Oxidative Stress in Male Infertility: Causes, Effects in Assisted Reproductive Techniques, and Protective Support of Antioxidants
Abstract
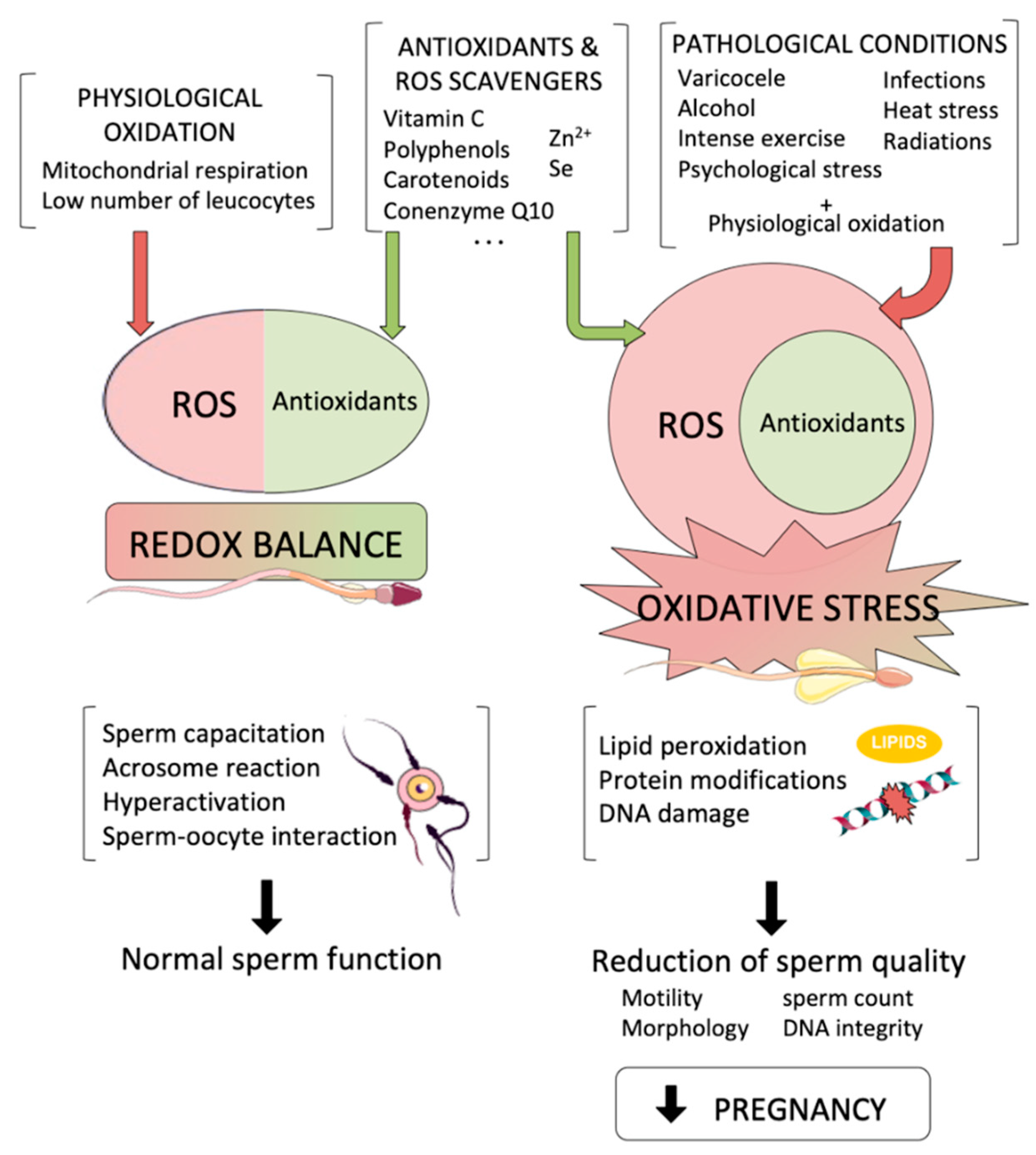
:1. Introduction
2. Sources of Oxidative Stress with Potential Effects to the Male Germline
2.1. Endogenous Sources of ROS
2.2. Exogenous Sources of ROS
2.2.1. Varicocele
2.2.2. Infections and Leucocytospermia
2.2.3. Alcohol and Tobacco
2.2.4. Physical Exercise and Heat Stress
2.2.5. Radiations and Pollution
3. Effects of Oxidative Stress to Sperm Components
3.1. Lipid Peroxidation
3.2. Protein Modifications
3.3. Sperm DNA Damage
4. Effects of Oxidative Stress on Fertility Treatments
4.1. Natural Pregnancy
4.2. Assisted Reproduction Techniques
4.2.1. Intrauterine Insemination
4.2.2. IVF and ICSI
5. Protection against Oxidative Damage and Designed Treatments for Fertility Improvement
5.1. Seminal Plasma
5.2. Varicocelectomy
5.3. Antioxidants
5.3.1. Use of Antioxidants for Natural Pregnancy
5.3.2. Use of Antioxidants for Intrauterine Insemination
5.3.3. Use of Antioxidants for IVF/ICSI
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Louis, J.F.; Thoma, M.E.; Sørensen, D.N.; McLain, A.C.; King, R.B.; Sundaram, R.; Keiding, N.; Buck Louis, G.M. The prevalence of couple infertility in the United States from a male perspective: Evidence from a nationally representative sample. Andrology 2013, 1, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Datta, J.; Palmer, M.J.; Tanton, C.; Gibson, L.J.; Jones, K.G.; Macdowall, W.; Glasier, A.; Sonnenberg, P.; Field, N.; Mercer, C.H.; et al. Prevalence of infertility and help seeking among 15000 women and men. Hum. Reprod. 2016, 31, 2108–2118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polis, C.B.; Cox, C.M.; Tunçalp, Ö.; McLain, A.C.; Thoma, M.E. Estimating infertility prevalence in low-to-middle-income countries: An application of a current duration approach to Demographic and Health Survey data. Hum. Reprod. 2017, 32, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, M.N.; Flaxman, S.R.; Boerma, T.; Vanderpoel, S.; Stevens, G.A. National, Regional, and Global Trends in Infertility Prevalence Since 1990: A Systematic Analysis of 277 Health Surveys. PLoS Med. 2012, 9, e1001356. [Google Scholar] [CrossRef]
- Bushnik, T.; Cook, J.L.; Yuzpe, A.A.; Tough, S.; Collins, J. Estimating the prevalence of infertility in Canada. Hum. Reprod. 2012, 27, 738–746. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [Green Version]
- Mehra, B.L.; Skandhan, K.P.; Prasad, B.S.; Pawankumar, G.; Singh, G.; Jaya, V. Male infertility rate: A retrospective study. Urologia 2018, 85, 22–24. [Google Scholar] [CrossRef]
- Vander Borght, M.; Wyns, C. Fertility and Infertility: Definition and Epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- Krausz, C.; Escamilla, A.R.; Chianese, C. Genetics of male infertility: From research to clinic. Reproduction 2015, 150, R159–R174. [Google Scholar] [CrossRef]
- Sharma, P.; Ghanghas, P.; Kaushal, N.; Kaur, J.; Kaur, P. Epigenetics and oxidative stress: A twin-edged sword in spermatogenesis. Andrologia 2019, 51, e13432. [Google Scholar] [CrossRef]
- Jodar, M.; Selvaraju, S.; Sendler, E.; Diamond, M.P.; Krawetz, S.A. Reproductive Medicine Network The presence, role and clinical use of spermatozoal RNAs. Hum. Reprod. Update 2013, 19, 604–624. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.F.S.; Østergren, P.; Dupree, J.M.; Ohl, D.A.; Sønksen, J.; Fode, M. Varicocele and male infertility. Nat. Rev. Urol. 2017, 14, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Soraggi, S.; Riera, M.; Rajpert-De Meyts, E.; Schierup, M.H.; Almstrup, K. Evaluating genetic causes of azoospermia: What can we learn from a complex cellular structure and single-cell transcriptomics of the human testis? Hum. Genet. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.D.; Sharma, R.; Henkel, R.; Agarwal, A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia 2018, 50, e13012. [Google Scholar] [CrossRef]
