The Efficacy of Sunitinib Treatment of Renal Cancer Cells Is Associated with the Protein PHAX In Vitro
Abstract
1. Introduction
2. Results
2.1. Regulatory Impact Factor Analysis
2.2. PHAX Protein Expression Is Increased in High Grade ccRCC Tumours As Compared to Adjacent Normal Kidney (NK) Cells
2.3. Sunitinib Induces PHAX Protein Expression in Tumour Cells and Vascular Endothelial Cells in ccRCC
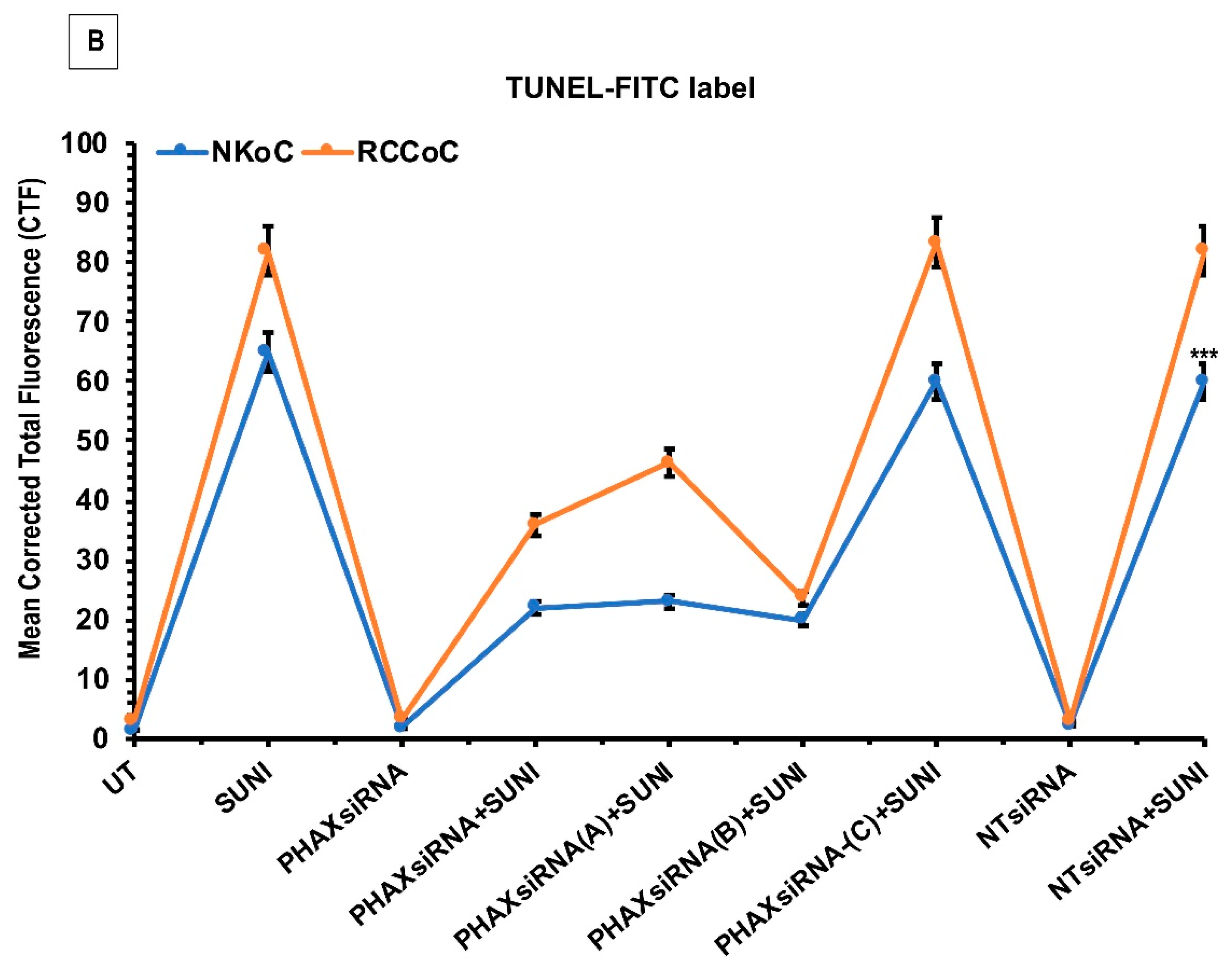
2.4. Sunitinib Induces Increased Cell Death in Tumour Cells and in Vascular Endothelial Cells in ccRCC Organ Culture
2.5. PHAX siRNA Knockdown Attenuates Sunitinib-Induced Cell Death in ccRCC Organ Culture
3. Discussion
4. Methods
4.1. Antibodies and Reagents
4.2. Tissue Collection
4.3. Nucleic Acid Extraction
4.4. Gene-Expression Analysis
4.5. Regulatory Impact Factor (RIF) Analysis
4.6. ccRCC and Adjacent Non-Tumour Kidney (NK) Organ Cultures
4.7. Immunohistochemical Staining for PHAX
4.8. Combined Immunofluorescence for PHAX and Cytokeratin or CD31
4.9. Cell Death and Cell Proliferation Assays
4.10. Transfection with Specific siRNAs in ccRCC Organ Cultures
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ccRCC | Clear cell renal cell carcinoma |
| mccRCC | Metastatic ccRCC |
| RIF | Regulatory impact factor |
| pH3S10 | Anti-phosphorylated histone H3S10 |
| TdT | Terminal transferase enzyme |
| siRNA | Short interfering RNA |
| DMSO | Dimethyl sulfoxide |
| DAB | Diaminobenzidine |
| CK | Cytokeratin |
| CTF | Corrected total fluorescence |
| MA | Minus average |
| DE | Differential expression |
| PIF | Phenotypic impact factor |
| TECs | Tubular epithelial cells |
| D-HSCORE | Digital histological score |
| NK | Normal kidney |
| CTF | Corrected total fluorescence |
| UT | Untreated |
References
- Kidney Cancer Incidence Statistics. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/kidney-cancer/incidence (accessed on 3 February 2018).
- Mitchell, T.J.; Rossi, S.H.; Klatte, T.; Stewart, G.D. Genomics and clinical correlates of renal cell carcinoma. World J. Urol. 2018, 36, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Renal Cell Carcinoma: ESMO Clinical Practice Guidelines | ESMO. Available online: https://www.esmo.org/Guidelines/Genitourinary-Cancers/Renal-Cell-Carcinoma (accessed on 13 January 2019).
