Effects of Ascorbic Acid and ?-1,3-Glucan on Survival, Physiological Response and Flesh Quality of Cultured Tiger Grouper (Epinephelus fuscoguttatus) during Simulated Transport in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Tiger Grouper
2.2. Experimental Design
2.2.1. Experiment 1: Transport Temperature Determination
2.2.2. Experiment 2: Anti-Stress Agent Exposure
2.3. Serum Cortisol Assessment
2.4. Analysis of Enzymatic Activity
2.4.1. Metabolic and Antioxidant Enzyme Activities
2.4.2. Immunological Enzyme Activity
2.5. Real-Time PCR
2.6. Biochemical Analysis
2.6.1. Chemical Composition of Muscle
2.6.2. Serum Biochemical Testing
2.7. Nucleotides
2.8. Free Amino Acids (FAAs) Assessment
2.9. Statistical Analysis
3. Results and Discussion
3.1. Pre-Experiment: Selection of Tiger Grouper Transport Temperature and Ascorbic Acid Addition
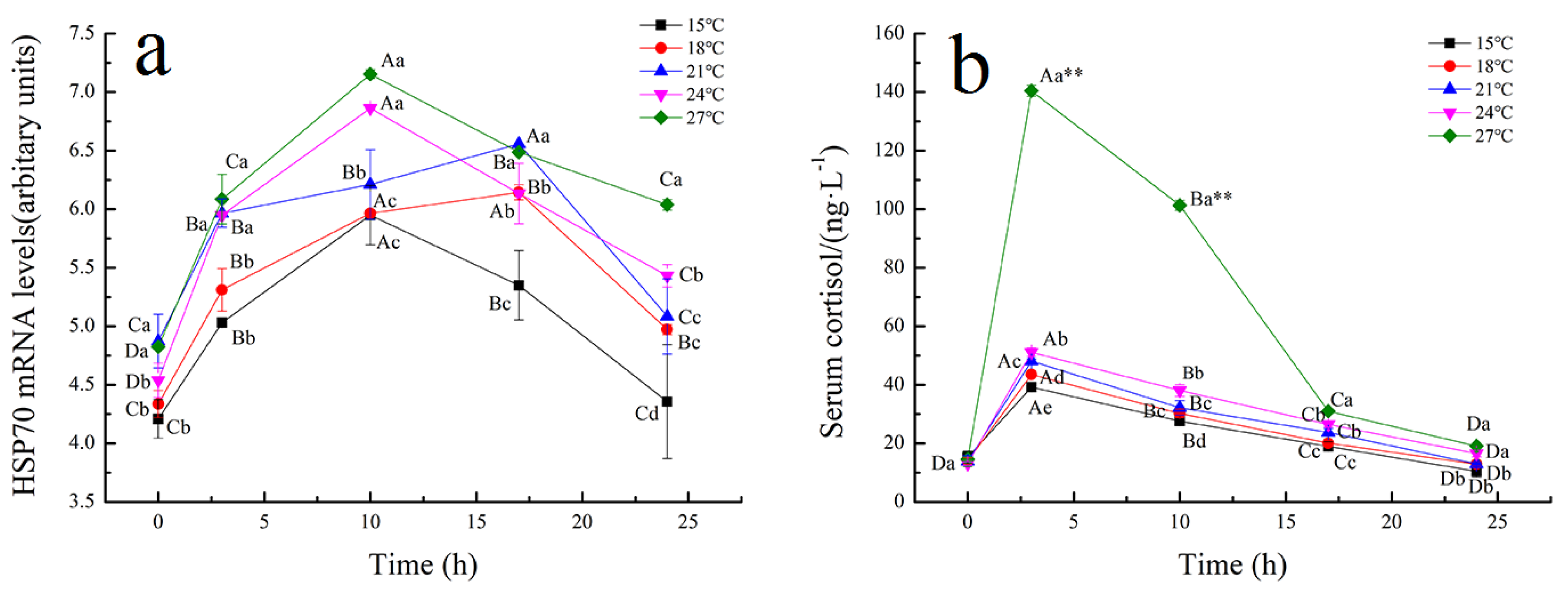
3.2. Effect of Temperature on Stress Responses of Tiger Grouper during Simulated Transport
3.3. Effect of Temperature on Antioxidant Enzyme of Tiger Grouper during Simulated Transport
3.4. Effect of Temperature on Metabolic and Immune Enzyme Activity of Tiger Grouper during Simulated Transport
3.5. Effect of Temperature on the Relative Expression of Immune Indexes of Tiger Grouper during Simulated Transport
3.6. Effect of Ascorbic Acid and β-1,3-Glucan Addition on Stress Responses of Tiger Grouper during Simulated Transport
3.7. Effect of Ascorbic Acid and β-1,3-Glucan Addition on Relative Expression of Non-Specific Immune Indexes of Tiger Grouper during Simulated Transport
3.8. Effect of Ascorbic Acid and β-1,3-Glucan Addition on Serum Biochemical Parameters of Tiger Grouper during Simulated Transport
3.9. Effect of Ascorbic Acid and β-1,3-Glucan Addition on Nutritional Indexes of Tiger Grouper during Simulated Transport
3.10. Effect of Ascorbic Acid and β-1,3-Glucan Addition on Free Amino Acids of Tiger Grouper during Simulated Transport
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Hoseinifar, S.H.; Sun, Y.; Wang, A.; Zhou, Z.J. Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front. Microbiol. 2018, 9, 2429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Steele, J.C.; Meng, X.Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Deng, M.; Wang, Q.; Yang, Y.; Yang, Y.; Nie, X. Residues and health risk assessment of quinolones and sulfonamides in cultured fish from Pearl River Delta, China. Aquaculture 2016, 458, 38–46. [Google Scholar] [CrossRef]
- Lee, M.K.; Nam, J. The determinants of live fish consumption frequency in South Korea. Food Res. Int. 2019, 120, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Purbosari, N.; Warsiki, E.; Syamsu, K.; Santoso, J. Natural versus synthetic anesthetic for transport of live fish: A review. Aquac. Fish. 2019, 4, 129–133. [Google Scholar] [CrossRef]
- Amar, E.C.; Apines-Amar, M.J.S.; Faisan, J.P. Dietary onion or ginger modulates the stress response and susceptibility to Vibrio harveyi JML1 infection in brown-marbled grouper (Epinephelus fuscoguttatus) juveniles. J. Aquat. Anim. Health 2017, 30, 39–49. [Google Scholar] [CrossRef]
- Afero, F.; Miao, S.; Perez, A.A. Economic analysis of tiger grouper (Epinephelus fuscoguttatus) and humpback grouper Cromileptesaltivelis commercial cage culture in Indonesia. Aquacult. Int. 2010, 18, 725–739. [Google Scholar] [CrossRef]
- Noor, N.M.; Defoirdt, T.; Alipiah, N.; Karim, M.; Daud, H.; Natrah, I. Quorum sensing is required for full virulence of Vibrio campbellii towards tiger grouper (Epinephelus fuscoguttatus) larvae. J. Fish Dis. 2019, 42, 489–495. [Google Scholar] [CrossRef]
