Mitochondrial microRNAs: A Putative Role in Tissue Regeneration
Abstract
:Simple Summary
Abstract
1. Regenerative Biology
Clinical Approaches
2. Mitochondria miRNA Biology
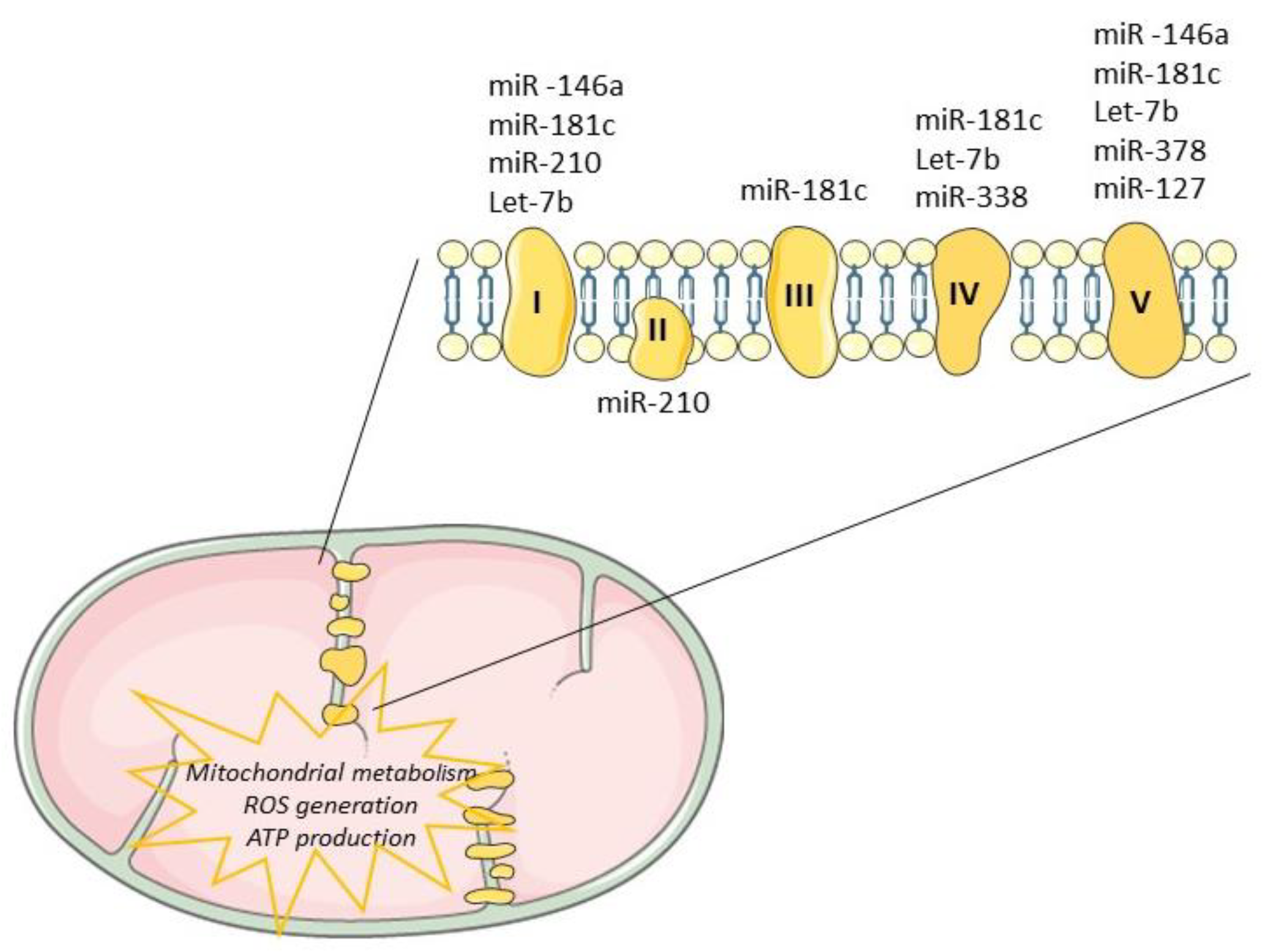
3. Mitochondrial miRNAs—Potential Contribution for Regeneration
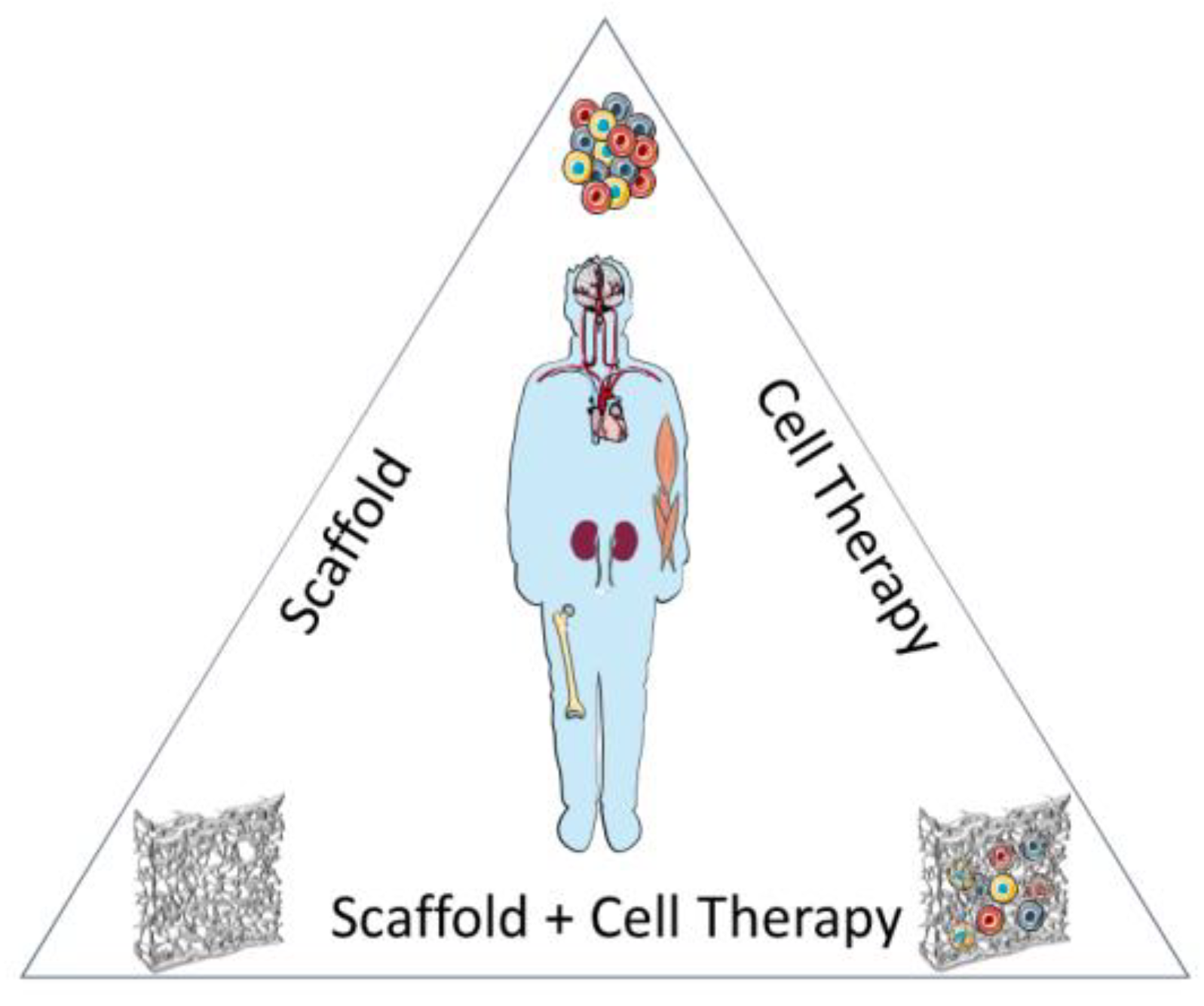
4. Emerging Therapies in Regenerative Medicine
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Krafts, K.P. Tissue repair: The hidden drama. Organogenesis 2010, 6, 225–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, E.M.; Reddien, P.W. The Cellular Basis for Animal Regeneration. Dev. Cell 2011, 21, 172–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, A.; Qin, H.; Fu, X. What determines the regenerative capacity in animals? Bioscience 2016, 66, 735–746. [Google Scholar] [CrossRef]
- McCusker, C.D.; Gardiner, D.M. Understanding positional cues in salamander limb regeneration: Implications for optimizing cell-based regenerative therapies. DMM Dis. Models Mech. 2014, 7, 593–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goessling, W.; North, T.E. Repairing quite swimmingly: Advances in regenerative medicine using zebrafish. DMM Dis. Models Mech. 2014, 7, 769–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunderland, M.E. Regeneration: Thomas Hunt Morgan’s window into development. J. Hist. Biol. 2010. [Google Scholar] [CrossRef]
- Agata, K.; Saito, Y.; Nakajima, E. Unifying principles of regeneration I: Epimorphosis versus morphallaxis. Dev. Growth Differ. 2007, 49, 73–78. [Google Scholar] [CrossRef]
- Londono, R.; Sun, A.X.; Tuan, R.S.; Lozito, T.P. Tissue Repair and Epimorphic Regeneration: An Overview. Curr. Pathobiol. Rep. 2018, 6, 61–69. [Google Scholar] [CrossRef]
- Jaźwińska, A.; Sallin, P. Regeneration versus scarring in vertebrate appendages and heart. J. Pathol. 2016, 238, 233–246. [Google Scholar] [CrossRef] [Green Version]
- Baddour, J.A.; Sousounis, K.; Tsonis, P.A. Organ repair and regeneration: An overview. Birth Defects Res. Part C Embryo Today Rev. 2012, 96, 1–29. [Google Scholar] [CrossRef]
- Sampogna, G.; Guraya, S.Y.; Forgione, A. Regenerative medicine: Historical roots and potential strategies in modern medicine. J. Microsc. Ultrastruct. 2015, 3, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahla, R.S. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int. J. Cell Biol. 2016, 2016, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Zhang, J.; Smuga-Otto, K.; Tian, S.; Yu, J.; Stewart, R.; Thomson, J.A. Protein Kinase C Mediated Extraembryonic Endoderm Differentiation of Human Embryonic Stem Cells. Stem Cells 2012, 30, 461–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shroff, G.; Gupta, R. Human embryonic stem cells in the treatment of patients with spinal cord injury. Ann. Neurosci. 2015. [Google Scholar] [CrossRef] [Green Version]
- Shiba, Y.; Fernandes, S.; Zhu, W.-Z.Z.; Filice, D.; Muskheli, V.; Kim, J.; Palpant, N.J.; Gantz, J.; Moyes, K.W.; Reinecke, H.; et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 2012, 489, 322–325. [Google Scholar] [CrossRef]
- Fernandes, S.; Chong, J.J.H.; Paige, S.L.; Iwata, M.; Torok-Storb, B.; Keller, G.; Reinecke, H.; Murry, C.E. Comparison of human embryonic stem cell-derived cardiomyocytes, cardiovascular progenitors, and bone marrow mononuclear cells for cardiac repair. Stem Cell Rep. 2015. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Holton, K.L.; Lanza, R. Efficient Differentiation of Functional Hepatocytes from Human Embryonic Stem Cells. Stem Cells 2008, 26, 1117–1127. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.; Kapacee, Z.; Peng, J.; Lu, S.; Lucas, R.J.; Hardingham, T.E.; Kimber, S.J. Cartilage Repair Using Human Embryonic Stem Cell-Derived Chondroprogenitors. Stem Cells Transl. Med. 2014, 3, 1287–1294. [Google Scholar] [CrossRef]
- Lo, B.; Parham, L. Ethical Issues in Stem Cell Research. Endocr. Rev. 2009, 30, 204–213. [Google Scholar] [CrossRef]
