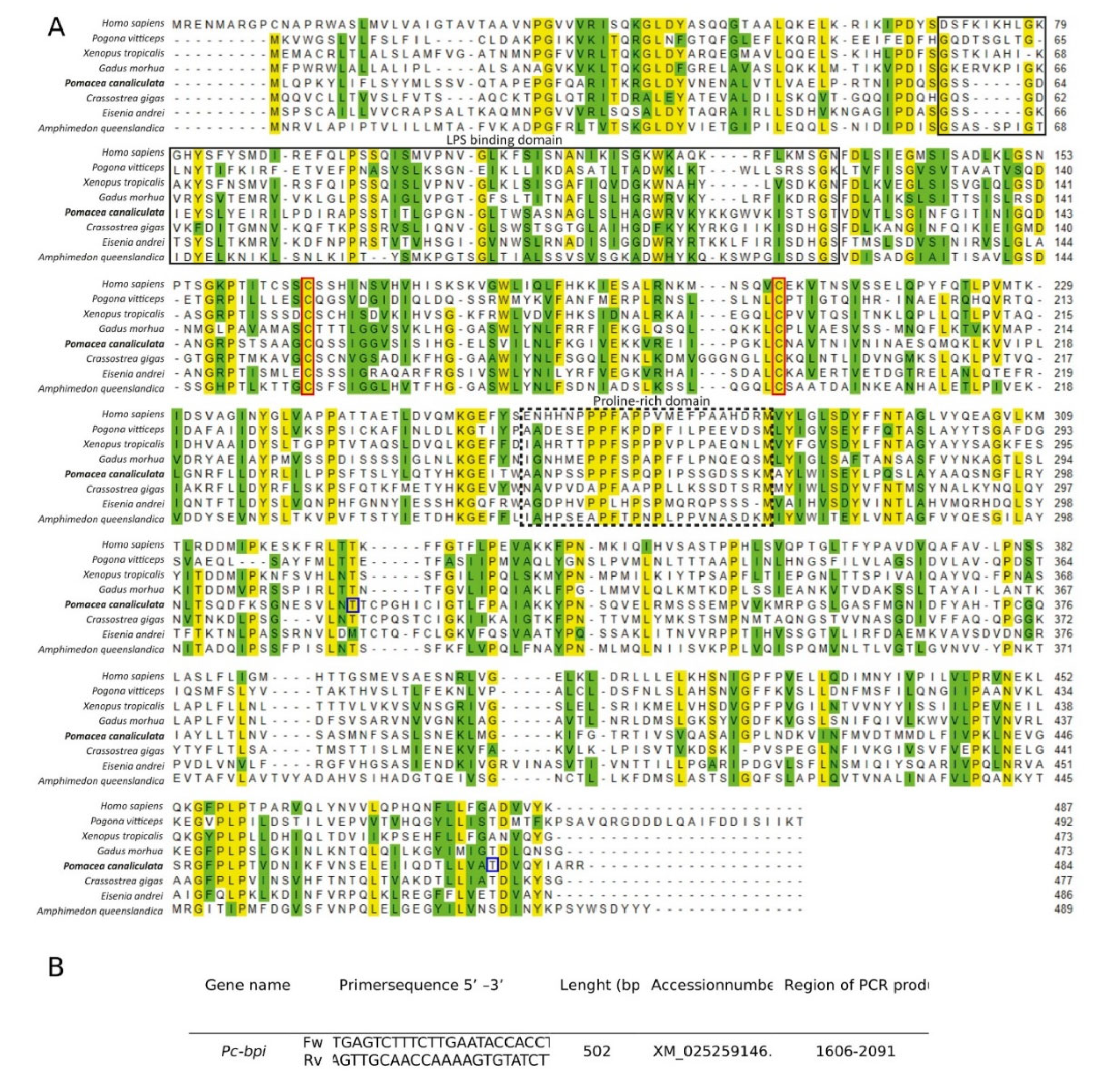
3.3. Pc-bpi Coding Sequence Identification and Analysis
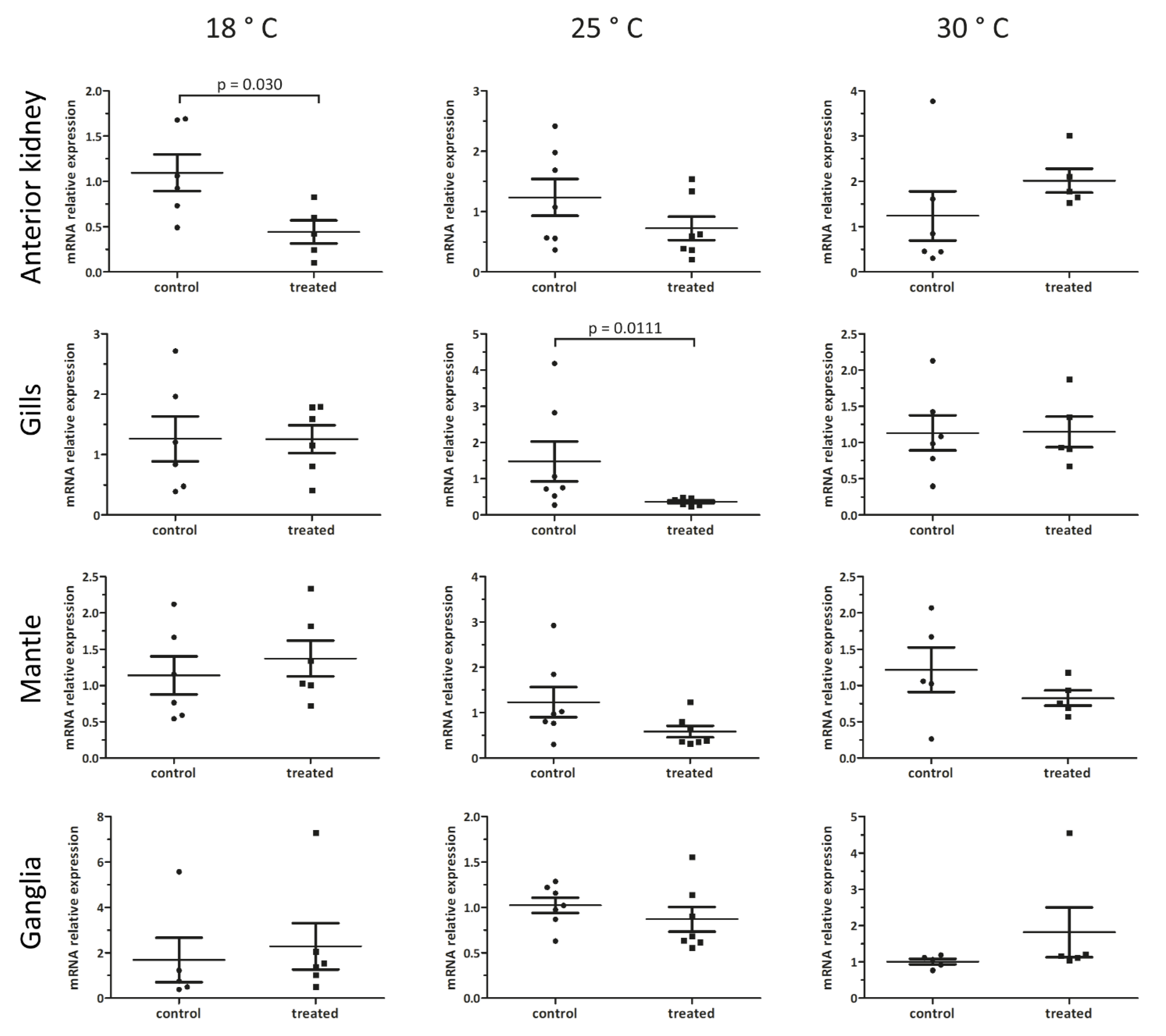
3.4. Organ- and Temperature-Dependent Effects of Nemaslug® on Pc-bpi mRNA Levels
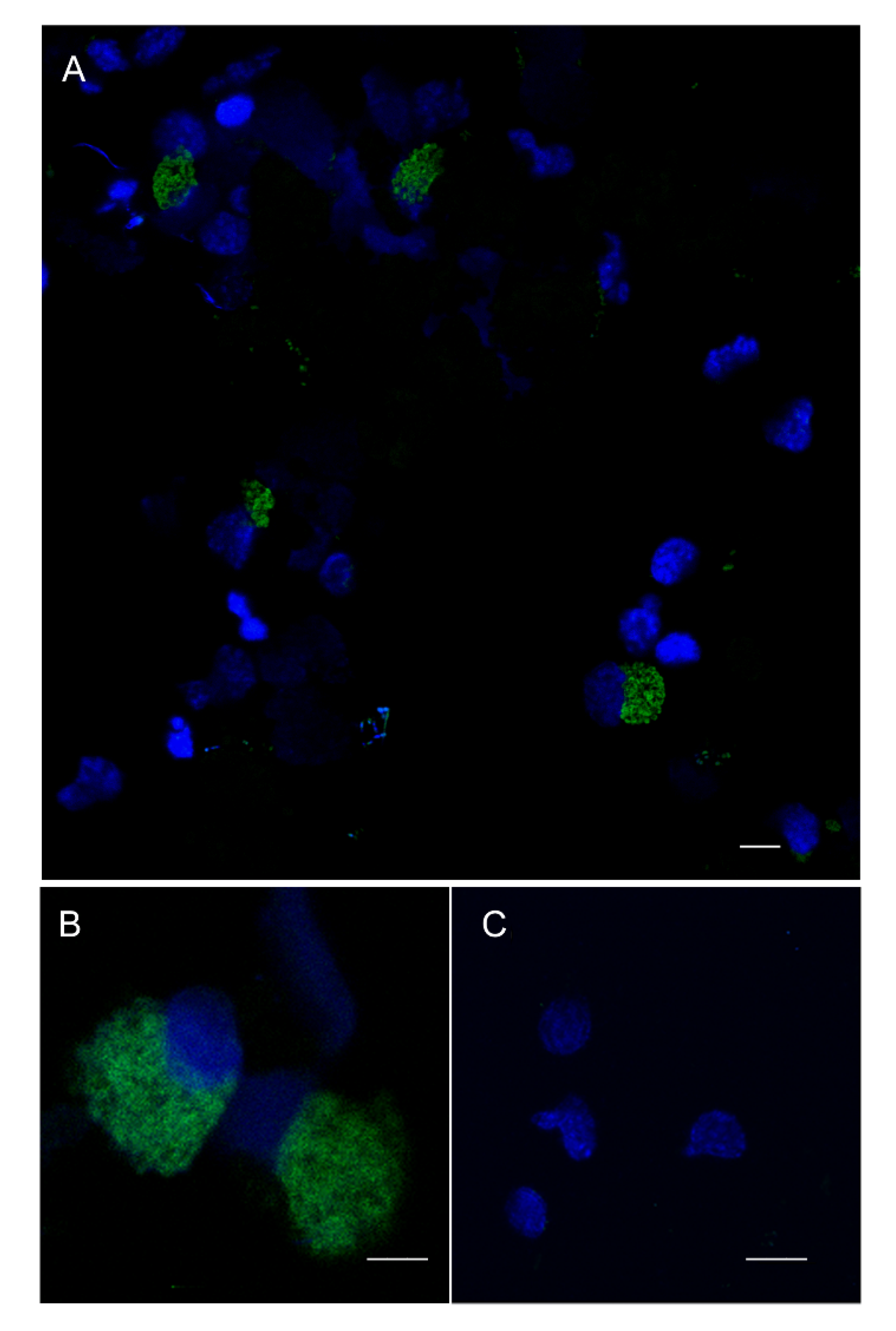
3.5. FISH Analysis of Pc-bpi Expression in P. canaliculata Hemocytes
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Zhang, Y.; Ren, Y.; Wang, H.; Li, S.; Jiang, F.; Yin, L.; Qiao, X.; Zhang, G.; Qian, W.; et al. The genome of the golden apple snail Pomacea canaliculata provides insight into stress tolerance and invasive adaptation. Gigascience 2018, 7, 101. [Google Scholar] [CrossRef]
- Sun, J.; Mu, H.; Ip, J.C.H.; Li, R.; Xu, T.; Accorsi, A.; Sánchez Alvarado, A.; Ross, E.; Lan, Y.; Sun, Y.; et al. Signatures of divergence, invasiveness, and terrestrialization revealed by four apple snail genomes. Mol. Biol. Evol. 2019, 36, 1507–1520. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Guo, Y.H.; Nguyen, H.M.; Sinuon, M.; Sayasone, S.; Lo, N.C.; Zhou, X.N.; Andrews, J.R. Invasive Pomacea snails as important intermediate hosts of Angiostrongylus cantonensis in Laos, Cambodia and Vietnam: Implications for outbreaks of eosinophilic meningitis. Acta Trop. 2018, 183, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Malagoli, D. Going beyond a static picture: The apple snail Pomacea canaliculata can tell us the life history of molluscan hemocytes. Invertebr. Surv. J. 2018, 15, 61–65. [Google Scholar]
- Accorsi, A.; Bucci, L.; de Eguileor, M.; Ottaviani, E.; Malagoli, D. Comparative analysis of circulating hemocytes of the freshwater snail Pomacea canaliculata. Fish Shellfish Immunol. 2013, 34, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Cueto, J.A.; Rodriguez, C.; Vega, I.A.; Castro-Vazquez, A. Immune defenses of the invasive apple snail Pomacea canaliculata (Caenogastropoda, Ampullariidae): Phagocytic hemocytes in the circulation and the kidney. PLoS ONE 2015, 10, e0123964. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, A.; Ottaviani, E.; Malagoli, D. Effects of repeated hemolymph withdrawals on the hemocyte populations and hematopoiesis in Pomacea canaliculata. Fish Shellfish Immunol. 2014, 38, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, A.; Benatti, S.; Ross, E.; Nasi, M.; Malagoli, D. A prokineticin-like protein responds to immune challenges in the gastropod pest Pomacea canaliculata. Dev. Comp. Immunol. 2017, 72, 37–43. [Google Scholar] [CrossRef]
- Rodriguez, C.; Prieto, G.I.; Vega, I.A.; Castro-Vazquez, A. Functional and evolutionary perspectives on gill structures of an obligate air-breathing, aquatic snail. Peer J. 2019, 7, e7342. [Google Scholar] [CrossRef]
- Rodriguez, C.; Prieto, G.I.; Vega, I.A.; Castro-Vazquez, A. Assessment of the kidney and lung as immune barriers and hematopoietic sites in the invasive apple snail Pomacea canaliculata. Peer J. 2018, 6, e5789. [Google Scholar] [CrossRef]
- Yao, T.; Lu, J.; Ye, L.; Wang, J. Molecular characterization and immune analysis of a defensin from small abalone, Haliotis diversicolor. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 235, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, E.; Accorsi, A.; Rigillo, G.; Malagoli, D.; Blom, J.M.; Tascedda, F. Epigenetic modification in neurons of the mollusc Pomacea canaliculata after immune challenge. Brain Res. 2013, 1537, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Huang, Y.; Tang, Y.; Zhang, Q.; Li, X.; Gao, S.; Hua, W.; Zhang, R. Survey of Angiostrongylus cantonensis infection status in host animals and populations in Shenzhen, 2016–2017. Vector Borne Zoonotic Dis. 2019, 19, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhou, H.C.; Dong, Y.; Zhang, T.; Sun, Y.Y.; Zhong, J.F.; Cao, Y.L.; Shao, S.W.; Pan, Y.L.; Dong, H.Y. New nodule type found in the lungs of Pomacea canaliculata, an intermediate host of Angiostrongylus cantonensis. Iran J. Parasitol. 2018, 13, 362–368. [Google Scholar] [PubMed]