- Fui, C.F.; Miura, A.; Nakagawa, Y.; Kato, K.; Sakamoto, W.; Takii, K.; Miyashita, S.; Senoo, S. Aeration rate adjustment at night to prevent sinking syndrome-related death in the tiger grouper Epinephelus fuscoguttatus (Perciformes: Serranidae) larvae. Aquac. Res. 2016, 47, 165–175. [Google Scholar] [CrossRef]
- Manuel, R.; Boerrigter, J.; Roques, J.; van der Heul, J.; van den Bos, R.; Flik, G.; van de Vis, H. Stress in African catfish (Clarias gariepinus) following overland transportation. Fish Physiol. Biochem. 2014, 40, 33–44. [Google Scholar] [CrossRef]
- Iversen, M.H.; Eliassen, R.A. The effect of allostatic load on hypothalamic-pituitary-interrenal (HPI) axis before and after secondary vaccination in Atlantic salmon postsmolts (Salmo salar L.). Fish Physiol. Biochem. 2014, 40, 527–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacchi, L.; Lowrey, L.; Musharrafieh, R.; Crossey, K.; Larragoite, E.T.; Salinas, I. Effects of transportation stress and addition of salt to transport water on the skin mucosal homeostasis of rainbow trout (Oncorhynchus mykiss). Aquaculture 2015, 435, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bricknell, I.; Dalmo, R.A. The use of immunostimulants in fish larval aquaculture. Fish Shellfish Immun. 2005, 19, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Salbego, J.; Becker, A.G.; Gonçalves, J.F.; Menezes, C.C.; Heldwein, C.G.; Spanevello, R.M.; Loro, V.L.; Schetinger, M.R.C.; Morsch, V.M.; Heinzmann, B.M. The essential oil from Lippia alba induces biochemical stress in the silver catfish (Rhamdia quelen) after transportation. Neotrop. Ichthyol. 2014, 12, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Souza, D.M.; Martins, Á.C.; Jensen, L.; Monserrat, J.M.; Wasielesky, W., Jr.; Garcia, L. Effects of water temperature on oxidative stress parameters in the pink shrimp Farfantepenaeus brasiliensis during transport. Aquaculture 2013, 416, 310–314. [Google Scholar] [CrossRef]
- Wu, S.M.; Tseng, Y.J.; Lin, J.J.; Pan, B.S. Mitigation of stress and water deterioration with a root extract of Glycine tomentella during simulated transport of orange-spotted grouper (Epinephelus coioides). Aquaculture 2020, 514, 734485. [Google Scholar] [CrossRef]
- Taheri Mirghaed, A.; Ghelichpour, M. Effects of anesthesia and salt treatment on stress responses, and immunological and hydromineral characteristics of common carp (Cyprinus carpio, Linnaeus, 1758) subjected to transportation. Aquaculture 2019, 501, 1–6. [Google Scholar] [CrossRef]
- Vilhena, C.S.; do Nascimento, L.A.S.; de Aguiar Andrade, E.H.; da Silva, J.K.d.R.; Hamoy, M.; Torres, M.F.; Barbas, L.A.L. Essential oil of Piper divaricatum induces a general anaesthesia-like state and loss of skeletal muscle tonus in juvenile tambaqui, Colosso mamacropomum. Aquaculture 2019, 510, 169–175. [Google Scholar] [CrossRef]
- De Oliveira, C.P.B.; Lemos, C.H.d.P.; Felix e Silva, A.; de Souza, S.A.; Albinati, A.C.L.; Lima, A.O.; Copatti, C.E. Use of eugenol for the anaesthesia and transportation of freshwater angelfish (Pterophyllum scalare). Aquaculture 2019, 513, 734409. [Google Scholar] [CrossRef]
- De Oliveira, C.P.B.; Lemos, C.H.d.P.; Vidal, L.V.O.; Couto, R.D.; Pereira, D.S.P.; Copatti, C.E. Anaesthesia with eugenol in hybrid Amazon catfish (Pseudoplatystoma reticulatum × Leiarius marmoratus) handling: Biochemical and haematological responses. Aquaculture 2019, 501, 255–259. [Google Scholar] [CrossRef]