- Nombela-Arrieta, C.; Ritz, J.; Silberstein, L.E. The elusive nature and function of mesenchymal stem cells. Nat. Rev. Mol. Cell Biol. 2011, 12, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.C.W.W.; Baily, J.E.; Corselli, M.; Díaz, M.E.; Sun, B.; Xiang, G.; Gray, G.A.; Huard, J.; Péault, B. Human myocardial pericytes: Multipotent mesodermal precursors exhibiting cardiac specificity. Stem Cells 2015, 33, 557–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanska, A.; Kenyon, C.; Christian, H.C.; Buckley, C.; Shaw, I.; Mullins, J.J.; Péault, B. Human kidney pericytes produce renin. Kidney Int. 2016, 90, 1251–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deskins, D.L.; Bastakoty, D.; Saraswati, S.; Shinar, A.; Holt, G.E.; Young, P.P. Human Mesenchymal Stromal Cells: Identifying Assays to Predict Potency for Therapeutic Selection. Stem Cells Transl. Med. 2013, 2, 151–158. [Google Scholar] [CrossRef]
- Singaravelu, K.; Padanilam, B.J. In Vitro Differentiation of MSC into Cells with a Renal Tubular Epithelial-Like Phenotype. Ren. Fail. 2009, 31, 492–502. [Google Scholar] [CrossRef] [Green Version]
- Körbling, M.; Robinson, S.; Estrov, Z.; Champlin, R.; Shpall, E. Umbilical cord blood-derived cells for tissue repair. Cytotherapy 2005, 7, 258–261. [Google Scholar] [CrossRef]
- Weiss, M.L.; Troyer, D.L. Stem Cells in the Umbilical Cord. Stem Cells Rev. 2013, 2, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Ma, Q.H. Repairing neural injuries using human umbilical cord blood. Mol. Neurobiol. 2013, 47, 938–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copeland, N.; Harris, D.; Gaballa, M.A. Human umbilical cord blood stem cells, myocardial infarction and stroke. Clin. Med. 2009, 9, 342–345. [Google Scholar] [CrossRef]
- Hong, B.; Lee, S.; Shin, N.; Ko, Y.; Kim, D.; Lee, J.; Lee, W. Bone regeneration with umbilical cord blood mesenchymal stem cells in femoral defects of ovariectomized rats. Osteoporos. Sarcopenia 2018, 4, 95–101. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, M.; Umegaki-Arao, N.; Guo, Z.; Liu, L.; Higgins, C.A.; Christiano, A.M. Generation of 3D Skin Equivalents Fully Reconstituted from Human Induced Pluripotent Stem Cells (iPSCs). PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohyama, M.; Okano, H. Promise of human induced pluripotent stem cells in skin regeneration and investigation. J. Invest. Dermatol. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.; Zheng, Y.; Burrows, M.; Liu, S.; Wei, Z.; Nace, A.; Guo, W.; Kumar, S.; Cotsarelis, G.; Xu, X. Generation of folliculogenic human epithelial stem cells from induced pluripotent stem cells. Nat. Commun. 2014, 5, 3071. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, K.J.; Shamis, Y.; Hayman, R.B.; Margvelashvili, M.; Dong, S.; Carlson, M.W.; Garlick, J.A. Epigenetic and phenotypic profile of fibroblasts derived from induced pluripotent stem cells. PLoS ONE 2011, 6, e17128. [Google Scholar] [CrossRef] [Green Version]
- Itoh, M.; Kiuru, M.; Cairo, M.S.; Christiano, A.M. Generation of keratinocytes from normal and recessive dystrophic epidermolysis bullosa-induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2011, 108, 8797–8802. [Google Scholar] [CrossRef] [Green Version]
- Neofytou, E.; O’Brien, C.G.; Couture, L.A.; Wu, J.C. Hurdles to clinical translation of human induced pluripotent stem cells. J. Clin. Invest. 2015, 125, 2551–2557. [Google Scholar] [CrossRef] [Green Version]
- Daley, W.P.; Peters, S.B.; Larsen, M. Extracellular matrix dynamics in development and regenerative medicine. J. Cell Sci. 2008, 121, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Hosseinkhani, M.; Mehrabani, D.; Karimfar, M.H.; Bakhtiyari, S.; Manafi, A.; Shirazi, R. Tissue engineered scaffolds in regenerative medicine. World J. Plast. Surg. 2014, 3, 3–7. [Google Scholar]
- Yang, D.; Zhao, Z.; Bai, F.; Wang, S.; Tomsia, A.P.; Bai, H. Promoting Cell Migration in Tissue Engineering Scaffolds with Graded Channels. Adv. Healthc. Mater. 2017, 6, 1700472. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Wang, Z.Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9. [Google Scholar] [CrossRef]
- Mitrousis, N.; Fokina, A.; Shoichet, M.S. Biomaterials for cell transplantation. Nat. Rev. Mater. 2018, 3, 441–456. [Google Scholar] [CrossRef]
