Impact of Cytokines and Phosphoproteins in Response to Chronic Joint Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Collection of Tissue Samples
2.3. Sample Preparation
2.4. Cytokine and Phosphoprotein Measurement
2.5. Data Processing and Statistical Analysis
2.6. Network Evaluation with Ingenuity Pathway Analysis (IPA)
2.7. Network Centrality Parameter Analysis
3. Results
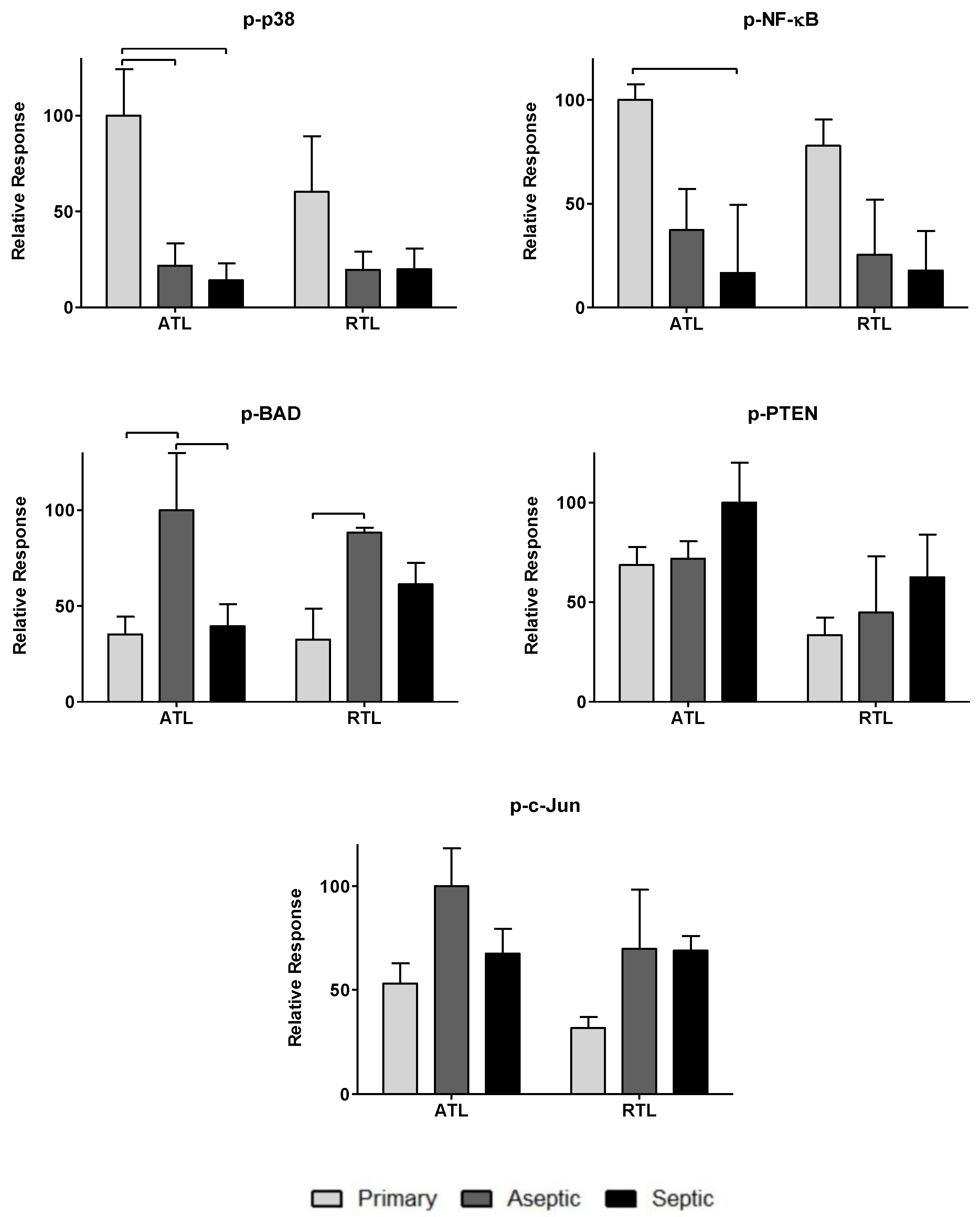
3.1. Relative Spatial Cytokine Responses
3.2. Relative Spatial Phosphoprotein Responses
3.3. IPA-Generated Networks
3.4. Normalized Radiality of All 30 Nodes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, H.; Zhou, J.; Zhong, Y.; Ali, M.M.; McGuire, F.; Nagarkatti, P.S.; Nagarkatti, M. Role of cytokines as a double-edged sword in sepsis. In Vivo 2013, 27, 669–684. [Google Scholar] [PubMed]
- Cavaillon, J.M.; Adib-Conquy, M.; Fitting, C.; Adrie, C.; Payen, D. Cytokine cascade in sepsis. Scand. J. Infect. Dis. 2003, 35, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.; Mostertz, J.; Hochgräfe, F. Proteomic discovery of host kinase signaling in bacterial infections. Proteomics Clin. Appl. 2016, 10, 994–1010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, J.; Ye, J.; Ashraf, U.; Chen, Z.; Zhu, B.; He, W.; Xu, Q.; Wei, Y.; Chen, H.; et al. Quantitative label-free phosphoproteomics reveals differentially regulated protein Phosphorylation involved in West Nile virus-induced host inflammatory response. J. Proteome Res. 2015, 14, 5157–5168. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, H.; Yin, Y.L.; Guo, W.Z.; Ma, Y.Q.; Wang, Y.B.; Shu, C.; Dong, L.Q. Role of interleukin-6 to differentiate sepsis from non-infectious systemic inflammatory response syndrome. Cytokine 2016, 88, 126–135. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Seah, C.C.; Phillips, T.J.; Howard, C.E.; Panova, I.P.; Hayes, C.M.; Asandra, A.S.; Park, H.Y. Chronic wound fluid suppresses proliferation of dermal fibroblasts through a Ras-mediated signaling pathway. J. Investig. Dermatol. 2005, 124, 466–474. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Rämet, M.; Lanot, R.; Zachary, D.; Manfruelli, P. JNK signaling pathway is required for efficient wound healing in Drosophila. Dev. Biol. 2002, 241, 145–156. [Google Scholar] [CrossRef]
- Brady, R.A.; Mocca, C.P.; Plaut, R.D.; Takeda, K.; Burns, D.L. Comparison of the immune response during acute and chronic Staphylococcus aureus infection. PLoS ONE 2018, 13, e0195342. [Google Scholar] [CrossRef]
- Li, C.; Renz, N.; Trampuz, A. Management of periprosthetic joint infection. Hip Pelvis 2018, 30, 138–146. [Google Scholar] [CrossRef]
- Seebach, E.; Kubatzky, K.F. Chronic implant-related bone infections—Can immune modulation be a therapeutic strategy? Front. Immunol. 2019, 10, 1724. [Google Scholar] [CrossRef]
