Abstract
(1) Background: Direct-acting antiviral therapy for chronic hepatitis C virus (HCV) infection is associated with high sustained virologic response (SVR) and overcomes negative predictive factors, including steatosis, in patients without human immunodeficiency virus (HIV) coinfection. The impact of steatosis on SVR in patients with HIV–HCV coinfection is unknown. (2) Methods: A retrospective analysis of patients treated with direct-acting antivirals was performed. Demographic, laboratory and direct-acting antiviral regimen data were prospectively collected. Metabolic syndrome and its components—diabetes mellitus, hypertension, dyslipidemia and obesity—were assessed. Hepatic steatosis (≥5%) was defined by liver biopsy or controlled attenuation parameter (CAP) measurement during vibration-controlled transient elastography (VCTE). (3) Results: A total of 151 HIV–HCV-coinfected patients on combined antiretroviral therapy and direct-acting antiviral therapy were included in this analysis. Prevalence of steatosis by liver biopsy (n = 34) or CAP (≥263 db/m) during VCTE (n = 92) was 27% and was independently associated with obesity (OR 3.11; 95% CI 1.43–6.82; p = 0.004) and the metabolic syndrome (OR 1.08; 95% CI 1.01–0.15; p = 0.01). The overall SVR rate (n = 148) was 95% and was not impacted by the presence of steatosis (p = 0.42). (4) Conclusions: Hepatic steatosis is common in HIV–HCV coinfection, correlates with obesity and the metabolic syndrome and does not impact SVR.
1. Introduction
Hepatic steatosis is common in patients living with human immunodeficiency virus (HIV), including those coinfected with chronic hepatitis C virus (HCV) [1,2]. In studies of HIV patients at risk for steatosis (e.g., increased liver enzymes) without HCV using liver histology as reference standard, steatosis was detected in 60%–69% [2,3,4,5] compared to 23%–75% of those with HIV–HCV coinfection [1] and 40%–86% of those with chronic HCV without HIV [6]. Similar to those without HIV, the development of steatosis in patients with HIV is often secondary to the presence of the metabolic syndrome and its components (diabetes, obesity, hypertension, and dyslipidemia), HIV-related lipodystrophy, microbial dysbiosis and mitochondrial damage leading to insulin resistance and dyslipidemia [7,8,9]. Furthermore, some of the HIV therapies, specifically older nucleoside/nucleotide analogue reverse transcriptase inhibitors and protease inhibitors, cause lipolysis, and this increase in free fatty acids results in mitochondrial dysfunction [10]. These medications also lead to insulin resistance via modulation of adipose and intestinal tissue; leptin, interleukin-6, interleukin-1, tumor necrosis factor alpha and peroxisome proliferator-activated receptor gamma are increased where adiponectin is decreased [7,11]. The mechanism of hepatic steatosis in patients with HCV genotype 1 (GT1) as well as HIV is believed to be through insulin resistance [12]; hence, the observed association of steatosis with obesity in this cohort. A meta-analysis of 34 studies found a positive correlation between HCV and the development of diabetes [13]. It is plausible that via increased insulin resistance, the presence of diabetes is associated with steatosis; interestingly, this association was not found in this cohort. Furthermore, there is evidence that HCV itself, especially genotype 3 (GT3), can lead to steatosis [14]. In addition to factors mentioned above, high alcohol consumption and HCV GT3 are risk factors associated with steatosis in HCV patients [6,15,16,17,18,19].
Liver biopsy is the reference standard for diagnosing hepatic fibrosis and steatosis; however, it is invasive, expensive and affected by biopsy size (sampling error) and interobserver variation among pathologists [20]. Consequently, non-invasive serum-based tests and imaging techniques, such as vibration-controlled transient elastography (VCTE) to measure fibrosis with controlled attenuation parameter (CAP) measurement to assess hepatic steatosis, respectively, have replaced liver biopsy in most centers [21,22]. However, because VCTE with CAP may not be available in many clinical settings, other non-invasive serum-based assessments of steatosis, such as the hepatic steatosis index (HSI) have been developed [23,24] but not assessed in those with HIV.