- Tosic, J.; Walton, A. Metabolism of spermatozoa. The formation and elimination of hydrogen peroxide by spermatozoa and effects on motility and survival. Biochem. J. 1950, 47, 199–212. [Google Scholar] [CrossRef] [Green Version]
- Lamirande, E.; Harakat, A.; Gagnon, C. Human Sperm Capacitation Induced by Biological Fluids and Progesterone, but Not by NADH or NADPH, Is Associated with the Production of Superoxide Anion. J. Androl. 1998, 19, 215–225. [Google Scholar]
- Herrero, M.B.; de Lamirande, E.; Gagnon, C. Nitric Oxide Regulates Human Sperm Capacitation and Protein-Tyrosine Phosphorylation in Vitro. Biol. Reprod. 1999, 61, 575–581. [Google Scholar] [CrossRef]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef]
- Griveau, J.F.; Lannou, D.L. Reactive oxygen species and human spermatozoa: Physiology and pathology. Int. J. Androl. 1997, 20, 61–69. [Google Scholar] [CrossRef]
- Camello-Almaraz, C.; Gomez-Pinilla, P.J.; Pozo, M.J.; Camello, P.J. Mitochondrial reactive oxygen species and Ca2+ signaling. Am. J. Physiol. Cell Physiol. 2006, 291, C1082–C10828. [Google Scholar] [CrossRef] [Green Version]
- Aitken, R.J.; Buckingham, D.W.; Carreras, A.; Irvine, D.S. Superoxide dismutase in human sperm suspensions: Relationship with cellular composition, oxidative stress, and sperm function. Free Radic. Biol. Med. 1996, 21, 495–504. [Google Scholar] [CrossRef]
- Said, T.M.; Agarwal, A.; Sharma, R.K.; Mascha, E.; Sikka, S.C.; Thomas, A.J. Human sperm superoxide anion generation and correlation with semen quality in patients with male infertility. Fertil. Steril. 2004, 82, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Trocchia, S.; Abdel-Gawad, F.K.; Ciarcia, G. Roles of Reactive Oxygen Species in the Spermatogenesis Regulation. Front. Endocrinol. 2014, 5, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzyściak, W. Activity of selected aromatic amino acids in biological systems. Acta Biochim. Pol. 2011, 58, 461–466. [Google Scholar] [CrossRef] [Green Version]
- Mehta, A.; Goldstein, M. Microsurgical varicocelectomy: A review. Asian J. Androl. 2013, 15, 56–60. [Google Scholar] [CrossRef] [Green Version]
- Kroese, A.C.; de Lange, N.M.; Collins, J.; Evers, J.L. Surgery or embolization for varicoceles in subfertile men. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef] [Green Version]
- Plante, M.; de Lamirande, E.; Gagnon, C. Reactive oxygen species released by activated neutrophils, but not by deficient spermatozoa, are sufficient to affect normal sperm motility. Fertil. Steril. 1994, 62, 387–393. [Google Scholar] [CrossRef]
- WHO. WHO Laboratory Manual for the Examination and Precesssing of Human Semen, 5th ed.; World Heal Organ Press: Geneva, Switzerland, 2010. [Google Scholar]
- Henkel, R. The impact of oxidants on sperm function. Andrologia 2005, 37, 205–206. [Google Scholar] [CrossRef]
- Lobascio, A.M.; De Felici, M.; Anibaldi, M.; Greco, P.; Minasi, M.G.; Greco, E. Involvement of seminal leukocytes, reactive oxygen species, and sperm mitochondrial membrane potential in the DNA damage of the human spermatozoa. Andrology 2015, 3, 265–270. [Google Scholar] [CrossRef]
- Seshadri, S.; Flanagan, B.; Vince, G.; Lewis Jones, D.I. Leucocyte subpopulations in the seminal plasma and their effects on fertilisation rates in an IVF cycle. Andrologia 2012, 44, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.A.; Agarwal, A.; Kandirali, E.; Sharma, R.K.; Thomas, A.J.; Nada, E.A.; Evenson, D.P.; Alvarez, J.G. Leukocytospermia is associated with increased reactive oxygen species production by human spermatozoa. Fertil. Steril. 2002, 78, 1215–1224. [Google Scholar] [CrossRef]
