A High-Resolution Mass Spectrometry-Based Quantitative Metabolomic Workflow Highlights Defects in 5-Fluorouracil Metabolism in Cancer Cells with Acquired Chemoresistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture, Generation of 5-FU Resistant Cells
2.2. FACS Cell Death Assay
2.3. Extraction of 5-FU and Its Metabolites from Media
2.4. Extraction of 5-FU and Its Metabolites from Cells
2.5. Preparation of Media and Cell Extract Samples for Mass Spectrometry Coupled with Reverse Phase UHPLC
2.6. Ultra High Performance Liquid Chromatography Coupled with High-Resolution Mass Spectrometry
2.7. Preparation of 5-FU and FdUMP Standards for Generation of Calibration Curve to Determine LOD and LOQ
2.8. Identifying and Quantifying 5-FU and its Associated Metabolites
2.8.1. Selection of Precursor Mass for tSIM-ddMS2 and Detection of 5-FU and Its Associated Metabolites in Samples Using Accurate Precursor Mass
- (a)
- Molecular formula of the compounds were first entered in the isotope simulation section with the deduction of one Hydrogen (H) from the formula and the software gave the m/z as well as the isotope pattern as shown in Supplementary Figure S1 for FURD and Supplementary Figure S2 for FdUMP.
- (b)
- An m/z [M-H]- targeted inclusion list (Table 2) of all the eight precursor masses was then used as a target for the tSIM-ddMS2 experiment.
- (c)
- For data analysis, specific mass range filter in Qual browser was used to view the elution of the compounds that had similar m/z of target precursor at a 5 ppm mass error.
- (d)
- Within this broad range of mass filter, an additional mass filter was applied, which was accurate up to three decimal points, as shown in Table 2 for each compound. This helped in the reduction of nonspecific peaks and limited the search to a definite range around the target [26] to get the clear peak of the target precursor at specific retention time, as shown in Supplementary Figure S1A.
2.8.2. Selection of Fragment Ions (Transitions) for Identification and Quantification of 5-FU and Its Associated Metabolites
- (a)
- The ‘canonical SMILES’ formula were first obtained from PubChem [36].
- (b)
- To generate theoretical spectra, the canonical smiles formula was subjected in the compound structure section within the spectra prediction utility of the CFM-ID 3.0 online tool.
- (c)
- The search was processed with Spectra type ‘ESI’ with negative ion mode and [M-H]- adduct type from the drop-down menus.
2.8.3. Detection of 5-FU and Associated Metabolites Using Accurate Precursor and Fragment Masses
- (a)
- Selection of MS2 for specific m/z [M-H] shown in Table 2 at a retention time when the peak for precursor mass was observed
- (b)
- The fragment ions from the MS2 spectrum were visualized in Xcalibur Qual browser software (Thermo Fisher) to ensure clean signal to noise, free from interfering signal.
- (c)
- This way, in the same window, experimental data including the precursor along with fragment ion pattern obtained from it as well as the theoretically predicted isotope pattern using isotope simulation was visualized as shown in Supplementary Figure S1A.
- (d)
- To validate that the precursors and fragments are detected correctly, the untreated test sample was run alongside and the absence of matching precursor as well as matching fragment ion patterns signified that the process was precise (Supplementary Figure S1B).
2.8.4. Validation of the Correct Identification of the Compounds without Standards
- (a)
- The MS1 spectrum was copied from the Qual Browser software by clicking in the spectrum and copying the data and creating an excel file in ‘.csv’ format.
- (b)
- The precursor m/z data was obtained, and the file was then imported into SIRIUS 4.0.1 software following the steps mentioned in the user manual.
- (c)
- The spectra file was dragged and dropped into the application window or alternatively imported using the import option. This gives the option to select the mass and intensity, where the default values are usually correct.
- (d)
- MS level was selected (‘MS1′ for precursors or ‘MS2′ for product m/z list) and ’ok’ was clicked.
- (e)
- The correct precursor molecular weight was selected from the dropdown option and the adduct was set to a negative ion format as ‘[M-H]’ and ‘ok’ was clicked to input the data.