The presence of steatosis resulted in lower sustained virologic response (SVR) during the interferon-alpha treatment era of HCV infection [25,26]. The development of direct-acting antiviral (DAA) medications revolutionized the treatment of HCV infection, including those with HIV, resulting in high SVR (90%–95%) [27,28]. Past negative predictive factors associated with non-response when treated with interferon therapies, such as the metabolic syndrome, insulin resistance, advanced fibrosis, black race, GT1, and HIV–HCV coinfection, do not appear to be risk factors for relapse in the DAA era [27,28,29]. Because hepatic steatosis does not appear to be a risk factor for DAA treatment failure in those with HCV monoinfection [30], it is hypothesized that steatosis will not impact SVR in HIV–HCV coinfection.
The impact of hepatic steatosis on SVR in HIV–HCV-coinfected patients receiving DAA therapy is unknown. To address this gap in knowledge, the primary aims of the current study were to determine the prevalence and risk factors of hepatic steatosis in a cohort of HIV–HCV-coinfected patients undergoing DAA therapy and the impact of steatosis on SVR. The secondary aim was to assess the sensitivity and specificity of the HSI versus CAP for detecting steatosis to determine its utility in settings where VCTE and CAP are not available.
2. Materials and Methods
2.1. Study Design and Participants
This was a retrospective analysis of adult patients treated at Virginia Commonwealth University Health with DAAs between December 2014 and December 2018. Participants provided written informed consent prior to enrollment. All patients were treated with DAAs in accordance with American Association for the Study of Liver Disease/Infectious Disease Society of America (AASLD-IDSA) Guidelines [31]. The study was approved by the HCV Treatment Registry institutional review board (IRB). Adult patients with HIV–HCV coinfection adherent with a DAA regimen were eligible for inclusion. Exclusion criteria included active evaluation for organ transplant, significant hepatic disease (e.g., acute hepatitis, decompensated cirrhosis), uncontrolled HIV (CD4 count <200 cells/mm3), and active alcohol use (men who reported drinking >4 drinks on any day or >14 drinks per week; and women who reported drinking >3 drinks on any day or >7 drinks per week; a standard drink was defined as 14 grams of ethanol) [32].
2.2. Study Outcomes
The primary outcomes were to determine the prevalence and risk factors of hepatic steatosis in a cohort of HIV–HCV-coinfected patients undergoing DAA therapy and the impact of steatosis on SVR 12 weeks after completion of therapy (SVR-12). The secondary aim was to assess the sensitivity and specificity of the HSI versus CAP for detecting steatosis to determine its utility in settings where VCTE and CAP are not available.
2.3. Data Collection and Laboratory Analysis
To ascertain the primary outcome, the presence of hepatic steatosis was measured by liver biopsy (≥5%) (n = 34), CAP (≥263 db/m) [33] during VCTE (n = 92) and the serologic-based testing score, the hepatic steatosis index (HSI) (>41) (n = 151) (HSI = 8 × (alanine aminotransferase level (U/L)/aspartate aminotransferase level (U/L) ratio) + BMI (+2, if female; +2, if diabetes mellitus)) [23]. The CAP threshold of 263 dB/m was validated in patients with non-alcoholic fatty liver disease (NAFLD) and chosen for this study because of its high sensitivity (90%) for detecting at least five percent steatosis compared to liver histology [34]. Lower CAP thresholds (238 dB/m [35] and 248 dB/m [33]) have also been validated for evaluating the presence of hepatic steatosis in patients with NAFLD [33,35] and HCV [33]. It is important to recognize that none of these thresholds, including 263 dB/m used in this study, are validated in patients with HIV or HIV–HCV coinfection. The lower CAP thresholds were included in a sensitivity analysis with a HSI > 41. Liver fibrosis was assessed by liver biopsy (n = 34) and VCTE (n = 117). Significant liver fibrosis by VCTE was defined as >9.0 kPa [20,36,37]. A higher threshold of 11 kPa was also used to define advanced fibrosis [34,38,39,40]. Additionally, the aspartate aminotransferase to platelet ratio index (APRI = (aspartate aminotransferase level (U/L)/aspartate aminotransferase upper limit of normal (U/L))/(platelet count (×109/L) × 100) and fibrosis 4 index for liver fibrosis (FIB-4 = (age (years) × aspartate aminotransferase level (U/L))/(platelet count (×109/L) × square root of alanine aminotransferase level (U/L)) were calculated based on baseline data [41]. Demographic and baseline characteristics including, age, gender, race, HCV genotype, liver fibrosis grade by biopsy and/or VCTE, liver CAP score, body mass index (BMI), history of obesity, diabetes, hypertension, the metabolic syndrome, hepatitis B virus (HBV), and alcohol use as well as laboratory, HIV combination antiretroviral therapy (cART) and DAA regimen data were prospectively collected. Diabetes and hypertension were classified as present if patients were taking antidiabetic or antihypertensive medications. Obesity was defined as BMI ≥ 30 kg/m2. The metabolic syndrome was defined as the presence of at least three of the following: BMI ≥ 30 kg/m2, diabetes, hypertension, or dyslipidemia (triglyceride level ≥ 150 mg/dL) [42]. SVR was defined as undetectable HCV RNA 12 weeks after completing DAA treatment (SVR-12).
