The Antiviral Properties of Cyclosporine. Focus on Coronavirus, Hepatitis C Virus, Influenza Virus, and Human Immunodeficiency Virus Infections
Abstract
1. Introduction
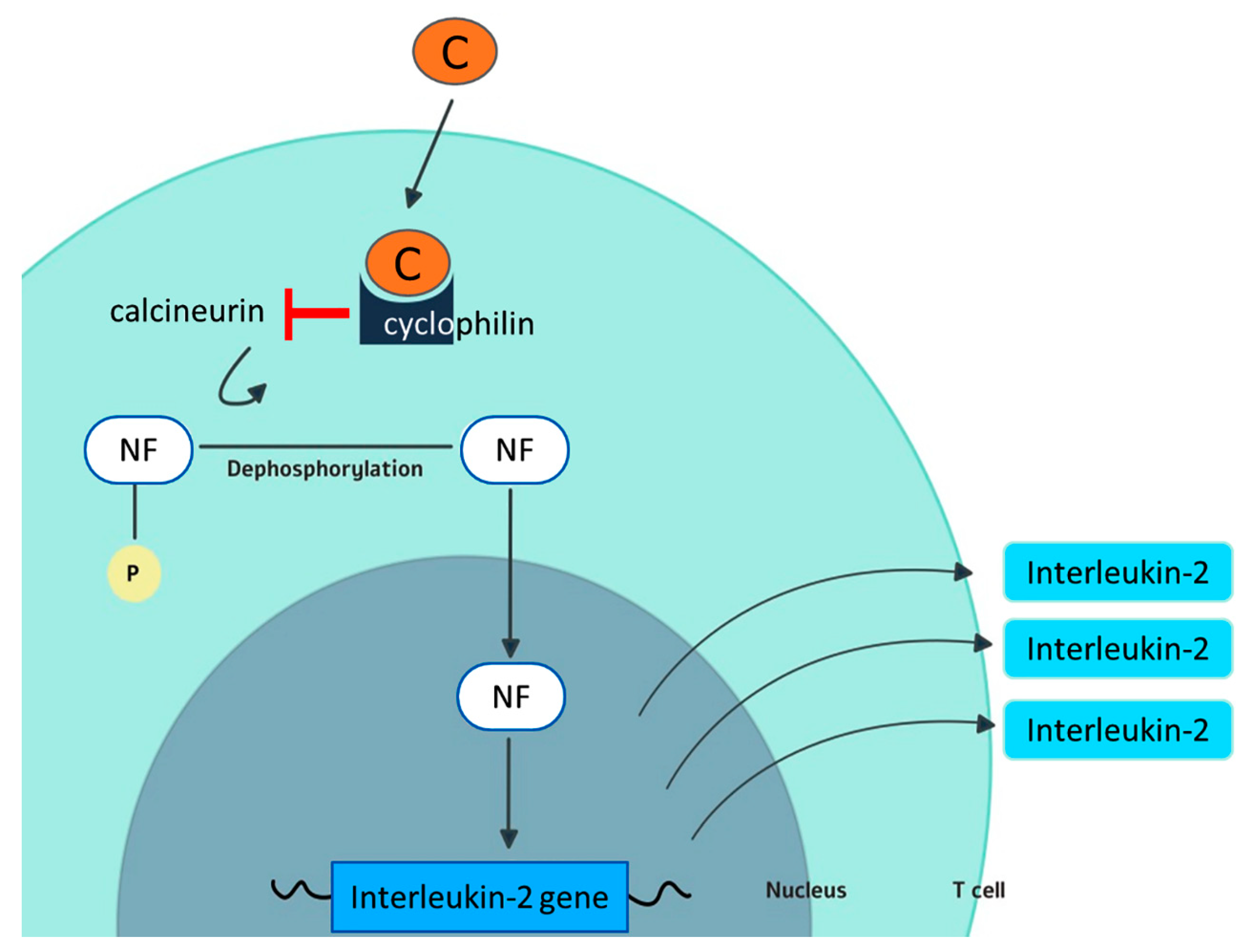
2. Cyclosporine and Cyclophilins
3. Cyclosporine and Viral Infections
3.1. Hepatitis B Virus
3.2. Hepatitis C Virus
3.3. Hepatitis D Virus
3.4. Hepatitis E Virus
3.5. Cytomegalovirus
3.6. Herpes Simplex Virus
3.7. Human Papilloma Virus
3.8. Influenza Virus
3.9. Rotavirus
3.10. Herpes Zoster Virus
3.11. Human Immunodeficiency Virus
3.11.1. Human Herpesvirus-8
3.11.2. Coronaviruses
4. Cyclosporine and Bacterial Diseases
5. Cyclosporine and Parasitic Diseases
6. Conclusions
Funding
Conflicts of Interest
References
- Amor, K.T.; Ryan, C.; Menter, A. The use of cyclosporine in dermatology: Part I. J. Am. Acad. Dermatol. 2010, 63, 925–946. [Google Scholar] [CrossRef]
- Ryan, C.; Amor, K.T.; Menter, A. The use of cyclosporine in dermatology: Part II. J. Am. Acad. Dermatol. 2010, 63, 949–972. [Google Scholar] [CrossRef]
- Tapia, C.; Nessel, T.A.; Zito, P.M. Cyclosporine. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2020. [Google Scholar]
- Lai, V.W.Y.; Chen, G.; Gin, D.; Sinclair, R. Cyclosporine for moderate-to-severe alopecia areata: A double-blind, randomized, placebo-controlled clinical trial of efficacy and safety. J. Am. Acad. Dermatol. 2019, 81, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, L.; Glowacka, P.; Goldust, M.; Sikora, M.; Sar-Pomian, M.; Rakowska, A.; Samochocki, Z.; Olszewska, M. Cyclosporine therapy during the COVID-19 pandemic. J. Am. Acad. Dermatol. 2020, 83, e151–e152. [Google Scholar] [CrossRef]
- Dawar, F.U.; Tu, J.; Khattak, M.N.; Mei, J.; Lin, L. Cyclophilin A: A Key Factor in Virus Replication and Potential Target for Anti-viral Therapy. Curr. Issues Mol. Biol. 2017, 21, 1–20. [Google Scholar] [CrossRef]
- Nigro, P.; Pompilio, G.; Capogrossi, M.C. Cyclophilin A: A key player for human disease. Cell Death Dis. 2013, 4, e888. [Google Scholar] [CrossRef] [PubMed]
- Ma-Lauer, Y.; Zheng, Y.; Malesevic, M.; Von Brunn, B.; Fischer, G.; Von Brunn, A. Influences of cyclosporin A and non-immunosuppressive derivatives on cellular cyclophilins and viral nucleocapsid protein during human coronavirus 229E replication. Antivir. Res. 2020, 173, 104620. [Google Scholar] [CrossRef] [PubMed]
- Colombo, D.; Chimenti, S.; Grossi, P.; Marchesoni, A.; Di Nuzzo, S.; Griseta, V.; Gargiulo, A.; Parodi, A.; Simoni, L.; Bellia, G. Prevalence of past and reactivated viral infections and efficacy of cyclosporine A as monotherapy or in combination in patients with psoriatic arthritis—Synergy study: A longitudinal observational study. BioMed. Res. Int. 2014, 2014, 941767. [Google Scholar] [CrossRef]