- (f)
- For analysis, we right clicked on the imported compound and compute was pressed. It is necessary to select the correct precursor ion mass in the dialogue box. For the compounds containing Fluorine, ‘F’ was added using the elements selection option, and the instrument was set to ‘Orbitrap.’
- (g)
- A comparison was performed to check if the isotopic patterns matched the precursors isotopes pattern, and were then identified as the target compounds matching to PubChem database [40].
- (h)
- The query was compared with the ‘all molecular formula’ option selected; however, SIRIUS generated a molecular formula with the elements that were selected in the compute dialogue box based on the ions m/z (precursor or fragment ions) and their mass, which might not be in the PubChem database. SIRIUS will consider all molecular formulas possible for the given specific precursor m/z, which will include the elements that are selected in the compute dialogue box [44].
- (i)
- The isotope scores obtained for each target compound precursor identification step was then tabulated to form a prediction score table.
- (j)
- To validate the fragment ions were fingerprint of the target precursors, the product mass spectrum data was copied from Skyline (Section 2.8.5) and as earlier, a .csv file was created.
- (k)
2.8.5. Relative Quantification of 5-FU and the Associated Metabolites
- (a)
- Inserting the transition within the insert menu.
- (b)
- In the insert window, small molecules were selected instead of peptides, and then, the values (precursor name, precursor molecular formula, precursor adduct as M-H for all compounds) were added.
- (c)
- Entry was checked for error using the option available within the software itself.
- (d)
- The precursor was then inserted, which appeared in the target section of the Skyline window.
- (e)
- Transition values representing the fragment ion m/z was then inserted for each precursor by following ‘right click’ in the precursor and then ‘add transition.’
- (f)
- In this window, the transition m/z was typed in both monoisotopic and average m/z spaces with the adduct set to [M-] representing negative ions.
- (g)
- To test the applicability of the approach, initial test samples data were obtained and analyzed following this procedure using Skyline and confirmed with XCalibur Qual Browser software (Thermo Fisher Scientific, version 3.1).
- (h)
- Quantification was based on the peak area (total area, including precursor and products) obtained for each compound using skyline software from each sample. The peak area results were collected and stored in excel sheet for all the standards and one FdUMP standard (0.780 ng/µL) was chosen and all the compounds were normalized to the peak area of this standard.
3. Results