2.4. Statistical Analyses
Descriptive statistics are presented using means and standard deviation (SD) for normally distributed continuous variables or medians and interquartile range (IQR) when data were skewed. Categorical variables were expressed as percent. For the primary analysis, the percent of participants who achieved SVR-12 was calculated. Univariate analysis was done to compare SVR-12 in those with and without hepatic steatosis. Differences in continuous variables were assessed by the t-test or the Mann–Whitney U test while differences in categorical variables were assessed by chi-square or Fisher exact test as appropriate. Factors on univariate analysis with p < 0.2 were used in the multivariate logistic regression analysis to identify independent factors associated with SVR-12 (the primary outcome) and hepatic steatosis (by biopsy or CAP > 263 dB/m). The relationship between the HSI and CAP was assessed by Spearman’s correlation. A sensitivity analysis of detecting ≥5% steatosis by a HSI > 41 versus various CAP thresholds (238, 248, and 263 dB/m) was used to determine the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of the HSI. All statistical analyses were conducted with JMP14 Pro. p < 0.05 (2-sided) was considered statistically significant.
3. Results
3.1. Study Participants
One hundred and fifty one HIV–HCV-coinfected patients on cART and DAAs were included in this analysis (Table 1). The median age was 57 years, 76% were male, 84% were black, 97% were HCV GT1, and 5% were infected with hepatitis B virus. The median alanine transaminase (ALT) level was 54 units per liter (U/L), the median body mass index (BMI) was 27 kg per square meter (kg/m2), and the median cluster of differentiation 4 (CD4) count was 559 (IQR 390–830) cells per cubic millimeter (cells/mm3). The prevalence of diabetes mellitus, hypertension, and obesity was 21%, 39%, and 34%, respectively, while metabolic syndrome was present in 33% of participants. The predominant cART used included a backbone nucleoside reverse transcriptase inhibitor (NRTI) (91%) in combination with a non-nucleoside reverse transcriptase inhibitor (NNRTI) (20%), protease inhibitor (PI) (24%), or an integrase inhibitor (II) (69%). The majority of those on a NRTI were on tenofovir (68%) or abacavir (25%) combined with emtricibine or lamivudine, while the majority of those on a PI were on darunavir (39%) or atazanavir (25%) combined with ritonavir or cobisistat. The predominant DAA used was ledipasvir/sofosbuvir (79%) with ribavirin (22%) for 12 weeks; the remaining participants received sofosbuvir/velpatasvir (8%), elbasvir/grazoprevir (6%), or glecaprevir/pibrentasvir (2%) for 12 weeks.

Table 1.
Demographic and laboratory characteristics of the cohort.