- Pounder, K.C.; Mitchell, J.L.; Thomson, J.S.; Pottinger, T.G.; Sneddon, L.U. Physiological and behavioural evaluation of common anaesthesia practices in the rainbow trout. Appl. Anim. Behav. Sci. 2018, 199, 94–102. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, X.; Yan, L.; Thi Tuyet Nga, M.; Zhang, X. Survival prediction system for waterless live Chinese Sturgeon transportation based on temperature related glucose changes. J. Food Process Eng. 2018, 41, e12646. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, Z.J.B.; Equipment, B. Nrf2 is involved in osmoregulation, antioxidation and immunopotentiation in Coilia nasus under salinity stress. Biotechnol. Biotechnol. Equip. 2019, 33, 1453–1463. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.L.; Ma, Q.; Sun, S.X.; Zhang, H.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y.J. Reduced oxidative stress increases acute cold stress tolerance in zebrafish. Comp. Biochem. Phys. 2019, 235, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Chen, T.; Shen, J. Effects of cold acclimation and storage temperature on crucian carp (Carassius auratusgi belio) in a waterless preservation. Fish Physiol. Biochem. 2014, 40, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Ye, C.X.; Guo, Z.X.; Wang, A.L. Immune and physiological responses of pufferfish (Takifugu obscurus) under cold stress. Fish Shellfish Immunol. 2017, 64, 137–145. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Yan, L.; Glamuzina, B.; Zhang, X. Development and evaluation of an intelligent traceability system for waterless live fish transportation. Food Control 2019, 95, 283–297. [Google Scholar] [CrossRef]
- Peng, S.; Shi, Z.; Fei, Y.; Gao, Q.; Sun, P.; Wang, J.J. Effect of high-dose vitamin C supplementation on growth, tissue ascorbic acid concentrations and physiological response to transportation stress in juvenile silver pomfret, Pampus argenteus. J. Appl. Ichthyol. 2013, 29, 1337–1341. [Google Scholar] [CrossRef]
- Balamurugan, J.; Kumar, T.T.A.; Prakash, S.; Meenakumari, B.; Balasundaram, C.; Harikrishnan, R.J. Clove extract: A potential source for stress free transport of fish. Aquaculture 2016, 454, 171–175. [Google Scholar] [CrossRef]
- Roosta, Z.; Hajimoradloo, A.; Ghorbani, R.; Hoseinifar, S.H. The effects of dietary vitamin C on mucosal immune responses and growth performance in Caspian roach (Rutilus rutilus caspicus) fry. Fish Physiol. Biochem. 2014, 40, 1601–1607. [Google Scholar] [CrossRef]
- Wan, J.; Ge, X.; Liu, B.; Xie, J.; Cui, S.; Zhou, M.; Xia, S.; Chen, R.J. Effect of dietary vitamin C on non-specific immunity and mRNA expression of three heat shock proteins (HSPs) in juvenile Megalobrama amblycephala under pH stress. Aquaculture 2014, 434, 325–333. [Google Scholar] [CrossRef]
- Chettri, J.K.; Kania, P.W.; Buchmann, K. Immunomodulation of rainbow trout (Oncorhynchus mykiss) fry by bath exposure to a β-glucan from Euglena gracilis. Aquac. Res. 2013, 44, 1407–1415. [Google Scholar] [CrossRef]
- Gopalakannan, A.; Arul, V. Enhancement of the innate immune system and disease-resistant activity in Cyprinus carpio by oral administration of β-glucan and whole cell yeast. Aquac. Res. 2010, 41, 662–670. [Google Scholar] [CrossRef]
- Kumari, J.; Sahoo, P.K.J. Dietary β-1,3 glucan potentiates innate immunity and disease resistance of Asian cat fish, Clariasbatrachus (L.). J. Fish Dis. 2006, 29, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yu, P.; Lin, L.; Li, L. Effects of dietary β-1,3-glucan, chitosan or raffinose on the growth, innate immunity and resistance of koi (Cyprinus carpio koi). Fish Shellfish Immunol. 2011, 31, 794. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.C.; Buentello, J.A.; Lii, D.M.G. Effects of dietary prebiotics on growth performance, immune response and intestinal morphology of red drum (Sciaenops ocellatus). Aquaculture 2010, 309, 253–257. [Google Scholar] [CrossRef]