3.1. Long Term Exposure of CRC Cells to Increasing Concentration of 5-FU Results in Acquired Resistance
3.2. Calibration Using Known Standards
3.3. Method Development for the Identification and Quantification of 5-FU and Its Associated Metabolites
3.4. Further Validation of the Accurate Identification of the Compounds
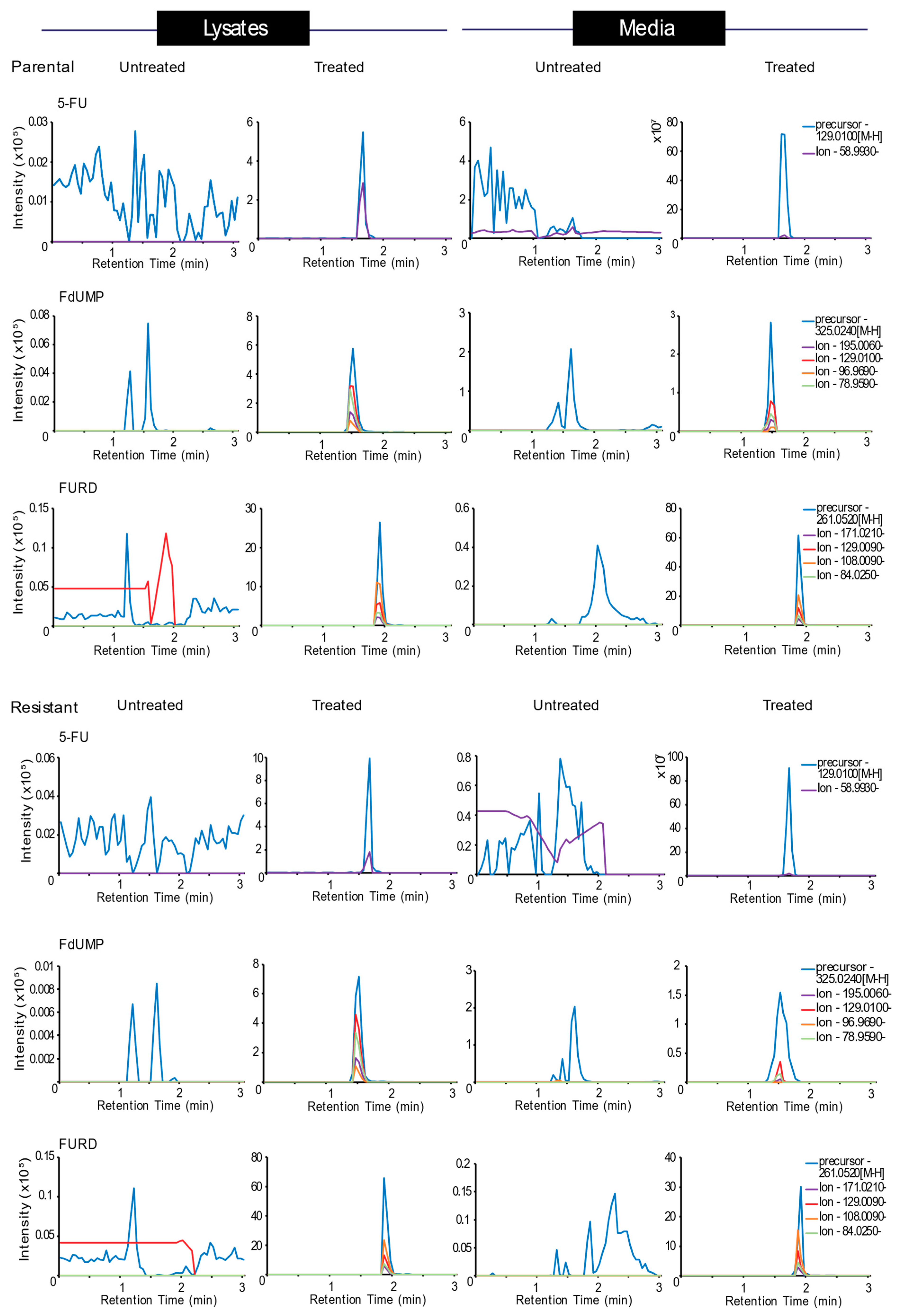
3.5. 5-FU Metabolism is Impaired in Resistant CRC Cells
3.6. FdUMP Sensitises 5-FU Resistant CRC Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics, 2009. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef]
- Weitz, J.; Koch, M.; Debus, J.; Hohler, T.; Galle, P.R.; Buchler, M.W. Colorectal cancer. Lancet 2005, 365, 153–165. [Google Scholar] [CrossRef]
- Chisanga, D.; Keerthikumar, S.; Pathan, M.; Ariyaratne, D.; Kalra, H.; Boukouris, S.; Mathew, N.A.; Saffar, H.A.; Gangoda, L.; Ang, C.S.; et al. Colorectal cancer atlas: An integrative resource for genomic and proteomic annotations from colorectal cancer cell lines and tissues. Nucleic Acids Res. 2016, 44, D969–D974. [Google Scholar] [CrossRef] [Green Version]
- Labianca, R.; Beretta, G.D.; Kildani, B.; Milesi, L.; Merlin, F.; Mosconi, S.; Pessi, M.A.; Prochilo, T.; Quadri, A.; Gatta, G.; et al. Colon cancer. Crit. Rev. Oncol. Hematol. 2010, 74, 106–133. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Oliveira, J. Primary colon cancer: ESMO clinical recommendations for diagnosis, adjuvant treatment and follow-up. Ann. Oncol. 2009, 20 (Suppl. 4), 49–50. [Google Scholar] [CrossRef] [PubMed]
- Tebbutt, N.C.; Cattell, E.; Midgley, R.; Cunningham, D.; Kerr, D. Systemic treatment of colorectal cancer. Eur. J. Cancer 2002, 38, 1000–1015. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.M.; Fazzone, W.; LaBonte, M.J.; Deng, J.; Neamati, N.; Ladner, R.D. Novel opportunities for thymidylate metabolism as a therapeutic target. Mol. Cancer Ther. 2008, 7, 3029–3037. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.S.; Wong, V.W.; Chan, C.M.; Ma, B.B.; Hui, E.P.; Wong, M.C.; Lam, M.Y.; Au, T.C.; Chan, W.H.; Cheuk, W.; et al. Identification of 5-fluorouracil response proteins in colorectal carcinoma cell line SW480 by two-dimensional electrophoresis and MALDI-TOF mass spectrometry. Oncol. Rep. 2008, 20, 89–98. [Google Scholar] [CrossRef]
- Agarwal, R.; Kaye, S.B. Ovarian cancer: Strategies for overcoming resistance to chemotherapy. Nat. Rev. Cancer 2003, 3, 502–516. [Google Scholar] [CrossRef]