3.2. Primary Outcomes
The liver disease characteristics are shown in Table 2. In participants who underwent liver biopsy (n = 34), 26% had mild fibrosis (score of 1), 30% had moderate or severe fibrosis (score of 2 or 3), and 43% had cirrhosis (score of 4). The prevalence of significant (>9 kPa) and advanced hepatic fibrosis (>11 kPa) by VCTE was 48% and 40%, respectively, with a median kPa of 8.65 overall. The prevalence of hepatic steatosis (≥5%) by liver biopsy (n = 34) or CAP (≥263 db/m) during VCTE (n = 92) was 27%. Using different CAP thresholds, the prevalence of steatosis increased as the CAP threshold decreased (15% with CAP ≥ 263 dB/m to 35% with CAP ≥ 238 dB/m).

Table 2.
Liver disease characteristics of the cohort.
Table 3 compares those with and without steatosis. On univariate analysis, steatosis was associated with increasing BMI (p = 0.013), obesity (p = 0.004), dyslipidemia (triglyceride level ≥ 150 mg/dL) (p = 0.01), and the metabolic syndrome (p = 0.01). On multivariate analysis, steatosis was independently associated with obesity (OR 3.11; 95% CI 1.43–6.82; p = 0.004) and the metabolic syndrome (OR 1.08; 95% CI 1.01–0.15; p = 0.01) but not with age, diabetes mellitus, dyslipidemia, hypertension, ALT, or cART type.

Table 3.
Demographic, laboratory and liver disease characteristics of the cohort by the presence or absence of steatosis.
SVR-12 data were available for 98% of the participants (n = 148). The overall SVR-12 rate in those with hepatic steatosis was 95% and similar to those without steatosis (93%). In addition, SVR-12 was not impacted by patient demographics, cART regimen, or liver fibrosis (all p > 0.05) (Figure 1).
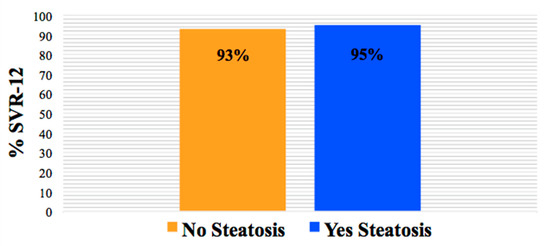
Figure 1.
SVR-12 rate by the presence and absence of hepatic steatosis (p > 0.05).
3.3. Secondary Outcomes
Hepatic steatosis by the HSI (>41) was present in 24% of participants and strongly correlated with CAP (≥263 db/m) (r = 0.41, p < 0.0001) (Figure 2).
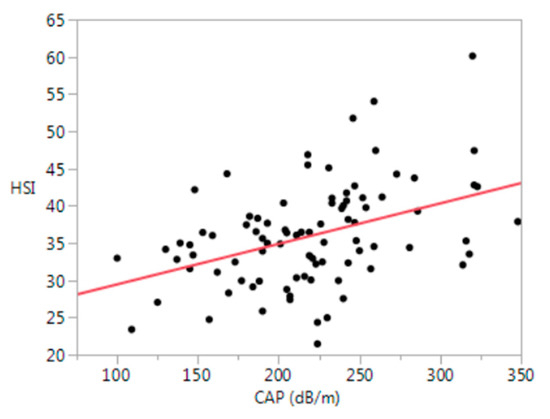
Figure 2.
Relationship between the HSI and CAP (r = 0.41, p < 0.0001). HSI = hepatic steatosis index (8 × (alanine aminotransferase level (U/L)/aspartate aminotransferase level (U/L)ratio) + BMI (+2, if female; +2, if diabetes mellitus)); CAP = controlled attenuation parameter; dB/m = decibel per meter.
However, the HSI (>41) had low sensitivity (39%) and positive predictive value (PPV) (50%) with good specificity (90%) and negative predictive value (NPV) (86%) for detecting steatosis versus CAP (≥263 db/m). Table 4 shows the sensitivity analysis of the HSI (>41) when CAP thresholds are lower. As the CAP threshold is lowered, the sensitivity and NPV increases and the specificity and PPV decreases. The HSI has the highest sensitivity and NPV at a CAP of 238 dB/m, and the highest specificity and PPV at a CAP of 263 dB/m.

Table 4.
Sensitivity analysis of the HSI (>41) versus CAP at different thresholds.