- Ahadian, S.; Khademhosseini, A. Smart scaffolds in tissue regeneration. Regen. Biomater. 2018, 5, 125–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, J.L.; Engler, A.J. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials 2011, 32, 1002–1009. [Google Scholar] [CrossRef] [Green Version]
- Bencherif, S.A.; Sands, R.W.; Bhatta, D.; Arany, P.; Verbeke, C.S.; Edwards, D.A.; Mooney, D.J. Injectable preformed scaffolds with shape-memory properties. Proc. Natl. Acad. Sci. USA 2012, 109, 19590–19595. [Google Scholar] [CrossRef] [Green Version]
- Tétreault, N.; De Guire, V. MiRNAs: Their discovery, biogenesis and mechanism of action. Clin. Biochem. 2013, 46, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- D’Aquila, P.; Bellizzi, D.; Passarino, G. Mitochondria in health, aging and diseases: The epigenetic perspective. Biogerontology 2015, 16, 569–585. [Google Scholar] [CrossRef]
- Hudson, G.; Gomez-Duran, A.; Wilson, I.J.; Chinnery, P.F. Recent Mitochondrial DNA Mutations Increase the Risk of Developing Common Late-Onset Human Diseases. PLoS Genet. 2014, 10, e1004369. [Google Scholar] [CrossRef] [PubMed]
- Dowling, D.K. Evolutionary perspectives on the links between mitochondrial genotype and disease phenotype. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Duarte, F.V.; Amorim, J.A.; Palmeira, C.M.; Rolo, A.P. Regulation of Mitochondrial Function and its Impact in Metabolic Stress. Curr. Med. Chem. 2015, 22, 2468–2479. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; Avadhani, N.G. Mitochondrial retrograde signaling at the crossroads of tumor bioenergetics, genetics and epigenetics. Mitochondrion 2013, 13, 577–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whelan, S.P.; Zuckerbraun, B.S. Mitochondrial signaling: Forwards, backwards, and in between. Oxid. Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfanner, N.; Warscheid, B.; Wiedemann, N. Mitochondrial proteins: From biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 2019, 20, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Hu, X.Q.; Zhang, L. Mitochondrial MiRNA in Cardiovascular Function and Disease. Cells 2019, 8, 1475. [Google Scholar] [CrossRef] [Green Version]
- Duarte, F.V.; Palmeira, C.M.; Rolo, A.P. The Emerging Role of MitomiRs in the Pathophysiology of Human Disease. In Advances in Experimental Medicine and Biology; Santulli, G., Ed.; Springer: New York, NY, USA, 2015; Volume 888, pp. 123–154. [Google Scholar]
- Duarte, F.V.; Palmeira, C.M.; Rolo, A.P. The Role of microRNAs in Mitochondria: Small Players Acting Wide. Genes 2014, 5, 865–886. [Google Scholar] [CrossRef] [Green Version]
- Tomasetti, M.; Neuzil, J.; Dong, L. MicroRNAs as regulators of mitochondrial function: Role in cancer suppression. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1441–1453. [Google Scholar] [CrossRef]
- Macgregor-Das, A.M.; Das, S. A microRNA’s journey to the center of the mitochondria. Am. J. Physiol. Circ. Physiol. 2018, 315, H206–H215. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Tian, T.; Chen, W.; Lv, X.; Lei, X.; Zhang, H.; Sun, S.; Cai, L.; Pan, G.; He, L.; et al. Mitochondrial miRNA Determines Chemoresistance by Reprogramming Metabolism and Regulating Mitochondrial Transcription. Cancer Res. 2019, 79, 1069–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinde, S.; Bhadra, U. A complex genome-MicroRNA interplay in human mitochondria. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, C.K.; Ghatak, S. miRNA control of tissue repair and regeneration. Am. J. Pathol. 2015, 185, 2629–2640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borralho, P.M.; Rodrigues, C.M.P.; Steer, C.J. Mitochondrial MicroRNAs and Their Potential Role in Cell Function. Curr. Pathobiol. Rep. 2014, 2, 123–132. [Google Scholar] [CrossRef]
- Sen, C.K. (Ed.) MicroRNA in Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780124055445. [Google Scholar]
- Yu, S.B.; Pekkurnaz, G. Mechanisms Orchestrating Mitochondrial Dynamics for Energy Homeostasis. J. Mol. Biol. 2018, 430, 3922–3941. [Google Scholar] [CrossRef]