- Akang, E.N.; Oremosu, A.A.; Osinubi, A.A.; James, A.B.; Biose, I.J.; Dike, S.I.; Idoko, K.M. Alcohol-induced male infertility: Is sperm DNA fragmentation a causative? J. Exp. Clin. Anat. 2017, 16, 53. [Google Scholar]
- Manzo-Avalos, S.; Saavedra-Molina, A. Cellular and Mitochondrial Effects of Alcohol Consumption. Int. J. Environ. Res. Public Health 2010, 7, 4281–4304. [Google Scholar] [CrossRef] [Green Version]
- Rompala, G.R.; Homanics, G.E. Intergenerational Effects of Alcohol: A Review of Paternal Preconception Ethanol Exposure Studies and Epigenetic Mechanisms in the Male Germline. Alcohol. Clin. Exp. Res. 2019, 43, 1032–1045. [Google Scholar] [CrossRef]
- Zhu, Q.; Meisinger, J.; Emanuele, N.V.; Emanuele, M.A.; LaPaglia, N.; Van Thiel, D.H. Ethanol exposure enhances apoptosis within the testes. Alcohol. Clin. Exp. Res. 2000, 24, 1550–1556. [Google Scholar] [CrossRef]
- Koh, P.O.; Kim, M.O. Ethanol Exposure Decreases Cell Proliferation and Increases Apoptosis in Rat Testes. J. Vet. Med. Sci. 2006, 68, 1013–1017. [Google Scholar] [CrossRef] [Green Version]
- Sadeghzadeh, M.; Shirpoor, A.; Naderi, R.; Kheradmand, F.; Gharalari, F.H.; Samadi, M.; Khalaji, N.; Gharaaghaji, R. Long-term ethanol consumption promotes changes in β-defensin isoform gene expression and induces structural changes and oxidative DNA damage to the epididymis of rats. Mol. Reprod. Dev. 2019, 86, 624–631. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A. Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. Int. J. Dev. Biol. 2013, 57, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Sakkas, D.; Alvarez, J.G. Sperm DNA fragmentation: Mechanisms of origin, impact on reproductive outcome, and analysis. Fertil. Steril. 2010, 93, 1027–1036. [Google Scholar] [CrossRef]
- Aparicio, I.M.; Espino, J.; Bejarano, I.; Gallardo-Soler, A.; Campo, M.L.; Salido, G.M.; Pariente, J.A.; Peña, F.J.; Tapia, J.A. Autophagy-related proteins are functionally active in human spermatozoa and may be involved in the regulation of cell survival and motility. Sci. Rep. 2016, 6, 33647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jiang, M.; Bi, Y.; Zhu, H.; Zhou, Z.; Sha, J. Autophagy and Apoptosis Act as Partners to Induce Germ Cell Death After Heat Stress in Mice. PLoS ONE 2012, 7, e41412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, R.; Zahid, N.; Amjad, S.; Baig, M.; Gazzaz, Z.J. Relationship Between Smoking Habit and Sperm Parameters Among Patients Attending an Infertility Clinic. Front. Physiol. 2019, 10, 1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, J.-B.; Wang, Z.-X.; Qiao, Z.-D. The hazardous effects of tobacco smoking on male fertility. Asian J. Androl. 2015, 17, 954–960. [Google Scholar]
- Santos, M.; Rodríguez-González, G.L.; Ibáñez, C.; Vega, C.C.; Nathanielsz, P.W.; Zambrano, E. Adult exercise effects on oxidative stress and reproductive programming in male offspring of obese rats. Am. J. Physiol. Integr. Comp. Physiol. 2015, 308, R219–R225. [Google Scholar] [CrossRef] [Green Version]
- Vaamonde, D.; Da Silva-Grigoletto, M.E.; García-Manso, J.M.; Barrera, N.; Vaamonde-Lemos, R. Physically active men show better semen parameters and hormone values than sedentary men. Eur. J. Appl. Physiol. 2012, 112, 3267–3273. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Mendiola, J.; Afeiche, M.; Jørgensen, N.; Swan, S.H.; Chavarro, J.E. Physical activity and television watching in relation to semen quality in young men. Br. J. Sports Med. 2015, 49, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Eisenberg, M.L.; Kim, S.; Chen, Z.; Sundaram, R.; Schisterman, E.F.; Louis, G.M.B. The relationship between male BMI and waist circumference on semen quality: Data from the LIFE study. Hum. Reprod. 2015, 30, 493–494. [Google Scholar] [CrossRef] [Green Version]