4. Discussion
The prevalence of hepatic steatosis is common in those with HIV, including those with HIV–HCV coinfection [1,2,3,4,5], and may be due to the high prevalence of the metabolic syndrome in those with HIV [43]. The prevalence of steatosis in the present cohort (27%) is at the lower end of the spectrum compared to past reports on HIV–HCV coinfection (23–75%) [1] and is related to BMI, obesity, and dyslipidemia, but not to diabetes and hypertension—two components of the metabolic syndrome. The prevalence of steatosis may be lower in this cohort because of the high proportion of black participants (81%) who have been observed to have a lower rate of steatosis compared to that in white and Hispanic individuals [44]. This discrepancy may also be due to the small sample size of the cohort. Steatosis was defined by CAP in the majority of patients, and the cutoff of 263 dB/m was chosen for maximum sensitivity to minimize the chance of falsely identifying those without steatosis [34]. When the threshold was reduced to 238 dB/m, the prevalence increased to 35%.
In the interferon era, hepatic steatosis negatively impacted SVR [25,26]. The development of DAAs has dramatically increased the ability to achieve SVR in chronic HCV [27,28,29,30]. Although DAAs have negated the presence of steatosis on SVR in those with HCV monoinfection [30], less is known about those with HIV–HCV coinfection. In this cohort, the SVR-12 rate was high, similar to that found in studies on participants with HCV monoinfection (90%–95%) [27,29], and not impacted by the presence of hepatic steatosis.
Liver biopsy, the gold standard for assessment of fibrosis and steatosis, has now been replaced by non-invasive methods such as the fibrosis 4 (FIB-4) index for liver fibrosis, the HSI, and VCTE [20,21,22,41]. In addition to biopsy and VCTE, the HSI was used to detect steatosis in this cohort because all of its components were collected as part of routine care. Although the HSI (>41) correlated with CAP (≥263 dB/m) as a continuous variable and had reasonable specificity compared to CAP (90% at a CAP of 263 dB/m), it had poor sensitivity and moderate positive predictive value, suggesting that it may be most useful to rule in steatosis rather than rule it out. The negative predictive value of the HSI (>41) for detecting steatosis was highest at a low CAP threshold (≥238 db/m). Based on the results, the HSI should not replace VCTE with CAP and should be used with caution in HIV–HCV-coinfected patients [23,24].
There are numerous limitations to this retrospective analysis. The cohort was predominantly black, male, and GT1—reflective of our institution’s coinfected population [18]; however, the results may not be generalizable to all those with HIV–HCV coinfection. Because SVR was high and few participants were infected with GT3, the interactions among GT3, steatosis, and SVR were not assessed. Past alcohol use among participants, prior to enrollment, was not recorded. In addition, liver biopsy was not performed on all participants; thus, VCTE with CAP was used to detect steatosis in the majority of patients. Because CAP was not available until 2016, CAP was not performed during VCTE on all patients undergoing DAA therapy. Although the HSI was used as another non-invasive measure of steatosis, it was not validated for patients with HIV and needs to be used with caution. The HSI was compared with CAP, but not with histology. The presence of non-alcoholic steatohepatitis (NASH) was not evaluated in this cohort. Lastly, given the high SVR-12 rate, a much larger sample size would be needed to detect significant differences in SVR-12 between those with and without steatosis.
5. Conclusions
Although hepatic steatosis is common in those with HIV–HCV coinfection undergoing DAA therapy and related to obesity, it has no impact on SVR. The HSI should not replace VCTE with CAP for detecting the presence of steatosis in HIV–HCV-coinfected patients. More research is needed to understand how eradication of HCV with DAAs affects hepatic steatosis and the potential progression to fibrosis, cirrhosis, and hepatocellular carcinoma [27]. Long-term follow-up of this HIV positive SVR-12 cohort to determine the impact of underlying steatosis on liver- and non-liver-related morbidity and mortality is ongoing.
Author Contributions
Conceptualization, R.K.S. and L.P.J.; methodology, R.K.S.; software, JMP 14.; validation, R.K.S., and L.P.J.; formal analysis, R.K.S.; investigation, R.K.S. and L.P.J.; resources, R.K.S.; data curation, R.K.S.; writing—original draft preparation, L.P.J. and R.K.S.; writing—review and editing, L.P.J. and R.K.S.; visualization, L.P.J.; supervision, R.K.S.; project administration, R.K.S.; funding acquisition, none. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to thank the HCV treatment staff for their support and the HIV providers for allowing us to care for their patients.