- Lüthje, F.L.; Jensen, L.K.; Jensen, H.E.; Skovgaard, K. The inflammatory response to bone infection—A review based on animal models and human patients. APMIS 2020, 128, 275–286. [Google Scholar] [CrossRef]
- Grant, S.S.; Hung, D.T. Persistent bacterial infections, antibiotic tolerance, and the oxidative stress response. Virulence 2013, 4, 273–283. [Google Scholar] [CrossRef]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef]
- Kalore, N.V.; Gioe, T.J.; Singh, J.A. Diagnosis and management of infected total knee arthroplasty. Open Orthop. J. 2011, 5, 86–91. [Google Scholar] [CrossRef]
- Dyskova, T.; Kriegova, E.; Slobodova, Z.; Zehnalova, S.; Kudelka, M.; Schneiderova, P.; Fillerova, R.; Gallo, J. Inflammation time-axis in aseptic loosening of total knee arthroplasty: A preliminary study. PLoS ONE 2019, 14, e0221056. [Google Scholar] [CrossRef]
- Morel, P.A.; Lee, R.E.C.; Faeder, J.R. Demystifying the cytokine network: Mathematical models point the way. Cytokine 2017, 98, 115–123. [Google Scholar] [CrossRef]
- Thomas, S.; Bonchev, D. A survey of current software for network analysis in molecular biology. Hum. Genom. 2010, 4, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Van Riel, N.A. Dynamic modelling and analysis of biochemical networks: Mechanism-based models and model-based experiments. Brief. Bioinf. 2006, 7, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Scardoni, G.; Laudanna, C. Centralities based analysis of complex networks. In New Frontiers in Graph Theory; Zhang, Y., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Abedi, M.; Gheisari, Y. Nodes with high centrality in protein interaction networks are responsible for driving signaling pathways in diabetic nephropathy. PeerJ 2015, 3, e1284. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Schork, N.J. Utility of network integrity methods in therapeutic target identification. Front. Genet. 2014, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, J.; Jagadish, T.; Haverland, N.A.; Madson, C.J.; Ciborowski, P.; Belshan, M. Alterations in the nuclear proteome of HIV-1 infected T-cells. Virology 2014, 468, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 definition of periprosthetic hip and knee infection: An evidence-based and validated criteria. J. Arthroplast. 2018, 33, 1309–1314. [Google Scholar] [CrossRef]
- Qasim, S.N.; Swann, A.; Ashford, R. The DAIR (debridement, antibiotics and implant retention) procedure for infected total knee replacement—A literature review. Sicot J. 2017, 3, 2. [Google Scholar] [CrossRef]
- Hulse, R.E.; Kunkler, P.E.; Fedynyshyn, J.P.; Kraig, R.P. Optimization of multiplexed bead-based cytokine immunoassays for rat serum and brain tissue. J. Neurosci. Methods 2004, 136, 87–98. [Google Scholar] [CrossRef]