- Hajizadeh Maleki, B.; Tartibian, B.; Chehrazi, M. The effects of three different exercise modalities on markers of male reproduction in healthy subjects: A randomized controlled trial. Reproduction 2017, 153, 157–174. [Google Scholar] [CrossRef] [Green Version]
- Mastaloudis, A.; Leonard, S.W.; Traber, M.G. Oxidative stress in athletes during extreme endurance exercise. Free Radic. Biol. Med. 2001, 31, 911–922. [Google Scholar] [CrossRef]
- Durairajanayagam, D.; Agarwal, A.; Ong, C. Causes, effects and molecular mechanisms of testicular heat stress. Reprod. Biomed. Online 2015, 30, 14–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parazzini, F.; Marchini, M.; Luchini, L.; Tozzi, L.; Mezzopane, R.; Fedele, L. Tight underpants and trousers and risk of dyspermia. Int. J. Androl. 1995, 18, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Agarwal, A.; Henkel, R. Radiations and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Nirala, J.P.; Behari, J.; Paulraj, R. Effect of electromagnetic irradiation produced by 3G mobile phone on male rat reproductive system in a simulated scenario. Indian J. Exp. Biol. 2014, 52, 890–897. [Google Scholar] [PubMed]
- Agarwal, A.; Desai, N.R.; Makker, K.; Varghese, A.; Mouradi, R.; Sabanegh, E.; Sharma, R. Effects of radiofrequency electromagnetic waves (RF-EMW) from cellular phones on human ejaculated semen: An in vitro pilot study. Fertil. Steril. 2009, 92, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Mailankot, M.; Kunnath, A.P.; Jayalekshmi, H.; Koduru, B.; Valsalan, R. Radio frequency electromagnetic radiation (RF-EMR) from GSM (0.9/1.8GHz) mobile phones induces oxidative stress and reduces sperm motility in rats. Clinics 2009, 64, 561–565. [Google Scholar] [CrossRef] [Green Version]
- Meena, R.; Kumari, K.; Kumar, J.; Rajamani, P.; Verma, H.N.; Kesari, K.K. Therapeutic approaches of melatonin in microwave radiations-induced oxidative stress-mediated toxicity on male fertility pattern of Wistar rats. Electromagn. Biol. Med. 2014, 33, 81–91. [Google Scholar] [CrossRef]
- Avendano, C.; Mata, A.; Sarmiento, C.A.S.; Doncel, G.F. Use of Laptop Computers Connected to Internet Through Wi-Fi Decreases Human Sperm Motility and Increases Sperm DNA Fragmentation. Fertil. Steril. 2012, 97, 39–44. [Google Scholar] [CrossRef]
- Kumar, S.; Behari, J.; Sisodia, R. Influence of electromagnetic fields on reproductive system of male rats. Int. J. Radiat. Biol. 2013, 89, 147–154. [Google Scholar] [CrossRef]
- Loseva, O.; Shubbar, E.; Haghdoost, S.; Evers, B.; Helleday, T.; Harms-Ringdahl, M. Chronic Low Dose Rate Ionizing Radiation Exposure Induces Premature Senescence in Human Fibroblasts that Correlates with up Regulation of Proteins Involved in Protection against Oxidative Stress. Proteomes 2014, 2, 341–362. [Google Scholar] [CrossRef] [Green Version]
- Einor, D.; Bonisoli-Alquati, A.; Costantini, D.; Mousseau, T.A.; Møller, A.P. Ionizing radiation, antioxidant response and oxidative damage: A meta-analysis. Sci. Total Environ. 2016, 548–549, 463–471. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.; Zarrilli, S.; Paesano, L.; Carbone, U.; Boggia, B.; Petretta, M.; Maisto, A.; Cimmino, F.; Puca, G.; Colao, A.; et al. Traffic pollutants affect fertility in men. Hum. Reprod. 2003, 18, 1055–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carré, J.; Gatimel, N.; Moreau, J.; Parinaud, J.; Léandri, R. Does air pollution play a role in infertility? A systematic review. Environ. Health 2017, 16, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubes, J.; Selevan, S.G.; Evenson, D.P.; Zudova, D.; Vozdova, M.; Zudova, Z.; Robbins, W.A.; Perreault, S.D. Episodic air pollution is associated with increased DNA fragmentation in human sperm without other changes in semen quality. Hum. Reprod. 2005, 20, 2776–2783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rengaraj, D.; Kwon, W.-S.; Pang, M.-G. Effects of Motor Vehicle Exhaust on Male Reproductive Function and Associated Proteins. J. Proteome Res. 2015, 14, 22–37. [Google Scholar] [CrossRef]