- Xiong, Y.M.; Yan, Z.H.; Zhang, J.E.; Hai-Yun Li, H.Y. Analysis of albumen gland proteins suggests survival strategies of developing embryos of Pomacea canaliculata. Mollusc. Res. 2018, 38, 99–104. [Google Scholar] [CrossRef]
- Boraldi, F.; Lofaro, F.D.; Accorsi, A.; Ross, E.; Malagoli, D. Toward the molecular deciphering of Pomacea canaliculata immunity: First proteomic analysis of circulating hemocytes. Proteomics 2019, 19, e1800314. [Google Scholar] [CrossRef]
- Rae, R.; Verdun, C.; Grewal, P.S.; Robertson, J.F.; Wilson, M.J. Biological control of terrestrial molluscs using Phasmarhabditis hermaphrodita--progress and prospects. Pest. Manag. Sci. 2007, 63, 1153–1164. [Google Scholar] [CrossRef]
- Grewal, P.S.; Grewal, S.K.; Tan, L.; Adams, B.J. Parasitism of molluscs by nematodes: Types of associations and evolutionary trends. J. Nematol. 2003, 35, 146–156. [Google Scholar]
- Whitaker, G.; Rae, R. Phasmarhabditis hermaphrodita does not affect non-target freshwater snails Lymnaea stagnalis, Bithynia tentaculata and Planorbarius corneus. Nematology 2015, 17, 679–683. [Google Scholar] [CrossRef]
- Bingle, C.D.; Craven, C.J. Meet the relatives: A family of BPI-and LBP-related proteins. Trends Immunol. 2004, 25, 53–55. [Google Scholar] [CrossRef]
- Beamer, L.J.; Carroll, S.F.; Eisenberg, D. The BPI/LBP family of proteins: A structural analysis of conserved regions. Protein Sci. 1998, 7, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J. Bactericidal/permeability-increasing protein (BPI) and lipopolysaccharide-binding protein (LBP): Structure, function and regulation in host defence against Gram-negative bacteria. Biochem. Soc. Trans. 2003, 31, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Canny, G.; Levy, O.; Furuta, G.T.; Narravula-Alipati, S.; Sisson, R.B.; Serhan, C.N.; Colgan, S.P. Lipid mediator-induced expression of bactericidal/permeability-increasing protein (BPI) in human mucosal epithelia. Proc. Natl. Acad. Sci. USA 2002, 99, 3902–3907. [Google Scholar] [CrossRef] [PubMed]