- Dong, C.; Wang, J. Immunostimulatory effects of dietary fructooligosaccharides on red swamp crayfish, Procambarusclarkii (Girard). Aquaculture 2013, 44, 1416–1424. [Google Scholar] [CrossRef]
- Fan, X.; Qin, X.; Zhang, C.; Zhu, Q.; Chen, J.; Chen, P.J. Metabolic and anti-oxidative stress responses to low temperatures during the waterless preservation of the hybrid grouper (Epinephelus fuscogutatus♀ × Epinephelus lanceolatus♂). Aquaculture 2019, 508, 10–18. [Google Scholar] [CrossRef]
- Seunghan, L.; Kumar, K.; Ali, H.; Jeongwhui, H.; Dae-Jung, K.; Bai, S.C. Synergistic effects of dietary supplementation of Bacillus subtilis WB60 and mannanoligosaccharide (MOS) on growth performance, immunity and disease resistance in Japanese eel, Anguilla japonica. Fish Shellfish Immunol. 2018, 83, 283–291. [Google Scholar]
- Ayanda, I.O.; Ekhator, U.I.; Bello, O.A. Determination of selected heavy metal and analysis of proximate composition in some fish species from Ogun River, Southwestern Nigeria. Heliyon 2019, 5, e02512. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Gao, Y.; Chen, X.; Huang, B. Determination of optimal fasting time before blood sampling to get baseline data on serum biochemical characteristics in juvenile turbot (Scophthalmus maximus). Aquaculture 2018, 487, 83–88. [Google Scholar] [CrossRef]
- Fang, S.; Zhou, Q.; Hu, Y.; Liu, F.; Mei, J.; Xie, J. Antimicrobial carvacrol incorporated in flaxseed gum-sodium alginate active films to improve the quality attributes of Chinese sea bass (Lateolabrax maculatus) during cold storage. Molecules 2019, 24, 3292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Li, P.; Fang, S.; Liu, W.; Mei, J.; Xie, J. Preservative effects of gelatin active coating enriched with eugenol emulsion on Chinese seabass (Lateolabrax maculatus) during superchilling (−0.9 °C) storage. Coatings 2019, 9, 489. [Google Scholar] [CrossRef] [Green Version]
- Kraitavin, W.; Yoshitake, K.; Igarashi, Y.; Mitsuyama, S.; Kinoshita, S.; Kambayashi, D.; Watabe, S.; Asakawa, S. Transcriptome analysis of yamame (Oncorhynchus masou) in normal conditions after heat stress. Biology 2019, 8, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eid, I.I.; Bhassu, S.; Goh, Z.H.; Khoo, L.T.; Tan, G.Y.A. Molecular characterization and gene evolution of the heat shock protein 70 gene in snakehead fish with different tolerances to temperature. Biochem. Syst. Ecol. 2016, 66, 137–144. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, B.; Ge, X.; Xie, J.; Xu, P.J. Effect of dietary carbohydrate on the growth performance, immune response, hepatic antioxidant abilities and heat shock protein 70 expression of Wuchang bream, Megalobrama amblycephala. J. Appl. Ichthyol. 2013, 29, 1348–1356. [Google Scholar] [CrossRef]
- Aedo, J.E.; Zuloaga, R.; Boltaña, S.; Molina, A.; Valdés, J.A. Membrane-initiated cortisol action modulates early pyruvate dehydrogenase kinase 2 (pdk2) expression in fish skeletal muscle. Comp. Biochem. Phys. 2019, 233, 24–29. [Google Scholar] [CrossRef]
- Song, J.; Brill, R.W.; McDowell, J.R. Plasticity in standard and maximum aerobic metabolic rates in two populations of an estuarine dependent teleost, spotted seatrout (Cynoscion nebulosus). Biology 2019, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Azambuja, C.R.; Mattiazzi, J.; Riffel, A.P.K.; Finamor, I.A.; de Oliveira Garcia, L.; Heldwein, C.G.; Heinzmann, B.M.; Baldisserotto, B.; Pavanato, M.A.; Llesuy, S.F. Effect of the essential oil of Lippia alba on oxidative stress parameters in silver catfish (Rhamdia quelen) subjected to transport. Aquaculture 2011, 319, 156–161. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Bagnyukova, T.V. Effects of different environmental oxygen levels on free radical processes in fish. Comp. Biochem. Phys. B 2006, 144, 283–289. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlahogianni, T.; Dassenakis, M.; Scoullos, M.J. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol. Environ. Saf. 2006, 64, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Paital, B.; Chainy, G.B.N. Antioxidant defenses and oxidative stress parameters in tissues of mud crab (Scylla serrata) with reference to changing salinity. Comp. Biochem. Phys. 2010, 151, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, M.; Wang, R.; Qian, Y. Effects of acute ammonia toxicity on oxidative stress, immune response and apoptosis of juvenile yellow catfish Pelteobagrus fulvidraco and the mitigation of exogenous taurine. Fish Shellfish Immunol. 2018, 79, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liang, X.; Zhang, Y.; Li, Y.; Cao, X.; Gao, J. Cloning of three heat shock protein genes (HSP70, HSP90α and HSP90β) and their expressions in response to thermal stress in loach (Misgurnus anguillicaudatus) fed with different levels of vitamin C. Fish Shellfish Immunol. 2017, 66, 103–111. [Google Scholar] [CrossRef]
- Dong, J.; Cheng, R.; Yang, Y.; Zhao, Y.; Wu, G.; Zhang, R.; Zhu, X.; Li, L.; Li, X. Effects of dietary taurine on growth, non-specific immunity, anti-oxidative properties and gut immunity in the Chinese mitten crab Eriocheir sinensis. Fish Shellfish Immunol. 2018, 82, 212–219. [Google Scholar] [CrossRef]
- Chen, Q.X.; Zheng, W.Z.; Lin, J.Y.; Shi, Y.; Xie, W.Z.; Zhou, H.M. Effect of metal ions on the activity of green crab (Scylla serrata) alkaline phosphatase. Int. J. Biochem. Cell B 2000, 32, 879–885. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.; Xu, W.; Mai, K.; Zhang, Y.; Liufu, Z. Effects of potential probiotic Bacillus subtilis T13 on growth, immunity and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2012, 32, 750–755. [Google Scholar] [CrossRef]
- Jia, R.; Liu, B.L.; Feng, W.R.; Han, C.; Huang, B.; Lei, J.L. Stress and immune responses in skin of turbot (Scophthalmus maximus) under different stocking densities. Fish Shellfish Immunol. 2016, 55, 131–139. [Google Scholar] [CrossRef]
- Ma, J.; Bu, Y.; Li, X.J. Immunological and histopathological responses of the kidney of common carp (Cyprinus carpio L.) sublethally exposed to glyphosate. Environ. Toxicol. Pharm. 2015, 39, 1–8. [Google Scholar] [CrossRef]
- Chen, X.X.; Guo, Z.; Jin, Q.; Qiao, S.; Li, R.; Li, X.; Deng, R.; Feng, W.H.; Zhang, G.P.J. Porcine reproductive and respiratory syndrome virus induces interleukin-1β through MyD88/ERK/AP-1 and NLRP3 inflammasome in microglia. Vet. Microbiol. 2018, 227, 82–89. [Google Scholar] [CrossRef]
- Klaper, R.; Arndt, D.; Setyowati, K.; Chen, J.; Goetz, F.J. Functionalization impacts the effects of carbon nanotubes on the immune system of rainbow trout, Oncorhynchus mykiss. Aqua. Toxicol. 2010, 100, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Lauriano, E.; Pergolizzi, S.; Capillo, G.; Kuciel, M.; Alesci, A.; Faggio, C. Immunohistochemical characterization of Toll-like receptor 2 in gut epithelial cells and macrophages of goldfish Carassius auratus fed with a high-cholesterol diet. Fish Shellfish Immunol. 2016, 59, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.; Takemura, A.; Tsuchiya, M.; Nakamura, S. Impact of different environmental factors on the circulating immunoglobulin levels in the Nile tilapia, Oreochromis niloticus. Aquaculture 2004, 241, 491–500. [Google Scholar] [CrossRef]
- Henrique, M.M.F.; Gomes, E.F.; Gouillou-Coustans, M.F.; Oliva-Teles, A.; Davies, S.J. Influence of supplementation of practical diets with vitamin C on growth and response to hypoxic stress of seabream, Sparus aurata. Aquaculture 2013, 161, 415–426. [Google Scholar] [CrossRef]