- Bahrami, N.; Goudarzi, M.; Hosseinzadeh, A.; Sabbagh, S.; Reiter, R.J.; Mehrzadi, S. Evaluating the protective effects of melatonin on di(2-ethylhexyl) phthalate-induced testicular injury in adult mice. Biomed. Pharmacother. 2018, 108, 515–523. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Coskun, S.; Al-Doush, I.; Al-Rajudi, T.; Al-Rouqi, R.; Abduljabbar, M.; Al-Hassan, S. Exposure to phthalates in couples undergoing in vitro fertilization treatment and its association with oxidative stress and DNA damage. Environ. Res. 2019, 169, 396–408. [Google Scholar] [CrossRef]
- Lafuente, R.; García-Blàquez, N.; Jacquemin, B.; Checa, M.A. Outdoor Air Pollution and Sperm Quality. Fertil. Steril. 2016, 106, 880–896. [Google Scholar] [CrossRef] [Green Version]
- Drevet, J.R.; Aitken, R.J. Oxidative damage to sperm DNA: Attack and defense. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2019; Volume 1166, pp. 107–117. [Google Scholar]
- Aitken, R.J.; Smith, T.B.; Jobling, M.S.; Baker, M.A.; De Iuliis, G.N. Oxidative stress and male reproductive health. Asian J. Androl. 2014, 16, 31–38. [Google Scholar] [CrossRef]
- Agarwal, A.; Makker, K.; Sharma, R. Clinical relevance of oxidative stress in male factor infertility: An update. Am. J. Reprod. Immunol. 2008, 59, 2–11. [Google Scholar] [CrossRef]
- Lenzi, A.; Gandini, L.; Picardo, M.; Tramer, F.; Sandri, G.; Panfili, E. Lipoperoxidation damage of spermatozoa polyunsaturated fatty acids (PUFA): Scavenger mechanisms and possible scavenger therapies. Front. Biosci. 2000, 5, E1–E15. [Google Scholar] [PubMed] [Green Version]
- O’Flaherty, C.; Matsushita-Fournier, D. Reactive oxygen species and protein modifications in spermatozoa. Biol. Reprod. 2017, 97, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Villani, P.; Eleuteri, P.; Grollino, M.G.; Rescia, M.; Altavista, P.; Spanò, M.; Pacchierotti, F.; Cordelli, E. Sperm DNA fragmentation induced by DNAse I and hydrogen peroxide: An in vitro comparative study among different mammalian species. Reproduction 2010, 140, 445–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribas-Maynou, J.; Benet, J. Single and double strand sperm DNA damage: Different reproductive effects on male fertility. Genes 2019, 10, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorostghoal, M.; Kazeminejad, S.R.; Shahbazian, N.; Pourmehdi, M.; Jabbari, A. Oxidative stress status and sperm DNA fragmentation in fertile and infertile men. Andrologia 2017, 49, e12762. [Google Scholar] [CrossRef]
- Ammar, O.; Haouas, Z.; Hamouda, B.; Hamdi, H.; Hellara, I.; Jlali, A.; Cheikh, H.B.; Mehdi, M. Relationship between sperm DNA damage with sperm parameters, oxidative markers in teratozoospermic men. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 233, 70–75. [Google Scholar] [CrossRef]
- Gawecka, J.E.; Marh, J.; Ortega, M.; Yamauchi, Y.; Ward, M.A.; Ward, W.S. Mouse zygotes respond to severe sperm DNA damage by delaying paternal DNA replication and embryonic development. PLoS ONE 2013, 8, e56385. [Google Scholar] [CrossRef] [Green Version]
- Toyoshima, M.; Shimura, T.; Adiga, S.-K.; Taga, M.; Shiraishi, K.; Inoue, M.; Yuan, Z.-M.; Niwa, O. Transcription-independent suppression of DNA synthesis by p53 in sperm-irradiated mouse zygotes. Oncogene 2005, 24, 3229–3235. [Google Scholar] [CrossRef] [Green Version]
- Lewis, S.E.M.; Aitken, R.J. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 2005, 322, 33–41. [Google Scholar] [CrossRef]