- Schultz, H.; Hume, J.; Zhang, D.S.; Gioannini, T.L.; Weiss, J.P. A novel role for the bactericidal/permeability increasing protein in interactions of gram-negative bacterial outer membrane blebs with dendritic cells. J. Immunol. 2007, 179, 2477–2484. [Google Scholar] [CrossRef]
- Baron, O.L.; Deleury, E.; Reichhart, J.M.; Coustau, C. The LBP/BPI multigenic family in invertebrates: Evolutionary history and evidences of specialization in mollusks. Dev. Comp. Immunol. 2016, 57, 20–30. [Google Scholar] [CrossRef]
- Seuffert, M.E.; Martin, P.R. Thermal limits for the establishment and growth of populations of the invasive apple snail Pomacea canaliculata. Biol. Invasions 2017, 19, 1169–1180. [Google Scholar] [CrossRef]
- Andrus, P.; Rae, R. Development of Phasmarhabditis hermaphrodita (and members of the Phasmarhabditis genus) as new genetic model nematodes to study the genetic basis of parasitism. J. Helminthol. 2019, 93, 319–331. [Google Scholar] [CrossRef]
- King, R.S.; Newmark, P.A. In situ hybridization protocol for enhanced detection of gene expression in the planarian Schmidtea mediterranea. BMC Dev. Biol. 2013, 13, 8. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Beamer, L.J.; Carroll, S.F.; Eisenberg, D. Crystal structure of human BPI and two bound phospholipids at 2.4 angstrom resolution. Science 1997, 276, 1861–1864. [Google Scholar] [CrossRef]
- Horwitz, A.H.; Leigh, S.D.; Abrahamson, S.; Gazzano-Santoro, H.; Liu, P.S.; Williams, R.E.; Carroll, S.F.; Theofan, G. Expression and characterization of cysteine-modified variants of an amino-terminal fragment of bactericidal/permeability-increasing protein. Protein Expr. Purif. 1996, 8, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.J.; Glen, D.M.; George, S.K. The rhabditid nematode Phasmarhabditis hermaphrodita as a potential biological control agent for slugs. Biocontr. Sci. Technol. 1993, 3, 503–511. [Google Scholar] [CrossRef]