- Couture, C.; Desjardins, P.; Zaniolo, K.; Germain, L.; Guérin, S.L. Enhanced wound healing of tissue-engineered human corneas through altered phosphorylation of the CREB and AKT signal transduction pathways. Acta Biomater. 2018, 73, 312–325. [Google Scholar] [CrossRef]
- Atalay, M.; Oksala, N.; Lappalainen, J.; Laaksonen, D.E.; Sen, C.K.; Roy, S. Heat shock proteins in diabetes and wound healing. Curr. Protein Pept. Sci. 2009, 10, 85–95. [Google Scholar] [CrossRef]
- Reid, M.B.; Li, Y.P. Cytokines and oxidative signalling in skeletal muscle. Acta Physiol. Scand. 2001, 171, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Guan, D.W.; Xiong, C.Y.; Cheng, Z.H.; Yu, T.S. Expression of c-jun during the incised wound healing in mice skin. Fa Yi Xue Za Zhi 2009, 25, 401–404. [Google Scholar] [PubMed]
- Hosokawa, R.; Urata, M.M.; Ito, Y.; Bringas, P.; Chai, Y. Functional significance of Smad2 in regulating basal keratinocyte migration during wound healing. J. Investig. Dermatol. 2005, 125, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Nédellec, S.; Renaudineau, Y.; Bordron, A.; Berthou, C.; Porakishvili, N.; Lydyard, P.M.; Pers, J.O.; Youinou, P. B cell response to surface IgM cross-linking identifies different prognostic groups of B-chronic lymphocytic leukemia patients. J. Immunol. 2005, 174, 3749–3756. [Google Scholar] [CrossRef]
- Vollmar, B.; El-Gibaly, A.M.; Scheuer, C.; Strik, M.W.; Bruch, H.P.; Menger, M.D. Acceleration of cutaneous wound healing by transient p53 inhibition. Lab. Invest. 2002, 82, 1063–1071. [Google Scholar] [CrossRef]
- Matsubayashi, Y.; Ebisuya, M.; Honjoh, S.; Nishida, E. ERK activation propagates in epithelial cell sheets and regulates their migration during wound healing. Curr. Biol. 2004, 14, 731–735. [Google Scholar] [CrossRef]
- Zhao, M. PTEN: A promising pharmacological target to enhance epithelial wound healing. Br. J. Pharmacol. 2007, 152, 1141–1144. [Google Scholar] [CrossRef]
- Downward, J. How BAD phosphorylation is good for survival. Nat. Cell Biol 1999, 1, E33–E35. [Google Scholar] [CrossRef]
- Lin, L.; White, S.A.; Hu, K. Role of p90RSK in kidney and other diseases. Int. J. Mol. Sci. 2019, 20, 972. [Google Scholar] [CrossRef]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef]
- Zhang, J.; Bárdos, T.; Shao, Q.; Tschopp, J.; Mikecz, K.; Glant, T.T.; Finnegan, A. IL-4 potentiates activated T cell apoptosis via an IL-2-dependent mechanism. J. Immunol. 2003, 170, 3495–3503. [Google Scholar] [CrossRef] [PubMed]