- Chai, S.; To, K.K.; Lin, G. Circumvention of multi-drug resistance of cancer cells by Chinese herbal medicines. Chin. Med. 2010, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, R.; Cao, Z.; Nedeva, C.; Naim, S.; Bachmann, D.; Rabachini, T.; Gangoda, L.; Shahi, S.; Glab, J.; Menassa, J.; et al. BCL-2 family protein BOK is a positive regulator of uridine metabolism in mammals. Proc. Natl. Acad. Sci. USA 2019, 116, 15469–15474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Triest, B.; Pinedo, H.M.; van Hensbergen, Y.; Smid, K.; Telleman, F.; Schoenmakers, P.S.; van der Wilt, C.L.; van Laar, J.A.; Noordhuis, P.; Jansen, G.; et al. Thymidylate synthase level as the main predictive parameter for sensitivity to 5-fluorouracil, but not for folate-based thymidylate synthase inhibitors, in 13 nonselected colon cancer cell lines. Clin. Cancer Res. 1999, 5, 643–654. [Google Scholar] [PubMed]
- Fukuda, H.; Takiguchi, N.; Koda, K.; Oda, K.; Seike, K.; Miyazaki, M. Thymidylate synthase and dihydropyrimidine dehydrogenase are related to histological effects of 5-fluorouracil and cisplatin neoadjuvant chemotherapy for primary gastric cancer patients. Cancer Investig. 2006, 24, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Maurer, H.H. Multi-analyte procedures for screening for and quantification of drugs in blood, plasma, or serum by liquid chromatography-single stage or tandem mass spectrometry (LC-MS or LC-MS/MS) relevant to clinical and forensic toxicology. Clin. Biochem. 2005, 38, 310–318. [Google Scholar] [CrossRef]
- Schlittenbauer, L.; Seiwert, B.; Reemtsma, T. A false positive finding in liquid chromatography/triple quadrupole mass spectrometry analysis by a non-isobaric matrix component: The case of benzotriazole in urine for human biomonitoring. Rapid Commun. Mass Spectrom. 2016, 30, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Schneider, B.B.; Covey, T.R. On the Nature of Mass Spectrometer Analyzer Contamination. J. Am. Soc. Mass Spectrom. 2017, 28, 2384–2392. [Google Scholar] [CrossRef]
- Zhang, H.; Gu, H.; Yan, F.; Wang, N.; Wei, Y.; Xu, J.; Chen, H. Direct Characterization of Bulk Samples by Internal Extractive Electrospray Ionization Mass Spectrometry. Sci. Rep. 2013, 3, 2495. [Google Scholar] [CrossRef] [Green Version]
- Ruhaak, L.R.; van der Burgt, Y.E.M.; Cobbaert, C.M. Prospective applications of ultrahigh resolution proteomics in clinical mass spectrometry. Expert Rev. Proteom. 2016, 13, 1063–1071. [Google Scholar] [CrossRef]
- Gertsman, I.; Gangoiti, J.A.; Barshop, B.A. Validation of a dual LC-HRMS platform for clinical metabolic diagnosis in serum, bridging quantitative analysis and untargeted metabolomics. Metabolomics 2014, 10, 312–323. [Google Scholar] [CrossRef] [Green Version]