Conflicts of Interest
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article. There are no sources of funding for this manuscript.
References
- Bani-Sadr, F.; Carrat, F.; Bedossa, P.; Piroth, L.; Cacoub, P.; Perronne, C.; Degott, C. Pol, Stanislas ANRS HC02—Ribavic Study team. Hepatic steatosis in HIV-HCV coinfected patients: Analysis of risk factors. AIDS 2006, 20, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Smith, P.G.; Brunt, E.M. Hepatic steatosis in human immunodeficiency virus: A prospective study in patients without viral hepatitis, diabetes, or alcohol abuse. J. Clin. Gastroenterol. 2013, 47, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Shur, N.F.; Tan, Y.; Goubet, S.; Fisher, M.; Gilleece, Y.; Verma, S. Non-viral liver disease burden in HIV-monoinfected individuals retrospective cohort study. AIDS Care 2016, 28, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Ingiliz, P.; Valantin, M.A.; Duvivier, C.; Medja, F.; Dominguez, S.; Charlotte, F.; Tubiana, R.; Poynard, T.; Katlama, C.; Lombès, A.; et al. Liver damage underlying unexplained aminotransferase elevation in human immunodeficiency virus-1 mono-infected patients on antiretroviral therapy. Hepatology 2009, 49, 436–442. [Google Scholar] [CrossRef]
- Sebastiani, G.; Rollet-Kurhajec, K.; Pexos, C.; Gilmore, N.; Klein, M. Incidence and predictors of hepatic steatosis and fibrosis by serum biomarkers in a large cohort of human immunodeficiency virus mono-infected patients. Open Forum Infect. Dis. 2015, 2. [Google Scholar] [CrossRef]
- Asselah, T.; Rubbia-Brandt, L.; Marcellin, P.; Negro, F. Steatosis in chronic hepatitis C: Why does it really matter? Gut 2006, 55, 123–130. [Google Scholar] [CrossRef]
- Lemoine, M.; Serfaty, L.; Capeau, J. From nonalcoholic fatty liver to nonalcoholic steatohepatitis and cirrhosis in HIV-infected patients: Diagnosis and management. Curr. Opin. Infect. Dis. 2012, 25, 10–16. [Google Scholar] [CrossRef]
- Mastroianni, C.M.; Lichtner, M.; Mascia, C.; Zuccala, P.; Vullo, V. Molecular mechanisms of liver fibrosis in HIV/HCV coinfection. Int. J. Mol. Sci. 2014, 15, 9184–9208. [Google Scholar] [CrossRef]
- Balagopal, A.; Philp, F.H.; Astemborski, J.; Block, T.M.; Mehta, A.; Long, R.; Kirk, G.D.; Mehta, S.H.; Cox, A.L.; Thomas, D.L.; et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology 2008, 135, 226–233. [Google Scholar] [CrossRef]
- Capeau, J.; Bouteloup, V.; Katlama, C.; Bastard, J.P.; Guiyedi, V.; Salmon-Ceron, D.; Protopopescu, C.; Leport, C.; Raffi, F.; Chêne, G.; et al. Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS 2012, 26, 303–314. [Google Scholar] [CrossRef]
- Poynard, T.; de Ledinghan, V.; Zarski, J.P.; Stanciu, C.; Munteanu, M.; Vergniol, J.; France, J.; Trifan, A.; Lenaour, G.; Vaillant, J.-C.; et al. Performance of elasto-fibro test, a combination between fibrotest and liver stiffness measurements for assessing the stage of liver fibrosis in patients with chronic hepatitis C. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Kralj, D.; Jukić, L.V.; Stojsavljević, S.; Duvnjak, M.; Smolić, M.; Čurčić, I.B. Hepatitis C virus, insulin resistance, and steatosis. J. Clin. Transl. Hepatol. 2016, 4, 66–75. [Google Scholar] [PubMed]
- White, D.L.; Ratziu, V.; El-Serag, H.B. Hepatitis C infection and risk of diabetes: A systemic review and meta-analysis. J. Hepatol. 2008, 49, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Vanni, E.; Abate, M.L.; Gentilcore, E.; Hickman, I.; Gambino, R.; Cassader, M.; Smedile, A.; Ferrannini, E.; Rizzetto, M.; Marchesini, G.; et al. Sites and mechanisms of insulin resistance in nonobese, nondiabetic patients with chronic hepatitis C. Hepatology 2009, 50, 697–706. [Google Scholar] [CrossRef]