- Motaparthi, K.; Stanisic, V.; Van Voorhees, A.S.; Lebwohl, M.G.; Hsu, S. From the Medical Board of the National Psoriasis Foundation: Recommendations for screening for hepatitis B infection prior to initiating anti-tumor necrosis factor-alfa inhibitors or other immunosuppressive agents in patients with psoriasis. J. Am. Acad. Dermatol. 2014, 70, 178–186. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.; Huang, S.; Song, Z.; Wu, J.; Zhang, E.; Zhu, Z.; Zhu, B.; Yin, Y.; Lin, Y.; et al. Immunosuppressive Drugs Modulate the Replication of Hepatitis B Virus (HBV) in a Hydrodynamic Injection Mouse Model. PLoS ONE 2014, 9, e85832. [Google Scholar] [CrossRef]
- Nkongolo, S.; Ni, Y.; Lempp, F.A.; Kaufman, C.; Lindner, T.; Esser-Nobis, K.; Lohmann, V.; Mier, W.; Mehrle, S.; Urban, S. Cyclosporin A inhibits hepatitis B and hepatitis D virus entry by cyclophilin-independent interference with the NTCP receptor. J. Hepatol. 2014, 60, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Watashi, K.; Sluder, A.; Daito, T.; Matsunaga, S.; Ryo, A.; Nagamori, S.; Iwamoto, M.; Nakajima, S.; Tsukuda, S.; Borroto-Esoda, K.; et al. Cyclosporin A and its analogs inhibit hepatitis B virus entry into cultured hepatocytes through targeting a membrane transporter, sodium taurocholate cotransporting polypeptide (NTCP). Hepatology 2014, 59, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
- Piaserico, S.; Messina, F.; Russo, F.P. Managing Psoriasis in Patients with HBV or HCV Infection: Practical Considerations. Am. J. Clin. Dermatol. 2019, 20, 829–845. [Google Scholar] [CrossRef] [PubMed]
- Gnarra, M.; De Simone, C.; Garcovich, M.; Garcovich, S. Low-Dose Cyclosporine A in the Treatment of Severe Atopic Dermatitis Complicated by Chronic Hepatitis C Virus Infection. Pediatr. Dermatol. 2017, 34, 374–376. [Google Scholar] [CrossRef]
- Nag, A.; Robotham, J.M.; Tang, H. Suppression of viral RNA binding and the assembly of infectious hepatitis C virus particles in vitro by cyclophilin inhibitors. J. Virol. 2012, 86, 12616–12624. [Google Scholar] [CrossRef]
- Giovanna Brunasso, A.M.; Michetti, P.; Fancelli, L.; Massone, C. Cyclosporine as monotherapy for psoriasis in the setting of chronic HCV infection: A forgotten therapeutical option. Hepat. Mon. 2012, 12, 349–352. [Google Scholar] [CrossRef]
- Bomm, L.; Zimmermann, C.C.; Souto, R.; Bressan, A.; Gripp, A. Use of cyclosporin in a patient with hepatitis C and pustular psoriasis. Bras. Dermatol. 2011, 86, S193–S195. [Google Scholar] [CrossRef]
- Liu, J.P.; Ye, L.; Wang, X.; Li, J.L.; Ho, W.Z. Cyclosporin A inhibits hepatitis C virus replication and restores interferon-alpha expression in hepatocytes. Transpl. Infect. Dis. 2011, 13, 24–32. [Google Scholar] [CrossRef]