- Sekar, D.; Johnson, J.; Biruntha, M.; Lakhmanan, G.; Gurunathan, D.; Ross, K. Biological and clinical relevance of microRNAs in mitochondrial diseases/dysfunctions. DNA Cell Biol. 2020, 39, 1379–1384. [Google Scholar] [CrossRef]
- Zhang, X.; Zuo, X.; Yang, B.; Li, Z.; Xue, Y.; Zhou, Y.; Huang, J.; Zhao, X.; Zhou, J.; Yan, Y.; et al. MicroRNA Directly Enhances Mitochondrial Translation during Muscle Differentiation. Cell 2014, 158, 607. [Google Scholar] [CrossRef] [Green Version]
- Wüst, S.; Dröse, S.; Heidler, J.; Wittig, I.; Klockner, I.; Franko, A.; Bonke, E.; Günther, S.; Gärtner, U.; Boettger, T.; et al. Metabolic Maturation during Muscle Stem Cell Differentiation Is Achieved by miR-1/133a-Mediated Inhibition of the Dlk1-Dio3 Mega Gene Cluster. Cell Metab. 2018, 27, 1026–1039.e6. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, A.; Prattichizzo, F.; Micolucci, L.; Ceriello, A.; Procopio, A.D.; Rippo, M.R. Mitochondrial (Dys) Function in Inflammaging: Do MitomiRs Influence the Energetic, Oxidative, and Inflammatory Status of Senescent Cells? Mediat. Inflamm. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Lin, S.; Luo, W.; Ye, Y.; Bekele, E.J.; Nie, Q.; Li, Y.; Zhang, X. Let-7b Regulates Myoblast Proliferation by Inhibiting IGF2BP3 Expression in Dwarf and Normal Chicken. Front. Physiol. 2017, 8, 477. [Google Scholar] [CrossRef] [Green Version]
- Zhai, L.; Wu, R.; Han, W.; Zhang, Y.; Zhu, D. miR-127 enhances myogenic cell differentiation by targeting S1PR3. Cell Death Dis. 2017, 8, e2707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsitkanou, S.; Della Gatta, P.A.; Russell, A.P. Skeletal Muscle Satellite Cells, Mitochondria, and MicroRNAs: Their Involvement in the Pathogenesis of ALS. Front. Physiol. 2016, 7, 403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Y.; Sun, Y.; Chen, J. IGF-II is regulated by microRNA-125b in skeletal myogenesis. J. Cell Biol. 2011, 192, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Zhang, W.R.; Wang, Y.M.; Liu, X.F.; Li, X.; Ding, X.B.; Guo, H. microRNA-128 regulates the proliferation and differentiation of bovine skeletal muscle satellite cells by repressing Sp1. Mol. Cell. Biochem. 2016, 414, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, H.; Das, S. Mitochondrial miRNA (MitomiR): A new player in cardiovascular health. Can. J. Physiol. Pharmacol. 2015, 93, 855–861. [Google Scholar] [CrossRef]
- Das, S.; Ferlito, M.; Kent, O.A.; Fox-Talbot, K.; Wang, R.; Liu, D.; Raghavachari, N.; Yang, Y.; Wheelan, S.J.; Murphy, E.; et al. Nuclear miRNA Regulates the Mitochondrial Genome in the Heart. Circ. Res. 2012, 110, 1596–1603. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Bedja, D.; Campbell, N.; Dunkerly, B.; Chenna, V.; Maitra, A.; Steenbergen, C. miR-181c Regulates the Mitochondrial Genome, Bioenergetics, and Propensity for Heart Failure In Vivo. PLoS ONE 2014, 9, e96820. [Google Scholar] [CrossRef] [Green Version]
- Banavath, H.N.; Roman, B.; Mackowski, N.; Biswas, D.; Afzal, J.; Nomura, Y.; Solhjoo, S.; O’Rourke, B.; Kohr, M.; Murphy, E.; et al. miR-181c Activates Mitochondrial Calcium Uptake by Regulating MICU1 in the Heart. J. Am. Heart Assoc. 2019, 8, e012919. [Google Scholar] [CrossRef]
- Aschrafi, A.; Schwechter, A.D.; Mameza, M.G.; Natera-Naranjo, O.; Gioio, A.E.; Kaplan, B.B. MicroRNA-338 Regulates Local Cytochrome c Oxidase IV mRNA Levels and Oxidative Phosphorylation in the Axons of Sympathetic Neurons. J. Neurosci. 2008, 28, 12581–12590. [Google Scholar] [CrossRef]