- Gallien, S.; Duriez, E.; Crone, C.; Kellmann, M.; Moehring, T.; Domon, B. Targeted proteomic quantification on quadrupole-orbitrap mass spectrometer. Mol. Cell. Proteom. 2012, 11, 1709–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Rúbies, A.; Centrich, F.; Granados, M.; Cortés-Francisco, N.; Caixach, J.; Companyó, R. Targeted analysis with benchtop quadrupole-orbitrap hybrid mass spectrometer: Application to determination of synthetic hormones in animal urine. Anal. Chim. Acta 2013, 780, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Carli, D.; Honorat, M.; Cohen, S.; Megherbi, M.; Vignal, B.; Dumontet, C.; Payen, L.; Guitton, J. Simultaneous quantification of 5-FU, 5-FUrd, 5-FdUrd, 5-FdUMP, dUMP and TMP in cultured cell models by LC-MS/MS. J. Chromatogr. B 2009, 877, 2937–2944. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Palacios, G.; Hartil, K.; Kurland, I.J. Advantages of tandem LC-MS for the rapid assessment of tissue-specific metabolic complexity using a pentafluorophenylpropyl stationary phase. J. Proteome Res. 2011, 10, 2104–2112. [Google Scholar] [CrossRef]
- Šmídová, B.; Šatínský, D.; Dostálová, K.; Solich, P. The pentafluorophenyl stationary phase shows a unique separation efficiency for performing fast chromatography determination of highbush blueberry anthocyanins. Talanta 2017, 166, 249–254. [Google Scholar] [CrossRef]
- Rathahao-Paris, E.; Alves, S.; Junot, C.; Tabet, J.-C.J.M. High resolution mass spectrometry for structural identification of metabolites in metabolomics. Metabolomics 2015, 12, 10. [Google Scholar] [CrossRef]
- Perez de Souza, L.; Naake, T.; Tohge, T.; Fernie, A.R. From chromatogram to analyte to metabolite. How to pick horses for courses from the massive web resources for mass spectral plant metabolomics. Gigascience 2017, 6, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Wohlgemuth, G.; Mehta, S.S.; Mejia, R.F.; Neumann, S.; Pedrosa, D.; Pluskal, T.; Schymanski, E.L.; Willighagen, E.L.; Wilson, M.; Wishart, D.S.; et al. SPLASH, a hashed identifier for mass spectra. Nat. Biotechnol. 2016, 34, 1099–1101. [Google Scholar] [CrossRef]
- MzCloud. Available online: https://www.mzcloud.org/ (accessed on 1 August 2018).
- Yang, X.; Neta, P.; Stein, S.E. Extending a Tandem Mass Spectral Library to Include MS2 Spectra of Fragment Ions Produced In-Source and MSn Spectra. J. Am. Soc. Mass Spectrom. 2017, 28, 2280–2287. [Google Scholar] [CrossRef]
- NIST-Tandem Mass Spectral Library. Available online: https://www.nist.gov/programs-projects/tandem-mass-spectral-library (accessed on 1 August 2018).
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Human Metabolome Database. Available online: http://www.hmdb.ca/metabolites (accessed on 1 July 2018).
- Allen, F.; Greiner, R.; Wishart, D. Competitive fragmentation modeling of ESI-MS/MS spectra for putative metabolite identification. Metabolomics 2015, 11, 98–110. [Google Scholar] [CrossRef] [Green Version]
- CFM-ID. Available online: http://cfmid.wishartlab.com/predict (accessed on 1 July 2018).
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 1 August 2018).