- Barros, M.M.; Falcon, D.R.; Ricardo, D.O.O. Non-specific immune parameters and physiological response of Nile tilapia fed β-glucan and vitamin C for different periods and submitted to stress and bacterial challenge. Fish Shellfish Immunol. 2014, 39, 188–195. [Google Scholar] [CrossRef]
- Steinbach, C.; Burkina, V.; Schmidt-Posthaus, H.; Stara, A.; Kolarova, J. Effect of the human therapeutic drug diltiazem on the haematological parameters, histology and selected enzymatic activities of rainbow trout Oncorhynchus mykiss. Chemosphere 2016, 157, 57–64. [Google Scholar] [CrossRef]
- Andreeva, A.M. The strategies of organization of the fish plasma proteome: With and without albumin. Russ. J. Mar. Biol. 2019, 45, 263–274. [Google Scholar] [CrossRef]
- Yancey, P.H. Nitrogen compounds as osmolytes. Fish Physiol. 2001, 20, 309–341. [Google Scholar]
- Osako, K.; Fujii, A.; Ruttanapornvareesakul, Y.; Nagano, N.; Kuwahara, K.; Okamoto, A. Differences in free amino acid composition between testis and ovary of sea urchin Anthocidarisc rassispina during gonadal development. Fish. Sci. 2007, 73, 660–667. [Google Scholar] [CrossRef]
- Hong, H.; Regenstein, J.M.; Luo, Y. The importance of ATP-related compounds for the freshness and flavor of post-mortem fish and shellfish muscle: A review. Crit. Rev. Food Sci. 2017, 57, 1787–1798. [Google Scholar] [CrossRef]
- Chen, D.W.; Zhang, M.J. Non-volatile taste active compounds in the meat of Chinese mitten crab (Eriocheir sinensis). Food Chem. 2007, 104, 1200–1205. [Google Scholar] [CrossRef]







| Samples | Anti-Stress Agent Addition |
|---|---|
| CK | Control |
| A | 25 mg/L ascorbic acid |
| A-G1 | 25 mg/L ascorbic acid + 2.4 mg/L β-1,3-glucan |
| A-G2 | 25 mg/L ascorbic acid + 3.2 mg/L β-1,3-glucan |
| A-G3 | 25 mg/L ascorbic acid + 4.0 mg/L β-1,3-glucan |
| Target Gene | Primer Sequence (5′-3′) |
|---|---|
| HSP70 | F: GACAAGAAGGTTGGGTCTGAAAGG |
| R: GGTTGACCATGCGGTTGTCGAAATCT | |
| IgM | F: GCCTCAGCGTCCTTCAGTTT |
| R: TGGCGTCCCAGTCCTGTTTGC | |
| IL-1β | F: AGGATGCCTGAGGGACTG |
| R: GGTAATCGTCTCCAGATGTAA |
| Temperature/°C | Keeping Alive Time/h | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 10 | 17 | 24 | 48 | 72 | |
| 10 | 100 | - | - | - | - | - | - |
| 13 | 100 | 100 | 100 | 100 | 85 | 65 | - |
| 15 | 100 | 100 | 100 | 100 | 100 | 100 | 95 |
| 18 | 100 | 100 | 100 | 100 | 100 | 100 | 90 |
| 21 | 100 | 100 | 100 | 100 | 100 | 95 | 85 |
| 24 | 100 | 100 | 100 | 100 | 100 | 85 | 80 |
| 27 | 100 | 100 | 100 | 100 | 100 | 85 | 75 |
| 30 | 100 | 100 | 100 | 100 | 90 | 50 | - |
| Transport | Samples | Creatine Kinase | Albumin | Total Protein | Uric Acid | Urea | Creatinine |
|---|---|---|---|---|---|---|---|
| Before transport | CK | 848.50 ± 0.25a | 11.00 ± 0.00a | 41.50 ± 0.00a | 13.00 ± 0.57b | 2.50 ± 0.12a | 17.00 ± 0.00a |
| A | 765.00 ± 0.13b | 7.00 ± 0.23c | 33.50 ± 0.71b | 13.50 ± 0.23b | 2.15 ± 0.16a | 18.00 ± 0.31a | |
| A-G1 | 227.00 ± 0.66c | 9.00 ± 0.16b | 32.00 ± 0.36b | 20.00 ± 0.06a | 2.35 ± 0.11a | 13.00 ± 0.06c | |
| A-G2 | 235.00 ± 0.57c | 7.50 ± 0.03c | 31.00 ± 0.00b | 21.00 ± 0.71a | 2.05 ± 0.08a | 13.00 ± 0.06c | |
| A-G3 | 221.00 ± 0.08c | 10.50 ± 0.00b | 32.00 ± 0.08b | 19.00 ± 0.35a | 2.10 ± 0.06a | 15.50 ± 0.24b | |
| After transport | CK | 1986.00 ± 0.58a | 26.00 ± 0.21a | 35.00 ± 0.32b | 14.00 ± 0.03c | 2.25 ± 0.00a | 18.50 ± 0.17a |
| A | 1181.50 ± 0.97b | 23.50 ± 0.00b | 31.00 ± 0.06c | 17.00 ± 0.21b | 2.05 ± 0.28a | 19.50 ± 0.00a | |
| A-G1 | 689.50 ± 0.69d | 12.00 ± 0.36c | 39.50 ± 0.00a | 21.50 ± 0.19a | 2.15 ± 0.14a | 14.00 ± 0.00c | |
| A-G2 | 391.50 ± 0.73e | 8.00 ± 0.42d | 33.00 ± 0.57c | 21.87 ± 0.09a | 1.94 ± 0.03a | 13.50 ± 0.25c | |