- Aschrafi, A.; Kar, A.N.; Natera-Naranjo, O.; MacGibeny, M.A.; Gioio, A.E.; Kaplan, B.B. MicroRNA-338 regulates the axonal expression of multiple nuclear-encoded mitochondrial mRNAs encoding subunits of the oxidative phosphorylation machinery. Cell. Mol. Life Sci. 2012, 69, 4017–4027. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, R.; Thapa, D.; Nichols, C.E.; Shepherd, D.L.; Stricker, J.C.; Croston, T.L.; Baseler, W.A.; Lewis, S.E.; Martinez, I.; Hollander, J.M. Translational Regulation of the Mitochondrial Genome Following Redistribution of Mitochondrial MicroRNA in the Diabetic Heart. Circ. Cardiovasc. Genet. 2015, 8, 785–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocum, D.L. Regenerative Biology and Medicine; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2012; ISBN 9780123848604. [Google Scholar]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction). J. Mol. Cell. Cardiol. 2016, 94, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, I.; Patel, A.; Sundaresan, N.R.; Gupta, M.P.; Solaro, R.J.; Nagalingam, R.S.; Gupta, M. A Novel Cardiomyocyte-enriched MicroRNA, miR-378, Targets Insulin-like Growth Factor 1 Receptor. J. Biol. Chem. 2012, 287, 12913–12926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrer, M.; Liu, N.; Grueter, C.E.; Williams, A.H.; Frisard, M.I.; Hulver, M.W.; Bassel-Duby, R.; Olson, E.N. Control of mitochondrial metabolism and systemic energy homeostasis by microRNAs 378 and 378. Proc. Natl. Acad. Sci. USA 2012, 109, 15330–15335. [Google Scholar] [CrossRef] [Green Version]
- Willers, I.M.; Martínez-Reyes, I.; Martínez-Diez, M.; Cuezva, J.M. miR-127-5p targets the 3′UTR of human β-F1-ATPase mRNA and inhibits its translation. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 838–848. [Google Scholar] [CrossRef]
- Sousounis, K.; Baddour, J.A.; Tsonis, P.A. Aging and Regeneration in Vertebrates. Curr. Top. Dev. Biol. 2014, 108, 217–246. [Google Scholar] [CrossRef]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z. Bin Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Ji, G.; Lv, K.; Chen, H.; Wang, T.; Wang, Y.; Zhao, D.; Qu, L.; Li, Y. MiR-146a Regulates SOD2 Expression in H2O2 Stimulated PC12 Cells. PLoS ONE 2013, 8, e69351. [Google Scholar] [CrossRef]
- Rippo, M.R.; Olivieri, F.; Monsurrò, V.; Prattichizzo, F.; Albertini, M.C.; Procopio, A.D. MitomiRs in human inflamm-aging: A hypothesis involving miR-181a, miR-34a and miR-146a. Exp. Gerontol. 2014, 56, 154–163. [Google Scholar] [CrossRef]
- Vianello, A.; Casolo, V.; Petrussa, E.; Peresson, C.; Patui, S.; Bertolini, A.; Passamonti, S.; Braidot, E.; Zancani, M. The mitochondrial permeability transition pore (PTP)—An example of multiple molecular exaptation? Biochim. Biophys. Acta Bioenerg. 2012, 1817, 2072–2086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, K.; An, T.; Zhai, M.; Huang, Y.; Wang, Q.; Wang, Y.; Zhang, R.; Wang, T.; Liu, J.; Zhang, Y.; et al. Mitochondrial miR-762 regulates apoptosis and myocardial infarction by impairing ND2. Cell Death Dis. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackl, M.; Brunner, S.; Fortschegger, K.; Schreiner, C.; Micutkova, L.; Mück, C.; Laschober, G.T.; Lepperdinger, G.; Sampson, N.; Berger, P.; et al. miR-17, miR-19b, miR-20a, and miR-106a are down-regulated in human aging. Aging Cell 2010, 9, 291–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuliani, A.; Cirilli, I.; Prattichizzo, F.; Mensà, E.; Fulgenzi, G.; Sabbatinelli, J.; Graciotti, L.; Olivieri, F.; Procopio, A.D.; Tiano, L.; et al. The mitomiR/Bcl-2 axis affects mitochondrial function and autophagic vacuole formation in senescent endothelial cells. Aging 2018, 10, 2855–2873. [Google Scholar] [CrossRef]
- Nishino, J.; Kim, I.; Chada, K.; Morrison, S.J. Hmga2 Promotes Neural Stem Cell Self-Renewal in Young but Not Old Mice by Reducing p16Ink4a and p19Arf Expression. Cell 2008, 135, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Mancini, M.; Saintigny, G.; Mahé, C.; Annicchiarico-Petruzzelli, M.; Melino, G.; Candi, E. MicroRNA-152 and -181a participate in human dermal fibroblasts senescence acting on cell adhesion and remodeling of the extra-cellular matrix. Aging 2012, 4, 843–853. [Google Scholar] [CrossRef] [Green Version]
- Purohit, P.K.; Saini, N. Mitochondrial microRNA (MitomiRs) in cancer and complex mitochondrial diseases: Current status and future perspectives. Cell. Mol. Life Sci. 2020, 1–17. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; Guijarro, L.G.; Casanova, C.; Coca, S.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N.; Asúnsolo, Á. The regulatory role of mitochondrial micrornas (Mitomirs) in breast cancer: Translational implications present and future. Cancers 2020, 12, 2443. [Google Scholar] [CrossRef]