- Huang, D.; Ding, Y.; Li, Y.; Luo, W.-M.; Zhang, Z.-F.; Snider, J.; Vandenbeldt, K.; Qian, C.-N.; Teh, B.T. Sunitinib acts primarily on tumor endothelium rather than tumor cells to inhibit the growth of renal cell carcinoma. Cancer Res. 2010, 70, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Joosten, S.C.; Hamming, L.; Soetekouw, P.M.; Aarts, M.J.; Veeck, J.; van Engeland, M.; Tjan-Heijnen, V.C. Resistance to sunitinib in renal cell carcinoma: From molecular mechanisms to predictive markers and future perspectives. Biochim. Biophys. Acta 2015, 1855, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, Y.; Akbani, R.; Ju, Z.; Roebuck, P.L.; Liu, W.; Yang, J.-Y.; Broom, B.M.; Verhaak, R.G.W.; Kane, D.W.; et al. TCPA: A resource for cancer functional proteomics data. Nat. Methods 2013, 10, 1046–1047. [Google Scholar] [CrossRef] [PubMed]
- Godovac-Zimmermann, J.; Brown, L.R. Perspectives for mass spectrometry and functional proteomics. Mass Spectrom. Rev. 2001, 20, 1–57. [Google Scholar] [CrossRef]
- Sidoli, S.; Kulej, K.; Garcia, B.A. Why proteomics is not the new genomics and the future of mass spectrometry in cell biology. J. Cell Biol. 2017, 216, 21–24. [Google Scholar] [CrossRef]
- Eden, E.; Navon, R.; Steinfeld, I.; Lipson, D.; Yakhini, Z. GOrilla: A tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics 2009, 10, 48. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Blais, A.; Dynlacht, B.D. Constructing transcriptional regulatory networks. Genes Dev. 2005, 19, 1499–1511. [Google Scholar] [CrossRef]
- Ohno, M.; Segref, A.; Bachi, A.; Wilm, M.; Mattaj, I.W. PHAX, a mediator of U snRNA nuclear export whose activity is regulated by phosphorylation. Cell 2000, 101, 187–198. [Google Scholar] [CrossRef]
- Al-Lamki, R.S.; Sadler, T.J.; Wang, J.; Reid, M.J.; Warren, A.Y.; Movassagh, M.; Lu, W.; Mills, I.G.; Neal, D.E.; Burge, J.; et al. Tumor necrosis factor receptor expression and signaling in renal cell carcinoma. Am. J. Pathol. 2010, 177, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Boulon, S.; Verheggen, C.; Jady, B.E.; Girard, C.; Pescia, C.; Paul, C.; Ospina, J.K.; Kiss, T.; Matera, A.G.; Bordonné, R.; et al. PHAX and CRM1 are required sequentially to transport U3 snoRNA to nucleoli. Mol. Cell 2004, 16, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, H.J.; Heyn, H.; Moutinho, C.; Esteller, M. CpG island hypermethylation-associated silencing of small nucleolar RNAs in human cancer. RNA Biol. 2012, 9, 881–890. [Google Scholar] [CrossRef]
- Hudson, N.J.; Reverter, A.; Dalrymple, B.P. A differential wiring analysis of expression data correctly identifies the gene containing the causal mutation. PLoS Comput. Biol. 2009, 5, e1000382. [Google Scholar] [CrossRef]