| A-G3 | 888.00 ± 0.29c | 12.50 ± 0.00c | 32.50 ± 0.69c | 22.05 ± 0.15a | 2.05 ± 0.00a | 17.00 ± 0.14b | |
| Recovery | CK | 273.00 ± 0.93c | 10.00 ± 0.32b | 38.50 ± 0.53c | 16.00 ± 0.32b | 2.35 ± 0.00a | 11.50 ± 0.27d |
| A | 769.00 ± 0.85a | 6.50 ± 0.31c | 46.00 ± 0.33a | 16.50 ± 0.31b | 2.40 ± 0.21a | 20.00 ± 0.32a | |
| A-G1 | 267.50 ± 0.23c | 13.00 ± 0.13a | 43.50 ± 0.00b | 17.00 ± 0.13b | 2.30 ± 0.18a | 18.00 ± 0.00b | |
| A-G2 | 225.00 ± 0.87d | 6.50 ± 0.19c | 32.50 ± 0.00d | 16.50 ± 0.19b | 2.30 ± 0.05a | 14.00 ± 0.00c | |
| A-G3 | 390.50 ± 0.25b | 11.00 ± 0.22b | 34.50 ± 0.22d | 22.00 ± 0.22a | 2.25 ± 0.17a | 18.50 ± 0.19b |
| Transport | Samples | Free Amino Acids | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Asp * | Thr # | Ser # | Glu * | Gly # | Ala # | Val | Met | Ile | ||
| Before transport | CK | 1.84 ± 0.25a | 7.12 ± 0.25c | 3.98 ± 0.17b | 4.06 ± 0.22b | 49.40 ± 0.49d | 38.22 ± 0.71a | 5.45 ± 0.31a | 2.96 ± 0.26a | 4.73 ± 0.34a |
| A | 1.76 ± 0.01a | 11.44 ± 0.06b | 4.87 ± 0.25a | 4.89 ± 0.71a | 86.09 ± 0.91a | 34.33 ± 0.59b | 4.30 ± 0.51b | 2.07 ± 0.21a | 3.01 ± 0.22b | |
| A-G1 | 1.65 ± 0.26a | 10.93 ± 0.18b | 3.77 ± 0.17b | 5.03 ± 0.14a | 75.24 ± 0.69b | 31.29 ± 0.06c | 5.98 ± 0.08a | 2.56 ± 0.18a | 3.97 ± 0.62a | |
| A-G2 | 1.79 ± 0.32a | 12.14 ± 0.21a | 4.05 ± 0.38b | 4.42 ± 0.61b | 71.25 ± 0.53b | 39.78 ± 0.71a | 5.09 ± 0.22a | 2.13 ± 0.11a | 4.17 ± 0.04a | |
| A-G3 | 1.84 ± 0.41a | 11.03 ± 0.01b | 4.97 ± 0.51a | 4.79 ± 0.25a | 66.42 ± 0.66c | 33.86 ± 0.81b | 4.97 ± 0.05a | 2.44 ± 0.01a | 3.57 ± 0.28b | |
| After transport | CK | 1.98 ± 0.03c | 5.87 ± 0.45c | 5.10 ± 0.05c | 8.57 ± 0.07a | 63.01 ± 0.21c | 42.56 ± 0.66b | 6.91 ± 0.06a | 3.32 ± 0.28a | 5.89 ± 0.41a |
| A | 3.37 ± 0.19a | 9.85 ± 0.06b | 7.62 ± 0.28a | 6.33 ± 0.21b | 124.35 ± 0.37a | 38.03 ± 0.08d | 5.13 ± 0.10b | 2.62 ± 0.01b | 2.50 ± 0.16c | |
| A-G1 | 2.38 ± 0.11b | 10.01 ± 0.32b | 7.99 ± 0.33a | 5.53 ± 0.03c | 75.44 ± 0.41b | 40.24 ± 0.19c | 6.02 ± 0.21a | 2.78 ± 0.06b | 3.11 ± 0.11b | |
| A-G2 | 1.93 ± 0.01c | 10.93 ± 0.22a | 6.46 ± 0.05b | 5.09 ± 0.10c | 78.03 ± 0.39b | 46.51 ± 0.73a | 5.67 ± 0.03b | 2.61 ± 0.01b | 3.55 ± 0.06b | |
| A-G3 | 1.70 ± 0.22c | 10.90 ± 0.06a | 5.48 ± 0.10c | 5.14 ± 0.19c | 74.29 ± 0.11b | 41.22 ± 0.37c | 5.13 ± 0.18b | 2.48 ± 0.12b | 3.71 ± 0.28b | |
| Recovery | CK | 1.82 ± 0.08a | 7.89 ± 0.18c | 4.77 ± 0.27a | 6.59 ± 0.47a | 58.98 ± 0.91c | 40.03 ± 0.57b | 6.84 ± 0.25a | 2.38 ± 0.18a | 4.84 ± 0.10a |
| A | 1.98 ± 0.10a | 11.19 ± 0.27a | 5.18 ± 0.27a | 5.94 ± 0.31b | 96.71 ± 0.43a | 35.28 ± 0.09d | 4.97 ± 0.33b | 2.46 ± 0.02a | 2.92 ± 0.16c | |
| A-G1 | 1.73 ± 0.02a | 10.52 ± 0.04b | 4.85 ± 0.03a | 4.96 ± 0.80c | 74.04 ± 0.09b | 37.90 ± 0.18c | 4.99 ± 0.09b | 2.10 ± 0.11a | 4.06 ± 0.71a | |
| A-G2 | 1.58 ± 0.16a | 11.88 ± 0.11a | 5.16 ± 0.18a | 4.83 ± 0.33c | 77.13 ± 0.36b | 41.64 ± 0.78a | 4.96 ± 0.18b | 1.92 ± 0.02a | 3.49 ± 0.39b | |
| A-G3 | 1.69 ± 0.22a | 11.85 ± 0.65a | 4.86 ± 0.20a | 4.31 ± 0.57c | 73.04 ± 0.74b | 37.49 ± 0.07c | 4.67 ± 0.21b | 2.23 ± 0.18a | 3.39 ± 0.27b | |
| Leu | Tyr | Phe | Lys | His | Arg | Pro# | Total | |||
| Before transport | CK | 7.52 ± 0.37a | 3.22 ± 0.02a | 2.88 ± 0.02a | 28.54 ± 0.54a | 3.86 ± 0.25a | 7.86 ± 0.71a | 6.27 ± 0.68a | 177.91 | |
| A | 7.73 ± 0.41a | 1.45 ± 0.01c | 1.57 ± 0.11c | 21.89 ± 0.48c | 3.55 ± 0.34b | 5.38 ± 0.42c | 5.49 ± 0.71b | 199.82 | ||
| A-G1 | 7.88 ± 0.68a | 1.73 ± 0.22b | 2.05 ± 0.18b | 25.33 ± 0.71b | 3.18 ± 0.33b | 6.93 ± 0.74bc | 5.93 ± 0.01a | 193.45 | ||
| A-G2 | 7.03 ± 0.31a | 1.98 ± 0.17b | 2.47 ± 0.17a | 26.09 ± 0.31b | 4.09 ± 0.06a | 6.41 ± 0.11b | 6.09 ± 0.39a | 198.98 | ||