- Matias, M.A.; Saunus, J.M.; Ivanovski, S.; Walsh, L.J.; Farah, C.S. Accelerated wound healing phenotype in Interleukin 12/23 deficient mice. J. Inflamm. 2011, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Kaviratne, M.; Hesse, M.; Leusink, M.; Cheever, A.W.; Davies, S.J.; McKerrow, J.H.; Wakefield, L.M.; Letterio, J.J.; Wynn, T.A. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. J. Immunol. 2004, 173, 4020–4029. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Feng, M.; Li, Y.; Liu, Z. Application of outlier mining in insider identification based on boxplot method. Proc. Comput. Sci. 2016, 91, 245–251. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Multiple significance tests: The Bonferroni method. BMJ 1995, 310, 170. [Google Scholar] [CrossRef]
- Ghosh, S.; Zang, S.; Mitra, P.S.; Ghimbovschi, S.; Hoffman, E.P.; Dutta, S.K. Global gene expression and ingenuity biological functions analysis on PCBs 153 and 138 induced human PBMC in vitro reveals differential mode(s) of action in developing toxicities. Environ. Int. 2011, 37, 838–857. [Google Scholar] [CrossRef]
- Bonnet, A.; Lagarrigue, S.; Liaubet, L.; Robert-Granié, C.; Sancristobal, M.; Tosser-Klopp, G. Pathway results from the chicken data set using GOTM, Pathway Studio and Ingenuity softwares. BMC Proc. 2009, 3. [Google Scholar] [CrossRef]
- Han, A.A.; Currie, H.N.; Loos, M.S.; Vrana, J.A.; Fabyanic, E.B.; Prediger, M.S.; Boyd, J.W. Spatiotemporal phosphoprotein distribution and associated cytokine response of a traumatic injury. Cytokine 2016, 79, 12–22. [Google Scholar] [CrossRef]
- Monastero, R.N.; Pentyala, S. Cytokines as biomarkers and their respective clinical cutoff levels. Int. J. Inflam. 2017, 2017, 4309485. [Google Scholar] [CrossRef]
- Gawel, D.R.; Serra-Musach, J.; Lilja, S.; Aagesen, J.; Arenas, A.; Asking, B.; Bengnér, M.; Björkander, J.; Biggs, S.; Ernerudh, J.; et al. A validated single-cell-based strategy to identify diagnostic and therapeutic targets in complex diseases. Genome Med. 2019, 11, 47. [Google Scholar] [CrossRef]
- Heim, C.E.; Vidlak, D.; Scherr, T.D.; Hartman, C.W.; Garvin, K.L.; Kielian, T. IL-12 promotes myeloid-derived suppressor cell recruitment and bacterial persistence during Staphylococcus aureus orthopedic implant infection. J. Immunol. 2015, 194, 3861–3872. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; McNally, A.K. Biocompatibility of implants: Lymphocyte/macrophage interactions. Semin. Immunopathol. 2011, 33, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Peranteau, W.H.; Zhang, L.; Muvarak, N.; Badillo, A.T.; Radu, A.; Zoltick, P.W.; Liechty, K.W. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J. Investig. Dermatol. 2008, 128, 1852–1860. [Google Scholar] [CrossRef]
- Han, A.A.; Currie, H.N.; Loos, M.S.; Scardoni, G.; Miller, J.V.; Prince, N.; Mouch, J.A.; Boyd, J.W. The impact of cytokine responses in the intra- and extracellular signaling network of a traumatic injury. Cytokine 2018, 106, 136–147. [Google Scholar] [CrossRef]