- Simon, L.; Lewis, S.E.M. Sperm DNA damage or progressive motility: Which one is the better predictor of fertilization in vitro? Syst. Biol. Reprod. Med. 2011, 57, 133–138. [Google Scholar] [CrossRef]
- Barbato, V.; Talevi, R.; Braun, S.; Merolla, A.; Sudhakaran, S.; Longobardi, S.; Gualtieri, R. Supplementation of sperm media with zinc, D-aspartate and co-enzyme Q10 protects bull sperm against exogenous oxidative stress and improves their ability to support embryo development. Zygote 2017, 25, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Zini, A. Are sperm chromatin and DNA defects relevant in the clinic? Syst. Biol. Reprod. Med. 2011, 57, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhao, J.-Y.; Xue, X.; Zhu, G.-X. The association between sperm DNA fragmentation and reproductive outcomes following intrauterine insemination, a meta analysis. Reprod. Toxicol. 2019, 86, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, A.; Van Avermaete, F.; Roelant, E.; Punjabi, U.; De Neubourg, D. The role of sperm DNA fragmentation testing in predicting intra-uterine insemination outcome: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 244, 8–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, C.; Li, T.; Xie, Y.; Guo, Y.; Yang, Q.; Liang, X.; Deng, C.; Liu, G. Sperm DNA fragmentation index influences assisted reproductive technology outcome: A systematic review and meta-analysis combined with a retrospective cohort study. Andrologia 2019, 51, e13263. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Zini, A.; Dyachenko, A.; Ciampi, A.; Carrell, D.T. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J. Androl. 2017, 19, 80–90. [Google Scholar]
- Zhao, J.; Zhang, Q.; Wang, Y.; Li, Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: A systematic review and meta-analysis. Fertil. Steril. 2014, 102, 998–1005.e8. [Google Scholar] [CrossRef]
- Osman, A.; Alsomait, H.; Seshadri, S.; El-Toukhy, T.; Khalaf, Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: A systematic review and meta-analysis. Reprod. Biomed. Online 2015, 30, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Cissen, M.; Van Wely, M.; Scholten, I.; Mansell, S.; De Bruin, J.P.; Mol, B.W.; Braat, D.; Repping, S.; Hamer, G. Measuring Sperm DNA Fragmentation and Clinical Outcomes of Medically Assisted Reproduction: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0165125. [Google Scholar] [CrossRef] [Green Version]
- Halpern, J.A.; Schlegel, P.N. Should A Couple with Failed In Vitro Fertilization/Intracytoplasmic Sperm Injection and Increased Sperm DNA Fragmentation Use Testicular Sperm for the Next Cycle? Eur. Urol. Focus 2018, 4, 299–300. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, L.; Jiang, H.; Chen, H.; Chen, Y.; Dai, Y. Sperm DNA fragmentation index and pregnancy outcome after IVF or ICSI: A meta-analysis. J. Assist. Reprod. Genet. 2015, 32, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, W.S. Function of sperm chromatin structural elements in fertilization and development. Mol. Hum. Reprod. 2010, 16, 30–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, K.; Muratani, M.; Araki, H.; Miura, F.; Suzuki, T.; Dohmae, N.; Katou, Y.; Shirahige, K.; Ito, T.; Ishii, S. Mapping of histone-binding sites in histone replacement-completed spermatozoa. Nat. Commun. 2018, 9, 3885. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Hada, M.; Fukuda, Y.; Inoue, E.; Makino, Y.; Katou, Y.; Shirahige, K.; Okada, Y. Re-evaluating the Localization of Sperm-Retained Histones Revealed the Modification-Dependent Accumulation in Specific Genome Regions. Cell Rep. 2018, 23, 3920–3932. [Google Scholar] [CrossRef] [PubMed]