- Reverter, A.; Hudson, N.J.; Nagaraj, S.H.; Pérez-Enciso, M.; Dalrymple, B.P. Regulatory impact factors: unraveling the transcriptional regulation of complex traits from expression data. Bioinforma. Oxf. Engl. 2010, 26, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Kehl, T.; Schneider, L.; Kattler, K.; Stöckel, D.; Wegert, J.; Gerstner, N.; Ludwig, N.; Distler, U.; Schick, M.; Keller, U.; et al. REGGAE: A novel approach for the identification of key transcriptional regulators. Bioinformatics 2018, 34, 3503–3510. [Google Scholar] [CrossRef] [PubMed]
- Karam, J.A.; Devine, C.E.; Urbauer, D.L.; Lozano, M.; Maity, T.; Ahrar, K.; Tamboli, P.; Tannir, N.M.; Wood, C.G. Phase 2 Trial of Neoadjuvant Axitinib in Patients with Locally Advanced Nonmetastatic Clear Cell Renal Cell Carcinoma. Eur. Urol. 2014. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Stewart, G.D.; Riddick, A.C.P.; Rae, F.; Marshall, C.; MacLeod, L.; O’Mahony, F.C.; Laird, A.; Alan McNeill, S.; O’Connor, K.M.; O’Donnell, M.; et al. Translational research will fail without surgical leadership: SCOTRRCC a successful surgeon-led Nationwide translational research infrastructure in renal cancer. Surg. J. R. Coll. Surg. Edinb. Irel. 2015, 13, 181–186. [Google Scholar] [CrossRef]
- Stewart, G.D.; O’Mahony, F.C.; Laird, A.; Eory, L.; Lubbock, A.L.R.; Mackay, A.; Nanda, J.; O’Donnell, M.; Mullen, P.; McNeill, S.A.; et al. Sunitinib Treatment Exacerbates Intratumoral Heterogeneity in Metastatic Renal Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 4212–4223. [Google Scholar] [CrossRef]
- Srigley, J.R.; Delahunt, B.; Eble, J.N.; Egevad, L.; Epstein, J.I.; Grignon, D.; Hes, O.; Moch, H.; Montironi, R.; Tickoo, S.K.; et al. The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia. Am. J. Surg. Pathol. 2013, 37, 1469–1489. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Chen, X.-Q.; Zhang, M.-N.; Chen, N.; Nie, L.; Xu, M.; Gong, J.; Shen, P.-F.; Su, Z.-Z.; Weng, X.; et al. SOX9 was involved in TKIs resistance in renal cell carcinoma via Raf/MEK/ERK signaling pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 3871–3881. [Google Scholar] [PubMed]
- Al-Lamki, R.S.; Wang, J.; Tolkovsky, A.M.; Bradley, J.A.; Griffin, J.L.; Thiru, S.; Wang, E.C.Y.; Bolton, E.; Min, W.; Moore, P.; et al. TL1A both promotes and protects from renal inflammation and injury. J. Am. Soc. Nephrol. JASN 2008, 19, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, C.; Huang, Y.; Jia, Z.; Cao, J. A novel multikinase inhibitor R8 exhibits potent inhibition on cancer cells through both apoptosis and autophagic cell death. Oncotarget 2017, 8, 87209–87220. [Google Scholar] [CrossRef]