- Tan, L.; Grewal, P.S. Pathogenicity of Moraxella osloensis, a bacterium associated with the nematode Phasmarhabditis hermaphrodita, to the slug Deroceras reticulatum. Appl. Environ. Microbiol. 2001, 67, 5010–5016. [Google Scholar] [CrossRef] [PubMed]
- Rae, R.G.; Tourna, M.; Wilson, M.J. The slug parasitic nematode Phasmarhabditis hermaphrodita associates with complex and variable bacterial assemblages that do not affect its virulence. J. Invertebr. Pathol. 2010, 104, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.J.; Rae, R. Susceptibility of the giant African snail (Achatina fulica) exposed to the gastropod parasitic nematode Phasmarhabditis hermaphrodita. J. Invertebr. Pathol. 2015, 127, 122–126. [Google Scholar] [CrossRef]
- Morris, A.; Green, M.; Martin, H.; Crossland, K.; Swaney, W.T.; Williamson, S.M.; Rae, R. A nematode that can manipulate the behaviour of slugs. Behav. Process. 2018, 151, 73–80. [Google Scholar] [CrossRef]
- Neyen, C.; Bretscher, A.J.; Binggeli, O.; Lemaitre, B. Methods to study Drosophila immunity. Methods 2014, 68, 116–128. [Google Scholar] [CrossRef]
- Gonzalez, M.; Gueguen, Y.; Destoumieux-Garzón, D.; Romestand, B.; Fievet, J.; Pugnière, M.; Roquet, F.; Escoubas, J.M.; Vandenbulcke, F.; Levy, O.; et al. Evidence of a bactericidal permeability increasing protein in an invertebrate, the Crassostrea gigas Cg-BPI. Proc. Natl. Acad. Sci. USA 2007, 104, 17759–17764. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Li, X.; Fu, D.; Chen, J.; Yu, Z. The second bactericidal permeability increasing protein (BPI) and its revelation of the gene duplication in the Pacific oyster, Crassostrea gigas. Fish Shellfish Immunol. 2011, 30, 954–963. [Google Scholar] [CrossRef]