- Lee, S.; Kim, M.S.; Jung, S.J.; Kim, D.; Park, H.J.; Cho, D. ERK activating peptide, AES16-2M promotes wound healing through accelerating migration of keratinocytes. Sci. Rep. 2018, 8, 14398. [Google Scholar] [CrossRef]
- François, F.; Godinho, M.J.; Grimes, M.L. CREB is cleaved by caspases during neural cell apoptosis. FEBS Lett. 2000, 486, 281–284. [Google Scholar] [CrossRef]
- Mayer, C.; Franz, A.; Harmsen, J.F.; Queitsch, F.; Behringer, M.; Beckmann, J.; Krauspe, R.; Zilkens, C. Soft-tissue damage during total knee arthroplasty: Focus on tourniquet-induced metabolic and ionic muscle impairment. J. Orthop. 2017, 14, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.H.M.; Fessler, M.B.; Chowdhury, S.M. Comparative and network-based proteomic analysis of low dose ethanol- and lipopolysaccharide-induced macrophages. PLoS ONE 2018, 13, e0193104. [Google Scholar] [CrossRef]






| ID | Sex | TKA/TKR | BMI (kg/m2) | Diabetic (Y/N) | CRP (mg/L) | Culture |
|---|---|---|---|---|---|---|
| P1 | F | TKA | 33.8 | N | N/A | Negative |
| P2 | F | TKA | 39.8 | N | N/A | Negative |
| P3 | F | TKA | 39.8 | N | N/A | Negative |
| P4 | M | TKA | 29.7 | Y | N/A | Negative |
| P5 | M | TKA | 24.6 | N | N/A | Negative |
| P6 | M | TKA | 27.2 | N | N/A | Negative |
| F1 | F | TKR—Aseptic | 28.2 | N | 4.3 | Negative |
| F2 | F | TKR—Aseptic | 29.8 | N | 0.2 | Negative |
| F3 | F | TKR—Aseptic | 33.9 | N | <1 | Negative |
| F4 | M | TKR—Aseptic | 40.4 | Y | 3.6 | Negative |
| F5 | M | TKR—Aseptic | 26.2 | N | 2.1 | Negative |
| F6 | F | TKR—Septic | 43.7 | N | 28.8 | S. epidermidis |
| F7 | F | TKR—Septic | 30.8 | Y | 161.4 | S. epidermidis |
| F8 | F | TKR—Septic | 41.9 | N | 21.7 | E. cloaecae |
| F9 | M | TKR—Septic | 36.2 | N | 33.5 | MSSA |
| F10 | M | TKR—Septic | 33.8 | Y | 3.8 | S. epidermidis |
| F11 | M | TKR—Septic | 31.9 | N | 111.9 | S. epidermidis |
| Target | Relevant Functions in Acute Wound Healing Response | |
|---|---|---|
| Phosphoprotein (site) | p-CREB (Ser133) | Inhibition of CREB via phosphorylation promotes wound closure [30] |
| p-HSP27 (Ser78) | Activation of HSP27 may inhibit stress-induced apoptosis [31] | |
| p-IκBα (Ser32/Ser36) | Pro-wound healing, inhibits actions of NF-κB [32] | |
| p-MEK1 (Ser217/Ser221) | Essential for migration of epithelial layers [33] | |
| p-S6RP (Ser235/Ser236) | Activated during proliferative growth phase [30] | |
| p-Smad2 (Ser465/Ser467) | Regulates keratinocyte migration during proliferation [34] | |
| p-Src (Tyr416) | Promotes keratinocyte migration in wound healing [32] | |
| p-Syk (Tyr352) | Important for cellular migration in wound healing [35] | |