- Derissen, E.J.; Jacobs, B.A.; Huitema, A.D.; Rosing, H.; Schellens, J.H.; Beijnen, J.H. Exploring the intracellular pharmacokinetics of the 5-fluorouracil nucleotides during capecitabine treatment. Br. J. Clin. Pharmacol. 2016, 81, 949–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böcker, S.; Letzel, M.C.; Lipták, Z.; Pervukhin, A. Decomposing Metabolomic Isotope Patterns. In Algorithms in Bioinformatics; Bücher, P., Moret, B.M.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 12–23. [Google Scholar]
- Böcker, S.; Letzel, M.C.; Lipták, Z.; Pervukhin, A. SIRIUS: Decomposing isotope patterns for metabolite identification. Bioinformatics 2009, 25, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Böcker, S.; Rasche, F. Towards de novo identification of metabolites by analyzing tandem mass spectra. Bioinformatics 2008, 24, i49–i55. [Google Scholar] [CrossRef]
- Duhrkop, K.; Scheubert, K.; Bocker, S. Molecular Formula Identification with SIRIUS. Metabolites 2013, 3, 506–516. [Google Scholar] [CrossRef]
- SIRIUS Documentation RElease 4.0.1. Available online: file:///C:/Users/SANJAY/Downloads/sirius-4.0.1-manual.pdf (accessed on 1 August 2018).
- Meusel, M.; Hufsky, F.; Panter, F.; Krug, D.; Müller, R.; Böcker, S. Predicting the Presence of Uncommon Elements in Unknown Biomolecules from Isotope Patterns. Anal. Chem. 2016, 88, 7556–7566. [Google Scholar] [CrossRef]
- MacLean, B.; Tomazela, D.M.; Shulman, N.; Chambers, M.; Finney, G.L.; Frewen, B.; Kern, R.; Tabb, D.L.; Liebler, D.C.; MacCoss, M.J. Skyline: An open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010, 26, 966–973. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Chen, X.; Yan, Z.; Qin, S.; Xu, J.; Lin, J.; Yang, C.; Shui, W. Exploring skyline for both MS(E) -based label-free proteomics and HRMS quantitation of small molecules. Proteomics 2014, 14, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Skyline Small Molecule Targets. Available online: https://skyline.ms/_webdav/home/software/Skyline/@files/tutorials/Skyline%20Small%20Molecule%20Targets.pdf (accessed on 1 August 2018).
- Şengül, Ü. Comparing determination methods of detection and quantification limits for aflatoxin analysis in hazelnut. J. Food Drug Anal. 2016, 24, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Uhrovčík, J. Strategy for determination of LOD and LOQ values—Some basic aspects. Talanta 2014, 119, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.D.; Fyrestam, J.; Lanekoff, I. Advances in mass spectrometry based single-cell metabolomics. Analyst 2019, 144, 782–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, S.; Emami Khoonsari, P.; Aftab, O.; Krishnan, S.; Strömbom, E.; Larsson, R.; Hammerling, U.; Spjuth, O.; Kultima, K.; Gustafsson, M.J.M. Mass spectrometry based metabolomics for in vitro systems pharmacology: Pitfalls, challenges, and computational solutions. Metabolomics 2017, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Kosovec, J.E.; Egorin, M.J.; Gjurich, S.; Beumer, J.H. Quantitation of 5-fluorouracil (5-FU) in human plasma by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 224–230. [Google Scholar] [CrossRef]