- Mitta, G.; Galinier, R.; Tisseyre, P.; Allienne, J.F.; Girerd-Chambaz, Y.; Guillou, F.; Bouchu, T.A.; Coustau, C. Gene discovery and expression analysis of immune-relevant genes from Biomphalaria glabrata hemocytes. Dev. Comp. Immunol. 2005, 29, 393–407. [Google Scholar] [CrossRef]
- Malagoli, D.; Casarini, L.; Sacchi, S.; Ottaviani, E. Stress and immune response in the mussel Mytilus galloprovincialis. Fish Shellfish Immunol. 2007, 23, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Dang, V.T.; Speck, P.; Benkendorff, K. Influence of elevated temperatures on the immune response of abalone, Haliotis rubra. Fish Shellfish Immunol. 2012, 32, 732–740. [Google Scholar] [CrossRef]
- Matozzo, V.; Giacomazzo, M.; Finos, L.; Marin, M.G.; Bargelloni, L.; Milan, M. Can ecological history influence immunomarker responses and antioxidant enzyme activities in bivalves that have been experimentally exposed to contaminants? A new subject for discussion in “eco-immunology” studies. Fish Shellfish Immunol. 2013, 35, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Henderson, S.; Miller-Ezzy, P.; Li, X.X.; Qin, J.G. Immune response to temperature stress in three bivalve species: Pacific oyster Crassostrea gigas, Mediterranean mussel Mytilus galloprovincialis and mud cockle Katelysia rhytiphora. Fish Shellfish Immunol. 2019, 86, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Seppälä, O.; Jokela, J. Maintenance of genetic variation in immune defense of a freshwater snail: Role of environmental heterogeneity. Evolution 2010, 64, 2397–2407. [Google Scholar]
- Seppälä, O.; Jokela, J. Immune defence under extreme ambient temperature. Biol. Lett 2011, 7, 119–122. [Google Scholar]
- Leicht, K.; Jokela, J.; Seppälä, O. An experimental heat wave changes immune defense and life history traits in a freshwater snail. Ecol. Evol. 2013, 3, 4861–4871. [Google Scholar] [CrossRef]