| p-c-Jun (Ser63) | Induces apoptosis of immune cells in skin wound healing [33] | |
| p-AKT (Ser473) | Phosphorylation of AKT promotes wound closure [30] | |
| p-p53 (Ser15) | Activated p53 accelerates cutaneous wound healing by increasing cell proliferation [36] | |
| p-p38 (Thr180/Tyr182) | Activated p38 involved in muscle catabolism [32] | |
| p-p70S6K (Ser380) | Growth factor associated with cell proliferation [37] | |
| p-PTEN (Ser380) | Pro-apoptotic, inhibits acute wound healing [38] | |
| p-ZAP-70 (Tyr319) | Stimulates cell migration during wound healing [35] | |
| p-BAD (Ser136) | Phosphorylation of BAD activates pro-apoptotic functions [39] | |
| p-ERK1/2 (Thr202/Tyr204) | Important for early proliferative response in wound healing [37] | |
| p-GSK-3α/β (Ser21/Ser9) | Controls wound healing and fibrosis progression [30] | |
| p-p90RSK (Ser380) | Downstream effector of MEK/ERK pathway in wound healing, regulator of cell migration [40] | |
| p-VEGFR2 (Tyr1175) | Stimulates angiogenic cascade during re-epithelialization [41] | |
| p-NF-κB p65 (Ser536) | Linked to muscle atrophy and catabolism [32] | |
| Cytokine | IL-1β | Early initiator of infection-driven inflammation [2] |
| IL-4 | Anti-inflammatory cytokine that activates Stat6, suppressing cell death [42] | |
| IL-6 | Initiator of early inflammatory response to implants and infection [2] | |
| IL-1α | Early recruitment of immune cells in response to infection [2] | |
| IL-10 | Down-regulator of several inflammatory cytokines (i.e., IL-1, IL-6, IL-12, IFN-γ, TNF-α) [43] | |
| IL-12p70 | Pro-inflammatory cytokine involved in adaptive immunity, produced by activated immune cells [43] | |
| IL-13 | Th2-associated cytokine critical in tissue remodeling [44] | |
| IFN-γ | Anti-inflammatory cytokine that has been associated with inhibition of wound healing [43] | |
| TNF-α | Early pro-inflammatory mediator of inflammation [2] |
| Primary TKA | Aseptic TKR | Septic TKR | |||
|---|---|---|---|---|---|
| IPA Network | p-Score | IPA Network | p-Score | IPA Network | p-Score |
| Cell-mediated immune response, cellular development, cellular function and maintenance | 72 | Inflammatory response, cellular movement, cell death and survival | 16 | Cellular movement, inflammatory response, hematological development and function | 16 |
| Cancer, organismal injury and abnormalities, cell cycle | 2 | Cell-mediated immune response, cellular development, cellular function and maintenance | 9 | Cell death and survival, organismal injury and abnormalities, cellular development | 9 |
| Node | ATL Primary TKA | ATL Aseptic TKR | ATL Septic TKR |
|---|---|---|---|
| p-CREB | 0.96 | 0.77 | 1.15 |
| p-HSP27 | 1.13 | 1.14 | 1.15 |
| p-IκBα | 1.13 | 1.16 | 1.10 |
| p-MEK1 | 1.13 | 1.08 | 1.10 |
| p-S6RP | 1.13 | 0.98 | 1.13 |