- Jusic, A.; Devaux, Y. Mitochondrial noncoding RNA-regulatory network in cardiovascular disease. Basic Res. Cardiol. 2020, 115, 1–17. [Google Scholar] [CrossRef]
- Chen, C.; Ponnusamy, M.; Liu, C.; Gao, J.; Wang, K.; Li, P. MicroRNA as a Therapeutic Target in Cardiac Remodeling. Biomed Res. Int. 2017, 2017, 1278436. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, S.; He, A.; Kong, S.W.; Lu, J.; Bejar, R.; Bodyak, N.; Lee, K.-H.; Ma, Q.; Kang, P.M.; Golub, T.R.; et al. MicroRNA-1 negatively regulates expression of the hypertrophy-associated calmodulin and Mef2a genes. Mol. Cell. Biol. 2009, 29, 2193–2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varrone, F.; Gargano, B.; Carullo, P.; Di Silvestre, D.; De Palma, A.; Grasso, L.; Di Somma, C.; Mauri, P.; Benazzi, L.; Franzone, A.; et al. The Circulating Level of FABP3 Is an Indirect Biomarker of MicroRNA-1. J. Am. Coll. Cardiol. 2013, 61, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Karakikes, I.; Chaanine, A.H.; Kang, S.; Mukete, B.N.; Jeong, D.; Zhang, S.; Hajjar, R.J.; Lebeche, D. Therapeutic cardiac-targeted delivery of miR-1 reverses pressure overload-induced cardiac hypertrophy and attenuates pathological remodeling. J. Am. Heart Assoc. 2013, 2, e000078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Wu, J.; Dong, H.; Khan, S.A.; Chu, M.-L.; Tsuda, T. Fibulin-2 deficiency attenuates angiotensin II-induced cardiac hypertrophy by reducing transforming growth factor-β signalling. Clin. Sci. 2014, 126, 275–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diniz, G.P.; Lino, C.A.; Guedes, E.C.; do Nascimento Moreira, L.; Barreto-Chaves, M.L.M. Cardiac microRNA-133 is down-regulated in thyroid hormone-mediated cardiac hypertrophy partially via Type 1 Angiotensin II receptor. Basic Res. Cardiol. 2015, 110, 49. [Google Scholar] [CrossRef]
- Carè, A.; Catalucci, D.; Felicetti, F.; Bonci, D.; Addario, A.; Gallo, P.; Bang, M.-L.; Segnalini, P.; Gu, Y.; Dalton, N.D.; et al. MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 2007, 13, 613–618. [Google Scholar] [CrossRef]
- Ganesan, J.; Ramanujam, D.; Sassi, Y.; Ahles, A.; Jentzsch, C.; Werfel, S.; Leierseder, S.; Loyer, X.; Giacca, M.; Zentilin, L.; et al. MiR-378 Controls Cardiac Hypertrophy by Combined Repression of Mitogen-Activated Protein Kinase Pathway Factors. Circulation 2013, 127, 2097–2106. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Zhang, X.; Fan, S.; Cui, G.; Shen, Z. MicroRNA-497 Inhibits Cardiac Hypertrophy by Targeting Sirt4. PLoS ONE 2016, 11, e0168078. [Google Scholar] [CrossRef]
- Ucar, A.; Gupta, S.K.; Fiedler, J.; Erikci, E.; Kardasinski, M.; Batkai, S.; Dangwal, S.; Kumarswamy, R.; Bang, C.; Holzmann, A.; et al. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat. Commun. 2012, 3, 1078. [Google Scholar] [CrossRef]
- Wang, K.; Lin, Z.-Q.; Long, B.; Li, J.-H.; Zhou, J.; Li, P.-F. Cardiac hypertrophy is positively regulated by MicroRNA miR-23a. J. Biol. Chem. 2012, 287, 589–599. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Wang, N.; Zhang, J.; He, H.-P.; Gong, H.-Q.; Zhang, R.; Song, T.-F.; Zhang, L.-N.; Guo, Z.-X.; Cao, D.-S.; et al. MicroRNA-29a-3p attenuates ET-1-induced hypertrophic responses in H9c2 cardiomyocytes. Gene 2016, 585, 44–50. [Google Scholar] [CrossRef]
- Vettori, S. Role of MicroRNAs in Fibrosis. Open Rheumatol. J. 2012, 6, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Z.; Sun, X.; Shan, H.; Wang, N.; Wang, J.; Ren, J.; Feng, S.; Xie, L.; Lu, C.; Yuan, Y.; et al. MicroRNA-101 Inhibited Postinfarct Cardiac Fibrosis and Improved Left Ventricular Compliance via the FBJ Osteosarcoma Oncogene/Transforming Growth Factor-β1 Pathway. Circulation 2012, 126, 840–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Wang, K.; Liao, Y.; Zeng, Q.; Li, Y.; Hu, F.; Liu, Y.; Meng, K.; Qian, C.; Zhang, Q.; et al. MicroRNA-101a inhibits cardiac fibrosis induced by hypoxia via targeting TGFβRI on cardiac fibroblasts. Cell. Physiol. Biochem. 2015, 35, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, J.; López, B.; Hermida, N.; Schroen, B.; San José, G.; Heymans, S.; Valencia, F.; Gómez-Doblas, J.J.; De Teresa, E.; Díez, J.; et al. microRNA-122 down-regulation may play a role in severe myocardial fibrosis in human aortic stenosis through TGF-β1 up-regulation. Clin. Sci. 2014, 126, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, W.; Xu, R.; Nie, Y.; Cao, X.; Meng, J.; Xu, X.; Hu, S.; Zheng, Z. MicroRNA-24 regulates cardiac fibrosis after myocardial infarction. J. Cell. Mol. Med. 2012, 16, 2150–2160. [Google Scholar] [CrossRef]