- Marchesini, G.; Bugianesi, E.; Forlani, G.; Cerrelli, F.; Lenzi, M.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; Melchionda, N.; et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003, 37, 917–923. [Google Scholar] [CrossRef]
- Schindhelm, R.K.; Diamant, M.; Dekker, J.M.; Tushuizen, M.E.; Teerlink, T.; Heine, R.J. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab. Res. Rev. 2006, 22, 437–443. [Google Scholar] [CrossRef]
- Farrell, G.C.; Larter, C.Z. Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology 2006, 43, S99–S112. [Google Scholar] [CrossRef]
- Sterling, R.K.; Contos, M.J.; Smith, P.G.; Stravitz, R.T.; Luketic, V.A.; Fuchs, M.; Shiffman, M.L.; Sanyal, A.J. Steatohepatitis: Risk factors and impact on disease severity in human immunodeficiency virus/hepatitis C virus coinfection. Hepatology 2008, 47, 1118–1127. [Google Scholar] [CrossRef]
- McGovern, B.H.; Ditelber, J.S.; Taylor, L.E.; Gandhi, R.T.; Christopoulos, K.A.; Chapman, S.; Schwartzapfel, B.; Rindler, E.; Fiorino, A.M.; Zaman, M.T.; et al. Hepatic steatosis is associated with fibrosis, nucleoside analogue use, and hepatitis C virus genotype 3 infection in HIV-seropositive patients. Clin. Infect. Dis. 2006, 43, 365–372. [Google Scholar] [CrossRef]
- Afdhal, N.H.; Bacon, B.R.; Patel, K.; Lawitz, E.J.; Gordon, S.C.; Nelson, D.R.; Challies, T.L.; Nasser, I.; Garg, J.; Wei, L.-J.; et al. Accurancy of fibroscan, compared with histology, in analysis of liver fibrosis in patients with hepatitis B or C: A United States multicenter study. Clin. Gastroenterol. Hepatol. 2015, 13, 772–779.e3. [Google Scholar] [CrossRef]
- Stern, C.; Castera, L. Non-invasive diagnosis of hepatic steatosis. Hepatol. Int. 2017, 11, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, G.; Mastrorosa, I.; Vergori, A.; Timelli, L.; Lorenzini, P.; Zaccarelli, M.; Cicalini, S.; Bellagamba, R.; Plazzi, M.M.; Mazzotta, V.; et al. Liver stiffness reduction and serum fibrosis score improvement in HIV/hepatitis C virus-coinfected patients treated with direct-acting antivirals. HIV Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.-H.; Cho, S.H.; Sung, M.-W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Fedchuk, L.; Nascimbeni, F.; Pais, R.; Charlotte, F.; Housset, C.; Ratziu, V.; LIDO Study Group. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2014, 40, 1209–1222. [Google Scholar] [CrossRef]
- Shah, S.R.; Patel, K.; Macellin, P.; Foster, G.R.; Manns, M.; Kottilil, S.; Healey, L.; Pulkstenis, E.; Subramanian, G.M.; McHutchison, J.G.; et al. Steatosis is an independent predictor of relapse following rapid virologic response in patients with HCV genotype 3. Clin. Gastroenterol. Hepatol. 2011, 9, 688–693. [Google Scholar] [CrossRef]
- Restivo, L.; Zampino, R.; Guerrera, B.; Ruggiero, L.; Adinolfi, L.E. Steatosis is the predictor of relapse in HCV genotype 3- but not 2- infected patients treated with 12 weeks of pegylated interferon-a-2a plus ribavirin and RVR. J. Viral.Hepat. 2012, 19, 346–353. [Google Scholar] [CrossRef]
- Villar, L.M.; Villela-Nogueira, C.A.; da Silva, A.P.; Scalioni, L.P. Metabolic Factors and Their Influence on the Clinical Course and Response to HCV Treatment. In Hepatitis C—From Infection to Cure; IntechOpen: London, UK, 2018; Chapter 6. [Google Scholar]