| p-Smad2 | 1.13 | 1.15 | 1.14 |
| p-Src | 1.13 | 1.15 | 1.15 |
| p-Syk | 1.13 | 1.11 | 0.95 |
| p-c-Jun | 1.04 | 0.77 | 1.03 |
| p-AKT | 1.10 | 0.99 | 1.08 |
| p-p53 | 1.13 | 1.00 | 1.06 |
| p-p38 | 1.13 | 1.05 | 1.06 |
| p-p70SK6 | 1.13 | 1.15 | 1.07 |
| p-PTEN | 1.09 | 1.02 | 0.76 |
| p-ZAP-70 | 1.13 | 1.16 | 1.07 |
| p-BAD | 0.96 | 0.77 | 1.15 |
| p-ERK1/2 | 1.13 | 1.15 | 1.13 |
| p-GSK-3a/b | 1.13 | 1.16 | 1.12 |
| p-p90RSK | 1.13 | 0.99 | 1.04 |
| p-VEGFR2 | 1.13 | 0.99 | 1.11 |
| p-NF-kB | 1.13 | 1.16 | 1.09 |
| IL-1b | 0.62 | 0.91 | 0.76 |
| IL-4 | 0.90 | 1.01 | 0.76 |
| IL-6 | 0.60 | 0.82 | 0.76 |
| IL-1a | 0.63 | 1.15 | 0.76 |
| IL-10 | 0.73 | 0.79 | 1.02 |
| IL-12p70 | 0.84 | 0.77 | 1.06 |
| IL-13 | 0.84 | 0.77 | 0.76 |
| IFN-y | 0.85 | 0.96 | 0.76 |
| TNF-a | 0.85 | 0.93 | 0.76 |
| Node | RTL Primary TKA | RTL Aseptic TKR | RTL Septic TKR |
|---|---|---|---|
| p-CREB | 1.12 | 0.79 | 1.06 |
| p-HSP27 | 0.87 | 1.12 | 1.03 |
| p-IκBα | 1.07 | 1.13 | 1.01 |
| p-MEK1 | 0.99 | 1.05 | 1.08 |
| p-S6RP | 1.06 | 0.98 | 1.02 |
| p-Smad2 | 1.12 | 1.11 | 1.10 |
| p-Src | 0.97 | 1.08 | 1.04 |
| p-Syk | 1.01 | 0.96 | 0.94 |
| p-c-Jun | 1.11 | 0.97 | 0.99 |
| p-AKT | 0.80 | 1.12 | 1.09 |
| p-p53 | 0.97 | 1.07 | 1.00 |
| p-p38 | 1.11 | 1.08 | 1.06 |
| p-p70SK6 | 0.80 | 1.13 | 1.00 |
| p-PTEN | 1.12 | 1.12 | 1.05 |
| p-ZAP-70 | 1.11 | 0.96 | 1.07 |
| p-BAD | 1.12 | 0.79 | 1.06 |
| p-ERK1/2 | 0.83 | 1.09 | 1.11 |
| p-GSK-3a/b | 1.12 | 1.12 | 1.02 |
| p-p90RSK | 1.10 | 1.00 | 0.95 |
| p-VEGFR2 | 1.09 | 1.13 | 1.10 |
| p-NF-kB | 1.04 | 1.12 | 1.04 |
| IL-1b | 0.85 | 0.99 | 1.08 |
| IL-4 | 1.04 | 1.03 | 1.00 |
| IL-6 | 0.82 | 0.89 | 1.11 |
| IL-1a | 0.84 | 1.09 | 1.11 |
| IL-10 | 0.95 | 0.64 | 0.70 |
| IL-12p70 | 0.96 | 0.92 | 1.07 |
| IL-13 | 1.01 | 0.74 | 0.59 |
| IFN-y | 1.01 | 0.75 | 0.73 |
| TNF-a | 1.00 | 1.00 | 0.80 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prince, N.; Penatzer, J.A.; Dietz, M.J.; Boyd, J.W. Impact of Cytokines and Phosphoproteins in Response to Chronic Joint Infection. Biology 2020, 9, 167. https://doi.org/10.3390/biology9070167
Prince N, Penatzer JA, Dietz MJ, Boyd JW. Impact of Cytokines and Phosphoproteins in Response to Chronic Joint Infection. Biology. 2020; 9(7):167. https://doi.org/10.3390/biology9070167
Chicago/Turabian StylePrince, Nicole, Julia A. Penatzer, Matthew J. Dietz, and Jonathan W. Boyd. 2020. "Impact of Cytokines and Phosphoproteins in Response to Chronic Joint Infection" Biology 9, no. 7: 167. https://doi.org/10.3390/biology9070167
APA StylePrince, N., Penatzer, J. A., Dietz, M. J., & Boyd, J. W. (2020). Impact of Cytokines and Phosphoproteins in Response to Chronic Joint Infection. Biology, 9(7), 167. https://doi.org/10.3390/biology9070167