- Mudrak, O.; Tomilin, N.; Zalensky, A. Chromosome architecture in the decondensing human sperm nucleus. J. Cell Sci. 2005, 118, 4541–4550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Rojo, S.; Fernández-Díez, C.; Lombó, M.; Herráez, M.P. Distribution of DNA damage in the sperm nucleus: A study of zebrafish as a model of histone-packaged chromatin. Theriogenology 2018, 122, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Jones, K.T.; Robertson, S.A. Reactive oxygen species and sperm function–in sickness and in health. J. Androl. 2012, 33, 1096–1106. [Google Scholar] [CrossRef]
- Rhemrev, J.P.T.; Van Overveld, F.W.; Haenen, G.R.; Teerlink, T.; Bast, A.; Vermeiden, J.P. Quantification of the Nonenzymatic Fast and Slow TRAP in a Postaddition Assay in Human Seminal Plasma and the Antioxidant Contributions of Various Seminal Compounds. J. Androl. 2000, 21, 913–920. [Google Scholar]
- Lazzarino, G.; Listorti, I.; Bilotta, G.; Capozzolo, T.; Amorini, A.M.; Longo, S.; Caruso, G.; Lazzarino, G.; Tavazzi, B.; Bilotta, P. Water- and Fat-Soluble Antioxidants in Human Seminal Plasma and Serum of Fertile Males. Antioxidants 2019, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- O’Flaherty, C. Peroxiredoxins: Hidden players in the antioxidant defence of human spermatozoa. Basic Clin. Androl. 2014, 24, 4. [Google Scholar] [CrossRef] [Green Version]
- Abdelbaki, S.A.; Sabry, J.H.; Al-Adl, A.M.; Sabry, H.H. The impact of coexisting sperm DNA fragmentation and seminal oxidative stress on the outcome of varicocelectomy in infertile patients: A prospective controlled study. Arab J. Urol. 2017, 15, 131–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baazeem, A.; Belzile, E.; Ciampi, A.; Dohle, G.; Jarvi, K.; Salonia, A.; Weidner, W.; Zini, A. Varicocele and Male Factor Infertility Treatment: A New Meta-Analysis and Review of the Role of Varicocele Repair. Eur. Urol. 2011, 60, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Lara-Cerrillo, S.; Gual-Frau, J.; Benet, J.; Abad, C.; Prats, J.; Amengual, M.J.; Ribas-Maynou, J.; García-Peiró, A. Microsurgical varicocelectomy effect on sperm telomere length, DNA fragmentation and seminal parameters. Hum. Fertil. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-L.; Liu, J.-J.; Li, J.-T.; Yang, Q.-A.; Zhang, J.-M. Melatonin therapy adds extra benefit to varicecelectomy in terms of sperm parameters, hormonal profile and total antioxidant capacity: A placebo-controlled, double-blind trial. Andrologia 2018, 50, e13033. [Google Scholar] [CrossRef] [PubMed]
- Smits, R.M.; Mackenzie-Proctor, R.; Yazdani, A.; Stankiewicz, M.T.; Jordan, V.; Showell, M.G. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2019, 3, CD007411. [Google Scholar] [CrossRef]
- Gharagozloo, P.; Aitken, R.J. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum. Reprod. 2011, 26, 1628–1640. [Google Scholar] [CrossRef] [Green Version]
- Ardestani Zadeh, A.; Arab, D.; Kia, N.S.; Heshmati, S.; Amirkhalili, S.N. The role of Vitamin E-Selenium-Folic Acid Supplementation in Improving Sperm Parameters After Varicocelectomy: A Randomized Clinical Trial. Urol. J. 2019, 16, 495–500. [Google Scholar]
- Nouri, M.; Amani, R.; Nasr-Esfahani, M.; Tarrahi, M.J. The effects of lycopene supplement on the spermatogram and seminal oxidative stress in infertile men: A randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2019, 33, 3203–3211. [Google Scholar] [CrossRef]
- Banday, M.N.; Lone, F.A.; Rasool, F.; Rashid, M.; Shikari, A. Use of antioxidants reduce lipid peroxidation and improve quality of crossbred ram sperm during its cryopreservation. Cryobiology 2017, 74, 25–30. [Google Scholar] [CrossRef]
- Gual-Frau, J.; Abad, C.; Amengual, M.J.; Hannaoui, N.; Checa, M.A.; Ribas-Maynou, J.; Lozano, I.; Nikolaou, A.; Benet, J.; García-Peiró, A.; et al. Oral antioxidant treatment partly improves integrity of human sperm DNA in infertile grade i varicocele patients. Hum. Fertil. 2015, 18, 225–229. [Google Scholar] [CrossRef]