- Afdhal, N.; Zeuzem, S.; Kwo, P.; Chojkier, M.; Gitlin, N.; Puoti, M.; Romero-Gomez, M.; Zarski, J.P.; Agarwal, K.; Buggisch, P.; et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N. Engl. J. Med. 2014, 370, 1889–1898. [Google Scholar] [CrossRef]
- Eslam, M.; Aparcero, R.; Kawaguchi, T.; Del Campo, J.A.; Sata, M.; Khattab, M.A.; Romero-Gomez, M. Meta-analysis: Insulin resistance and sustained virological response in hepatitis C. Aliment. Pharmacol. Ther. 2011, 34, 297–305. [Google Scholar] [CrossRef]
- Sheridan, D.A.; Neely, R.D.; Bassendine, M.F. Hepatitis C virus and lipids in the era of direct acting antivirals (DAAs). Clin. Res. Hepatol. Gastroenterol. 2013, 37, 10–16. [Google Scholar] [CrossRef]
- HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. AASLD. IDSA. 2019. Available online: www.hcvguidelines.org (accessed on 11 October 2019).
- Helping patients who drink too much: A clinician’s guide. In National Insitute on Alcohol Abuse and Alcoholism; NIH Publication: Bethesda, MD, USA, 2005.
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.G.; Mi, Y.Q.; de Ledinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.H.; Cardoso, A.C.; et al. Individual patient data meta-analysis of controlled attentuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; Vuppalanchi, R.; Van Natta, M.L.; Hallinan, E.; Kowdley, K.V.; Abdelmalek, M.; Neuschwander-Tetri, B.A.; Loomba, R.; Dasarathy, S.; Brandman, D.; et al. Vibration-controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Sasso, M.; Beaugrand, M.; de Ledinghen, V.; Douvin, C.; Marcellin, P.; Poupon, R.; Sandrin, L.; Miette, V. Controlled attenuation parameter (CAP): A novel VCTETM guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: Preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med. Biol. 2010, 36, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Matta, B.; Lee, T.H.; Patel, K. Use of Non-invasive Testing to Stage Liver Fibrosis in Patients with HIV. Curr. HIV/AIDS Rep. 2016, 13, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Vergara, S.; Macias, J.; Rivero, A.; Gutierrex-Valencia, A.; Gonzalez-Serrano, M.; Merino, D.; Ríos, M.J.; García-García, J.A.; Camacho, A.; López-Cortés, L.; et al. The use of transient elastometry for assessing liver fibrosis in patients with HIV hepatitis C virus coinfection. Clin. Infect. Dis. 2007, 45, 969–974. [Google Scholar] [CrossRef] [PubMed]
- De Franchis, R.; Baveno, V.I.F. Expanding consensus in portal hypertension: Report of the baveno VI consensus workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef] [PubMed]
- De Ledinghen, V.; Vergniol, J. Transient elastography (FibroScan). Gastreoenterol. Clin. Biol. 2008, 32, 58–67. [Google Scholar] [CrossRef]
- Bonder, A.; Afdhal, N. Utilization of FibroScan in clinical practice. Curr. Gastroenterol. Rep. 2014, 16, 372. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; S. Sulkowski, M.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [CrossRef]
- Gazzaruso, C.; Sacchi, P.; Garzaniti, A.; Fratino, P.; Bruno, R.; Filice, G. Prevalence of metabolic syndrome among HIV patients. Diabetes Care 2002, 25, 1253–1254. [Google Scholar] [CrossRef][Green Version]
- Zajac, M.; Muszalska, I.; Sobczak, A.; Dadej, A.; Tomczak, S.; Jelinska, A. Hepatitis C—New drugs and treatment prospects. Eur. J. Med. Chem. 2019, 165, 225–249. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).