- McEachran, A.D.; Balabin, I.; Cathey, T.; Transue, T.R.; Al-Ghoul, H.; Grulke, C.; Sobus, J.R.; Williams, A.J. Linking in silico MS/MS spectra with chemistry data to improve identification of unknowns. Sci. Data 2019, 6, 141. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Mizukoshi, T.; Hirayama, K.; Miyano, H. Comprehensive analytical method for the determination of hydrophilic metabolites by high-performance liquid chromatography and mass spectrometry. J. Agric. Food Chem. 2007, 55, 551–560. [Google Scholar] [CrossRef]
- Mani, D.R.; Abbatiello, S.E.; Carr, S.A. Statistical characterization of multiple-reaction monitoring mass spectrometry (MRM-MS) assays for quantitative proteomics. BMC Bioinform. 2012, 13 (Suppl. 16), S9. [Google Scholar] [CrossRef] [Green Version]
- Derissen, E.J.B.; Hillebrand, M.J.X.; Rosing, H.; Schellens, J.H.M.; Beijnen, J.H. Development of an LC–MS/MS assay for the quantitative determination of the intracellular 5-fluorouracil nucleotides responsible for the anticancer effect of 5-fluorouracil. J. Pharm. Biomed. Anal. 2015, 110, 58–66. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, Y.; Xu, S.-J.; Chen, W.-S. 5-Fluorouracil: Mechanisms of Resistance and Reversal Strategies. Molecules 2008, 13, 1551–1569. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, S.A.; Reiisi, S.; Ghiasi Tabari, P.; Shekari, A.; Aliakbari, F.; Azadfallah, E.; Elahian, F. Broad blocking of MDR efflux pumps by acetylshikonin and acetoxyisovalerylshikonin to generate hypersensitive phenotype of malignant carcinoma cells. Sci. Rep. 2018, 8, 3446. [Google Scholar] [CrossRef] [Green Version]
- Oguri, T.; Bessho, Y.; Achiwa, H.; Ozasa, H.; Maeno, K.; Maeda, H.; Sato, S.; Ueda, R. MRP8/ABCC11 directly confers resistance to 5-fluorouracil. Mol. Cancer Ther. 2007, 6, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riahi-Chebbi, I.; Souid, S.; Othman, H.; Haoues, M.; Karoui, H.; Morel, A.; Srairi-Abid, N.; Essafi, M.; Essafi-Benkhadir, K. The Phenolic compound Kaempferol overcomes 5-fluorouracil resistance in human resistant LS174 colon cancer cells. Sci. Rep. 2019, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Willingham, M.; Liu, J.; Gmeiner, W.H. Efficacy and safety of FdUMP[10] in treatment of HT-29 human colon cancer xenografts. Int. J. Oncol. 2002, 21, 303–308. [Google Scholar] [CrossRef] [PubMed]






| tSIM Settings | |
| MS1 resolution | 120,000 |
| MS1 AGC target | 200,000 |
| Max IT time (ms) | 200 |
| Isolation window | 4.0 m/z |
| Loop count | 4 |
| Isolation width | |
| ddMS2 Setting | |
| HCD NCE | 25 |
| Loop count | 4 |
| MS2 resolution | 15,000 |
| MS2 AGC target | 200,000 |
| Max IT time (ms) | 45 |
| Isolation window | 1.2 m/z |
| Charge exclusion | >2 |
| Target | Molecular Formula | Molecular Weight (g/mol) | m/z [M-H]- | Scan Filter |
|---|---|---|---|---|
| 5-FU | C4H3FN2O2 | 130.078 | 129.0109 | 127.0090–131.0090 |
| FdUMP | C9H12FN2O8P | 326.173 | 325.0211 | 323.0240–327.0240 |
| FURD | C9H11FN2O6 | 262.193 | 261.0529 | 259.0520–263.0520 |
| FdURD | C9H11FN2O5 | 246.194 | 245.0605 | 243.0570–247.0570 |
| FUTP | C9H14FN2O15P3 | 502.13 | 500.9517 | 498.9510–502.9510 |
| FdUTP | C9H14FN2O14P3 | 486.131 | 484.956 | 482.9560–486.9560 |
| dUMP | C9H13N2O8P | 308.183 | 307.0338 | 305.0330–309.0330 |