- Gilioli, G.; Pasquali, S.; Martín, P.R.; Carlsson, N.; Mariani, L.A. temperature-dependent physiologically based model for the invasive apple snail Pomacea canaliculata. Int. J. Biometeorol. 2017, 61, 1899–1911. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, G.; Liu, G.; Yang, Q.; Yu, X. Molecular cloning, characterization of Pomacea canaliculata HSP40 and its expression analysis under temperature change. J. Therm. Biol. 2019, 81, 59–65. [Google Scholar] [CrossRef]
- Nystrand, M.; Dowling, D.K. Dose-dependent effects of an immune challenge at both ultimate and proximate levels in Drosophila melanogaster. J. Evol. Biol. 2014, 27, 876–888. [Google Scholar] [CrossRef] [PubMed]

| Concentration (g/L) | Temperature (°C) | Food Intake |
|---|---|---|
| 0.17 | 18 | + |
| 25 | + | |
| 30 | + | |
| 1.7 | 18 | - |
| 25 | +/- | |
| 30 | + | |
| 17 | 18 | - |
| 25 | - | |
| 30 | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montanari, A.; Bergamini, G.; Ferrari, A.; Ferri, A.; Nasi, M.; Simonini, R.; Malagoli, D. The Immune Response of the Invasive Golden Apple Snail to a Nematode-Based Molluscicide Involves Different Organs. Biology 2020, 9, 371. https://doi.org/10.3390/biology9110371
Montanari A, Bergamini G, Ferrari A, Ferri A, Nasi M, Simonini R, Malagoli D. The Immune Response of the Invasive Golden Apple Snail to a Nematode-Based Molluscicide Involves Different Organs. Biology. 2020; 9(11):371. https://doi.org/10.3390/biology9110371
Chicago/Turabian StyleMontanari, Alice, Giulia Bergamini, Agnese Ferrari, Anita Ferri, Milena Nasi, Roberto Simonini, and Davide Malagoli. 2020. "The Immune Response of the Invasive Golden Apple Snail to a Nematode-Based Molluscicide Involves Different Organs" Biology 9, no. 11: 371. https://doi.org/10.3390/biology9110371
APA StyleMontanari, A., Bergamini, G., Ferrari, A., Ferri, A., Nasi, M., Simonini, R., & Malagoli, D. (2020). The Immune Response of the Invasive Golden Apple Snail to a Nematode-Based Molluscicide Involves Different Organs. Biology, 9(11), 371. https://doi.org/10.3390/biology9110371