| A-G3 | 7.97 ± 0.45a | 2.06 ± 0.28b | 1.99 ± 0.01c | 23.45 ± 0.78c | 3.74 ± 0.28a | 7.07 ± 0.02b | 5.77 ± 0.81ab | 185.94 | ||
| Aftertransport | CK | 7.83 ± 0.31a | 3.76 ± 0.28a | 3.46 ± 0.15a | 31.35 ± 0.59a | 4.30 ± 0.45a | 10.74 ± 0.33a | 8.18 ± 0.39a | 212.83 | |
| A | 7.76 ± 0.28a | 1.34 ± 0.01c | 2.28 ± 0.25b | 24.39 ± 0.63c | 3.63 ± 0.19b | 4.99 ± 0.41d | 6.07 ± 0.08c | 250.26 | ||
| A-G1 | 8.74 ± 0.41b | 1.43 ± 0.12c | 2.46 ± 0.06b | 29.39 ± 0.71b | 4.12 ± 0.06a | 6.63 ± 0.71c | 7.74 ± 0.73b | 214.01 | ||
| A-G2 | 7.01 ± 0.37a | 1.55 ± 0.08c | 2.49 ± 0.03b | 27.83 ± 0.39b | 4.23 ± 0.28a | 6.69 ± 0.07c | 6.41 ± 0.36c | 216.99 | ||
| A-G3 | 7.76 ± 0.63a | 2.38 ± 0.19b | 2.48 ± 0.11b | 24.87 ± 0.23c | 3.93 ± 0.09a | 7.98 ± 0.20b | 5.71 ± 0.51d | 205.16 | ||
| Recovery | CK | 6.82 ± 0.10b | 2.95 ± 0.01a | 1.79 ± 0.02b | 22.19 ± 0.33c | 3.60 ± 0.17a | 6.59 ± 0.38a | 5.19 ± 0.62c | 183.27 | |
| A | 6.98 ± 0.12b | 1.52 ± 0.02b | 1.98 ± 0.10ab | 22.75 ± 0.62c | 3.42 ± 0.31a | 5.21 ± 0.67b | 5.94 ± 0.17b | 214.43 | ||
| A-G1 | 7.65 ± 0.41a | 1.67 ± 0.13b | 2.30 ± 0.21a | 26.91 ± 0.15a | 2.75 ± 0.07b | 6.87 ± 0.37a | 6.79 ± 0.57a | 200.09 | ||
| A-G2 | 7.83 ± 0.57a | 1.86 ± 0.03b | 2.19 ± 0.09a | 24.50 ± 0.31b | 2.98 ± 0.35b | 6.61 ± 0.65a | 5.81 ± 0.08b | 204.37 | ||
| A-G3 | 7.23 ± 0.69a | 1.44 ± 0.02b | 2.26 ± 0.11a | 19.59 ± 0.09d | 3.64 ± 0.41a | 6.55 ± 0.52a | 4.94 ± 0.72c | 189.18 | ||
| Transport | Samples | IMP (mg/100 g) | TAV | AMP (mg/100 g) | TAV |
|---|---|---|---|---|---|
| Before transport | CK | 269.18 ± 0.78d | 10.77 | 13.24 ± 0.41ab | 0.26 |
| A | 273.40 ± 0.66c | 10.94 | 14.69 ± 0.59a | 0.29 | |
| A-G1 | 271.94 ± 0.96cd | 10.88 | 12.47 ± 0.65b | 0.25 | |
| A-G2 | 278.66 ± 0.55b | 11.15 | 13.93 ± 0.32ab | 0.28 | |
| A-G3 | 284.39 ± 0.47a | 11.38 | 13.54 ± 0.69ab | 0.27 | |
| Aftertransport | CK | 259.47 ± 0.84d | 10.38 | 9.55 ± 0.57b | 0.19 |
| A | 268.06 ± 0.28c | 10.72 | 11.23 ± 0.62ab | 0.22 | |
| A-G1 | 269.18 ± 0.71c | 10.77 | 11.37 ± 0.71ab | 0.23 | |
| A-G2 | 275.33 ± 0.49b | 11.01 | 12.99 ± 0.45a | 0.26 | |
| A-G3 | 280.91 ± 0.78a | 11.24 | 10.81 ± 0.66b | 0.22 | |
| Recovery | CK | 273.57 ± 0.67e | 10.94 | 14.43 ± 0.28a | 0.29 |
| A | 280.69 ± 0.71d | 11.23 | 10.98 ± 0.33c | 0.22 | |
| A-G1 | 301.42 ± 0.54c | 12.06 | 13.41 ± 0.19a | 0.27 | |
| A-G2 | 403.49 ± 0.66a | 16.14 | 12.79 ± 0.25b | 0.26 | |
| A-G3 | 390.24 ± 0.91b | 15.61 | 12.63 ± 0.36b | 0.25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Wang, Q.; Cao, J.; Mei, J.; Xie, J. Effects of Ascorbic Acid and ?-1,3-Glucan on Survival, Physiological Response and Flesh Quality of Cultured Tiger Grouper (Epinephelus fuscoguttatus) during Simulated Transport in Water. Biology 2020, 9, 37. https://doi.org/10.3390/biology9020037
Wu B, Wang Q, Cao J, Mei J, Xie J. Effects of Ascorbic Acid and ?-1,3-Glucan on Survival, Physiological Response and Flesh Quality of Cultured Tiger Grouper (Epinephelus fuscoguttatus) during Simulated Transport in Water. Biology. 2020; 9(2):37. https://doi.org/10.3390/biology9020037
Chicago/Turabian StyleWu, Bo, Qi Wang, Jie Cao, Jun Mei, and Jing Xie. 2020. "Effects of Ascorbic Acid and ?-1,3-Glucan on Survival, Physiological Response and Flesh Quality of Cultured Tiger Grouper (Epinephelus fuscoguttatus) during Simulated Transport in Water" Biology 9, no. 2: 37. https://doi.org/10.3390/biology9020037
APA StyleWu, B., Wang, Q., Cao, J., Mei, J., & Xie, J. (2020). Effects of Ascorbic Acid and ?-1,3-Glucan on Survival, Physiological Response and Flesh Quality of Cultured Tiger Grouper (Epinephelus fuscoguttatus) during Simulated Transport in Water. Biology, 9(2), 37. https://doi.org/10.3390/biology9020037