| TMP | C10H15N2O8P | 322.21 | 321.0497 | 319.0480–323.0480 |
| Compound | Molecular Weight (g/mol) | Retention Time (min) | Precursor (m/z) | Fragment (m/z) |
|---|---|---|---|---|
| 5-FU | 130.0173 | 1.6 | 129.01 | 58.993 |
| FdUMP | 326.0313 | 1.4 | 325.024 | 195.006 |
| 129.01 | ||||
| 96.969 | ||||
| 78.959 | ||||
| FURD | 262.0593 | 1.9 | 261.052 | 171.021 |
| 129.009 | ||||
| 108.009 | ||||
| 84.025 | ||||
| FdURD | 246.0643 | 2.1 | 245.057 | 155.025 |
| 129.009 | ||||
| 112.0204 | ||||
| FUTP | 501.9583 | - | 500.951 | 482.9407 |
| 158.900 | ||||
| 129.01 | ||||
| FdUTP | 485.9633 | - | 484.956 | 441.9506 |
| 256.80 | ||||
| 129.01 | ||||
| dUMP | 308.0403 | 1.4 | 307.033 | 195.006 |
| 111.02 | ||||
| 96.9696 | ||||
| TMP | 322.0553 | 1.5 | 321.048 | 195.005 |
| 125.0355 | ||||
| 96.9691 |
| Sirius Compound Prediction | ||||||
|---|---|---|---|---|---|---|
| Compound | PubChem | All Molecular Formula | ||||
| Score | Tree Score | Median mass Deviation (ppm) | Score | Tree Score | Median Mass Deviation (ppm) | |
| Standards (25 ng) | ||||||
| FdUMP Precursors | 100 | −3.75 | 12.05 | −3.57 | ||
| FdUMP Products | 100 | 37.55 | 0.27 | 14.41 | 14.75 | 0.78 |
| 5-FU Precursor | 33.33 | 0.43 | 97.91 | 0 | 0.43 | |
| 5-FU Products | 99.97 | 8.06 | −2.21 | 100 | 15.45 | 3.07 |
| Test Samples | ||||||
| 5-FU Precursor | 33.33 | 0.75 | 97.91 | 0.75 | ||
| 5-FU Products | 99.84 | 7.22 | −4.6 | 100 | 27.22 | −2.35 |
| FdUMP Precursors | 100 | −3.57 | 12.05 | −3.57 | ||
| FdUMP Products | 100 | 27.34 | −2.2 | 0.46 | 18.83 | −2.97 |
| FURD Precursor | 50 | −0.65 | 43.67 | −0.65 | ||
| FURD Products | 3.03 | 106.22 | −2.96 | 98.06 | 53.99 | 1.81 |
| FdURd Precursor | 12.8 | −0.2 | 77.78 | −0.2 | ||
| FdURD Products | 86.8 | 90.1 | −3.15 | 97.68 | 72.41 | 3.3 |
| dUMP Precursor | 20 | 0.8 | 12 | 0.8 | ||
| dUMP Products | 99.3 | 52.65 | 1.1 | 100 | 41.99 | −0.5 |
| TMP Precursors | 12.5 | 1.51 | 7.96 | 1.28 | ||
| TMP Products | 100 | 31.59 | 0.69 | 62.18 | 31.59 | 0.69 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahi, S.; Ang, C.-S.; Mathivanan, S. A High-Resolution Mass Spectrometry-Based Quantitative Metabolomic Workflow Highlights Defects in 5-Fluorouracil Metabolism in Cancer Cells with Acquired Chemoresistance. Biology 2020, 9, 96. https://doi.org/10.3390/biology9050096
Shahi S, Ang C-S, Mathivanan S. A High-Resolution Mass Spectrometry-Based Quantitative Metabolomic Workflow Highlights Defects in 5-Fluorouracil Metabolism in Cancer Cells with Acquired Chemoresistance. Biology. 2020; 9(5):96. https://doi.org/10.3390/biology9050096
Chicago/Turabian StyleShahi, Sanjay, Ching-Seng Ang, and Suresh Mathivanan. 2020. "A High-Resolution Mass Spectrometry-Based Quantitative Metabolomic Workflow Highlights Defects in 5-Fluorouracil Metabolism in Cancer Cells with Acquired Chemoresistance" Biology 9, no. 5: 96. https://doi.org/10.3390/biology9050096
APA StyleShahi, S., Ang, C.-S., & Mathivanan, S. (2020). A High-Resolution Mass Spectrometry-Based Quantitative Metabolomic Workflow Highlights Defects in 5-Fluorouracil Metabolism in Cancer Cells with Acquired Chemoresistance. Biology, 9(5), 96. https://doi.org/10.3390/biology9050096