- Cushing, L.; Kuang, P.P.; Qian, J.; Shao, F.; Wu, J.; Little, F.; Thannickal, V.J.; Cardoso, W.V.; Lü, J. miR-29 Is a Major Regulator of Genes Associated with Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2011, 45, 287–294. [Google Scholar] [CrossRef]
- Maurer, B.; Stanczyk, J.; Jüngel, A.; Akhmetshina, A.; Trenkmann, M.; Brock, M.; Kowal-Bielecka, O.; Gay, R.E.; Michel, B.A.; Distler, J.H.W.; et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010, 62, 1733–1743. [Google Scholar] [CrossRef]
- Du, B.; Ma, L.-M.; Huang, M.-B.; Zhou, H.; Huang, H.-L.; Shao, P.; Chen, Y.-Q.; Qu, L.-H. High glucose down-regulates miR-29a to increase collagen IV production in HK-2 cells. FEBS Lett. 2010, 584, 811–816. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Taylor, N.E.; Lu, L.; Usa, K.; Cowley, A.W.; Ferreri, N.R.; Yeo, N.C.; Liang, M. Renal Medullary MicroRNAs in Dahl Salt-Sensitive Rats. Hypertension 2010, 55, 974–982. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Li, Q.; Lian, X.; Zhu, Z.; Chen, X.; Pei, W.; Li, S.; Abbas, A.; Wang, Y.; Tian, L. MicroRNA-29b Mediates Lung Mesenchymal-Epithelial Transition and Prevents Lung Fibrosis in the Silicosis Model. Mol. Ther. Nucleic Acids 2019, 14, 20–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Xu, T.; Liu, Y.; Li, Y.; Yuan, J.; Yao, W.; Xu, Q.; Yan, W.; Ni, C. MiR-1224-5p mediates mitochondrial damage to affect silica-induced pulmonary fibrosis by targeting BECN1. Int. J. Mol. Sci. 2017, 18, 2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Liu, S.; Yuan, Z.-W.; Liu, J.-H.; Li, J.-M.; Chen, T.; Fang, K.-W. MicroRNA-27a Suppresses Detrusor Fibrosis in Streptozotocin-Induced Diabetic Rats by Targeting PRKAA2 Through the TGF-β1/Smad3 Signaling Pathway. Cell. Physiol. Biochem. 2018, 45, 1333–1349. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.; Tian, J.; Geng, J.; Li, X.; Tang, X.; Zhang, J.; Bai, X. MicroRNA-27a promotes renal tubulointerstitial fibrosis via suppressing PPARγ pathway in diabetic nephropathy. Oncotarget 2016, 7, 47760–47776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, X.; Chung, A.C.K.; Chen, H.-Y.; Meng, X.-M.; Lan, H.Y. Smad3-Mediated Upregulation of miR-21 Promotes Renal Fibrosis. J. Am. Soc. Nephrol. 2011, 22, 1668–1681. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Friggeri, A.; Yang, Y.; Milosevic, J.; Ding, Q.; Thannickal, V.J.; Kaminski, N.; Abraham, E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 2010, 207, 1589–1597. [Google Scholar] [CrossRef]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- McKelvey, K.J.; Powell, K.L.; Ashton, A.W.; Morris, J.M.; McCracken, S.A. Exosomes: Mechanisms of Uptake. J. Circ. Biomark. 2015, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.G.-E.E.; Cheng, K.; Marbán, E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2014, 2, 606–619. [Google Scholar] [CrossRef] [Green Version]
- Nojima, H.; Freeman, C.M.; Schuster, R.M.; Japtok, L.; Kleuser, B.; Edwards, M.J.; Gulbins, E.; Lentsch, A.B. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J. Hepatol. 2016, 64, 60–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz Ruiz, M.; Regueiro, J.R. New Tools in Regenerative Medicine: Gene Therapy. Adv. Exp. Med. Biol. 2012, 254–275. [Google Scholar] [CrossRef]
- Ishihara, A.; Bertone, A.L. Cell-mediated and direct gene therapy for bone regeneration. Expert Opin. Biol. Ther. 2012, 12, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Studeny, M.; Marini, F.C.; Champlin, R.E.; Zompetta, C.; Fidler, I.J.; Andreeff, M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-β delivery into tumors. Cancer Res. 2002, 62, 3603–3608. [Google Scholar]