- Negri, L.; Benaglia, R.; Monti, E.; Morenghi, E.; Pizzocaro, A.; Levi Setti, P.E. Effect of superoxide dismutase supplementation on sperm DNA fragmentation. Arch. Ital. Urol. Androl. 2017, 89, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Soto, J.C.; Domingo, J.C.; Cordobilla, B.; Nicolás, M.; Fernández, L.; Albero, P.; Gadea, J.; Landeras, J. Dietary supplementation with docosahexaenoic acid (DHA) improves seminal antioxidant status and decreases sperm DNA fragmentation. Syst. Biol. Reprod. Med. 2016, 62, 387–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Barranco, I.; Tvarijonaviciute, A.; Molina, M.F.; Martinez, E.A.; Rodriguez-Martinez, H.; Parrilla, I.; Roca, J. Seminal plasma antioxidants are directly involved in boar sperm cryotolerance. Theriogenology 2018, 107, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amidi, F.; Pazhohan, A.; Shabani Nashtaei, M.; Khodarahmian, M.; Nekoonam, S. The role of antioxidants in sperm freezing: A review. Cell Tissue Bank. 2016, 17, 745–756. [Google Scholar] [CrossRef]
- Martínez-Soto, J.C.; Landeras, J.; Gadea, J. Spermatozoa and seminal plasma fatty acids as predictors of cryopreservation success. Andrology 2013, 1, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Attallah, D.; El-Nashar, I.H.; Mahmoud, R.; Shaaban, O.M.; Salman, S.A. N-acytelcysteine prior to intrauterine insemination in couples with isolated athenozospermia: A randomized controlled trial. Fertil. Steril. 2013, 100, S462. [Google Scholar] [CrossRef]
- Comhaire, F.H.; Kunnen, M. Factors affecting the probability of conception after treatment of subfertile men with varicocele by transcatheter embolization with Bucrylate. Fertil. Steril. 1985, 43, 781–786. [Google Scholar] [CrossRef]
- Steiner, A.Z.; Hansen, K.R.; Barnhart, K.T.; Cedars, M.I.; Legro, R.S.; Diamond, M.P.; Krawetz, S.A.; Usadi, R.; Baker, V.L.; Coward, R.M.; et al. The effect of antioxidants on male factor infertility: The Males, Antioxidants, and Infertility (MOXI) randomized clinical trial. Fertil. Steril. 2020, 113, 552–560.e3. [Google Scholar] [CrossRef]
- Tremellen, K.; Miari, G.; Froiland, D.; Thompson, J. A randomised control trial examining the effect of an antioxidant (Menevit) on pregnancy outcome during IVF-ICSI treatment. Aust. N. Z. J. Obstet. Gynaecol. 2007, 47, 216–221. [Google Scholar] [CrossRef]
- Geva, E.; Bartoov, B.; Zabludovsky, N.; Lessing, J.B.; Lerner-Geva, L.; Amit, A. The effect of antioxidant treatment on human spermatozoa and fertilization rate in an in vitro fertilization program. Fertil. Steril. 1996, 66, 430–434. [Google Scholar] [CrossRef]
- Greco, E.; Romano, S.; Iacobelli, M.; Ferrero, S.; Baroni, E.; Minasi, M.G.; Ubaldi, F.; Rienzi, L.; Tesarik, J. ICSI in cases of sperm DNA damage: Beneficial effect of oral antioxidant treatment. Hum. Reprod. 2005, 20, 2590–2594. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribas-Maynou, J.; Yeste, M. Oxidative Stress in Male Infertility: Causes, Effects in Assisted Reproductive Techniques, and Protective Support of Antioxidants. Biology 2020, 9, 77. https://doi.org/10.3390/biology9040077
Ribas-Maynou J, Yeste M. Oxidative Stress in Male Infertility: Causes, Effects in Assisted Reproductive Techniques, and Protective Support of Antioxidants. Biology. 2020; 9(4):77. https://doi.org/10.3390/biology9040077
Chicago/Turabian StyleRibas-Maynou, Jordi, and Marc Yeste. 2020. "Oxidative Stress in Male Infertility: Causes, Effects in Assisted Reproductive Techniques, and Protective Support of Antioxidants" Biology 9, no. 4: 77. https://doi.org/10.3390/biology9040077
APA StyleRibas-Maynou, J., & Yeste, M. (2020). Oxidative Stress in Male Infertility: Causes, Effects in Assisted Reproductive Techniques, and Protective Support of Antioxidants. Biology, 9(4), 77. https://doi.org/10.3390/biology9040077