- Fuhrich, D.G.; Lessey, B.A.; Savaris, R.F. Comparison of HSCORE assessment of endometrial beta3 integrin subunit expression with digital HSCORE using computerized image analysis (ImageJ). Anal. Quant. Cytopathol. Histopathol. 2013, 35, 210–216. [Google Scholar]
- Al-Lamki, R.S.; Wang, J.; Vandenabeele, P.; Bradley, J.A.; Thiru, S.; Luo, D.; Min, W.; Pober, J.S.; Bradley, J.R. TNFR1- and TNFR2-mediated signaling pathways in human kidney are cell type-specific and differentially contribute to renal injury. FASEB J. 2005, 19, 1637–1645. [Google Scholar] [CrossRef]










| Probe | Gene | RIF1 Score | RIF2 Score | Combined RIF Score |
|---|---|---|---|---|
| ILMN_3260932 | LOC100130441 | −2.74 | 2.98 | 5.72 |
| ILMN_2190779 | PHAX | −2.37 | 3.18 | 5.55 |
| ILMN_2141030 | LOC641522 | −2.16 | 3.21 | 5.38 |
| ILMN_2110751 | CHRNA5 | −3.79 | 1.50 | 5.30 |
| ILMN_1787314 | ALS2CR14 | −2.99 | 2.28 | 5.27 |
| ILMN_2169839 | CNBP | −3.85 | 1.34 | 5.20 |
| ILMN_3179148 | LOC100128096 | −1.66 | 3.50 | 5.17 |
| ILMN_2053536 | RHBDL2 | −3.54 | 1.58 | 5.12 |
| ILMN_3279960 | LOC642784 | −3.04 | 2.03 | 5.08 |
| ILMN_1680774 | LOC730994 | −2.54 | 2.52 | 5.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Lamki, R.S.; Hudson, N.J.; Bradley, J.R.; Warren, A.Y.; Eisen, T.; Welsh, S.J.; Riddick, A.C.P.; O’Mahony, F.C.; Turnbull, A.; Powles, T.; et al. The Efficacy of Sunitinib Treatment of Renal Cancer Cells Is Associated with the Protein PHAX In Vitro. Biology 2020, 9, 74. https://doi.org/10.3390/biology9040074
Al-Lamki RS, Hudson NJ, Bradley JR, Warren AY, Eisen T, Welsh SJ, Riddick ACP, O’Mahony FC, Turnbull A, Powles T, et al. The Efficacy of Sunitinib Treatment of Renal Cancer Cells Is Associated with the Protein PHAX In Vitro. Biology. 2020; 9(4):74. https://doi.org/10.3390/biology9040074
Chicago/Turabian StyleAl-Lamki, Rafia S., Nicholas J. Hudson, John R. Bradley, Anne Y. Warren, Tim Eisen, Sarah J. Welsh, Antony C. P. Riddick, Fiach C. O’Mahony, Arran Turnbull, Thomas Powles, and et al. 2020. "The Efficacy of Sunitinib Treatment of Renal Cancer Cells Is Associated with the Protein PHAX In Vitro" Biology 9, no. 4: 74. https://doi.org/10.3390/biology9040074
APA StyleAl-Lamki, R. S., Hudson, N. J., Bradley, J. R., Warren, A. Y., Eisen, T., Welsh, S. J., Riddick, A. C. P., O’Mahony, F. C., Turnbull, A., Powles, T., SCOTRRCC Collaborative, Reverter, A., Harrison, D. J., & Stewart, G. D. (2020). The Efficacy of Sunitinib Treatment of Renal Cancer Cells Is Associated with the Protein PHAX In Vitro. Biology, 9(4), 74. https://doi.org/10.3390/biology9040074