- Nakamizo, A.; Marini, F.; Amano, T.; Khan, A.; Studeny, M.; Gumin, J.; Chen, J.; Hentschel, S.; Vecil, G.; Dembinski, J.; et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Dziak, R.; Mao, K.; Genco, R.; Swihart, M.; Li, C.; Yang, S. Integration of a novel injectable nano calcium sulfate/alginate scaffold and BMP2 gene-modified mesenchymal stem cells for bone regeneration. Tissue Eng. Part A 2013, 19, 508–518. [Google Scholar] [CrossRef] [Green Version]
- Bao, C.; Guo, J.; Lin, G.; Hu, M.; Hu, Z. TNFR gene-modified mesenchymal stem cells attenuate inflammation and cardiac dysfunction following MI. Scand. Cardiovasc. J. 2008. [Google Scholar] [CrossRef]
- Murphy, M.P.; Hartley, R.C. Mitochondria as a therapeutic target for common pathologies. Nat. Rev. Drug Discov. 2018, 17, 865–886. [Google Scholar] [CrossRef] [Green Version]


| miR | Target genes | Function | Reference |
|---|---|---|---|
| miR-1 | ↑ protein synthesis ↑ATP production | [69] | |
| EIF4E, Mef2a, Gata4, HDAC6 | Regulation of cardiac hypertrophy | [102,103] | |
| FABP3 | Heart enlargement and hypertrophy | [105] | |
| Fibulin-2 | ↓ TGFβ signaling ↓ extracellular matrix remodeling | [106] | |
| miR1/miR-133a | ↑ number of mitochondrial genesInfluence on mitochondria morphology | [70] | |
| ↑ cardiac stem cell differentiation | [86] | ||
| Let-7b | IGF-2 | ↓cell proliferation ↑ cell cycle arrest ↑ myofibroblast proliferation | [72] |
| Hmga2 | ↑ cell senescence | [98] | |
| miR-127 | S1P3 | ↑ cell differentiation | [73] |
| ATP5B | Control of bioenergetic cell pattern | [89] | |
| miR-125b | IGF-2 | ↓ stem cell differentiation | [75] |
| miR-128 | Sp1 | ↓ stem cell differentiation | [76] |
| miR-181c | COX1 | Altered mitochondrial metabolism and ROS generation | [79,80] |
| miR-181a | ↑ cell senescence | [99] | |
| miR-338 | Modulate COX-IV and subunits of the ATP synthase complex | [82,83] | |
| miR-378 | ATP6 | ↓ ATP synthase activity | [84] |
| IGF receptor 1 | ↑ apoptosis ↓ signaling in Akt cascade ↑ ROS generation | [87] | |
| miR-146-5p | ND1, ND2, ND4, ND5, ND6, ATP8, SOD3, Bcl-2 | ↑ ROS generation ↑ cell senescence | [71,92,93] |
| miR-762 | ND2 | ↓ intracellular ATP levels ↑ increased ROS production ↓ mitochondrial complex I enzyme activity | [95] |
| miR-19b, miR-20a, miR-17, miR-106 | Bcl-2 | ↑ permeability transition pore opening ↑ caspase-1 and 3 ↑ apoptosis | [96,97] |
| miR-133 | type 1 angiotensin II receptor, Cdc42, Rho-A and Nelf-A/WHSC2 | ↓ cardiac remodeling | [108,109] |
| miR-212/132 | Foxo3 | ↑ cardiac remodeling | [112] |
| miR-23a | Foxo3 | ↑ cardiac remodeling | [113] |
| miR-29a-3p | NFATc4 | ↓ hypertrophic response | [114] |
| miR-101 | TGFβRI and c-Fos | ↓ Extracellular matrix production ↓ fibroblast proliferation | [116,117] |
| miR-24 | furin | ↓ differentiation and migration of cardiac fibroblasts via TGFβ-smad2/3 | [120] |
| miR-29 family | Antifibrotic role | [120,121,122,123] | |
| miR-1224-3p | BECN1 | ↑ EMT ↓ gene expression of extracellular matrix-related genes | [125] |
| miR-21 | Smad7 | Pro-fibrotic effect | [128] |
| Spry1 | Pro-fibrotic effect | [130] | |
| miR-27a | TGFb | Pro-fibrotic effect | [126,127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, S.C.; Cardoso, R.M.S.; Duarte, F.V. Mitochondrial microRNAs: A Putative Role in Tissue Regeneration. Biology 2020, 9, 486. https://doi.org/10.3390/biology9120486
Rodrigues SC, Cardoso RMS, Duarte FV. Mitochondrial microRNAs: A Putative Role in Tissue Regeneration. Biology. 2020; 9(12):486. https://doi.org/10.3390/biology9120486
Chicago/Turabian StyleRodrigues, Sílvia C., Renato M. S. Cardoso, and Filipe V. Duarte. 2020. "Mitochondrial microRNAs: A Putative Role in Tissue Regeneration" Biology 9, no. 12: 486. https://doi.org/10.3390/biology9120486
APA StyleRodrigues, S. C., Cardoso, R. M. S., & Duarte, F. V. (2020). Mitochondrial microRNAs: A Putative Role in Tissue Regeneration. Biology, 9(12), 486. https://doi.org/10.3390/biology9120486