- Karayiannis, P.; Goldin, R.; Luther, S.; Carman, W.F.; Monjardino, J.; Thomas, H.C. Effect of cyclosporin-A in woodchucks with chronic hepatitis delta virus infection. J. Med. Virol. 1992, 36, 316–321. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Debing, Y.; Chen, K.; Van Der Laan, L.J.; Neyts, J.; Janssen, H.L.; Metselaar, H.J.; Peppelenbosch, M.P.; Pan, Q. Calcineurin inhibitors stimulate and mycophenolic acid inhibits replication of hepatitis E virus. Gastroenterology 2014, 146, 1775–1783. [Google Scholar] [CrossRef]
- Fuhrmann, S.; Lachmann, R.; Streitz, M.; Hetzer, R.; Volk, H.D.; Lehmkuhl, H.; Kern, F. Cyclosporin A and tacrolimus reduce T-cell polyfunctionality but not interferon-gamma responses directed at cytomegalovirus. Immunology 2012, 136, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Serrano, M.; Sánchez-Lázaro, I.; Almenar-Bonet, L.; Martínez-Dolz, L.; Portolés-Sanz, M.; Rivera-Otero, M.; Salvador-Sanz, A. Does the calcineurin inhibitor have influence on cytomegalovirus infection in heart transplantation? Clin. Transpl. 2014, 28, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Siak, J.; Chee, S.P. Cytomegalovirus Anterior Uveitis Following Topical Cyclosporine A. Ocul. Immunol. Inflamm. 2018, 26, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Song, X.; Deng, J.; Lv, L.; Ma, P.; Gao, B.; Zhou, X.; Zhang, Y.; Xu, J. Inhibition of cyclophilin A suppresses H2O2-enhanced replication of HCMV through the p38 MAPK signaling pathway. FEBS Open Bio 2016, 6, 961–971. [Google Scholar] [CrossRef]
- Lee, J.J.; Kim, M.K.; Wee, W.R. Adverse effects of low-dose systemic cyclosporine therapy in high-risk penetrating keratoplasty. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1111–1119. [Google Scholar] [CrossRef]
- Laohathai, C.; Weber, D.J.; Hayat, G.; Thomas, F.P. Chronic herpes simplex type-1 encephalitis with intractable epilepsy in an immunosuppressed patient. Infection 2016, 44, 121–125. [Google Scholar] [CrossRef]
- Kishore, B.N.; Ankadavar, N.S.; Kamath, G.H.; Martis, J. Varicella zoster with erythema multiforme in a young girl: A rare association. Indian J. Dermatol. 2014, 59, 299–301. [Google Scholar] [CrossRef]
- Sułowicz, J.; Wojas-Pelc, A.; Kuzniewski, M.; Ignacak, E.; Janda, K.; Sulowicz, W. Cutaneous viral infections in patients after kidney transplantation: Risk factors. Pol. Arch. Med. Wewn 2013, 123, 686–692. [Google Scholar] [CrossRef]
- Szubińska-Lelonkiewicz, D.; Osiak, M.; Wychowański, P.; Siewert-Gutowska, M.; Fiedor, P. Frequency of human papilloma virus (HPV) occurrence among pathological changes of the oral cavity in kidney allotransplant recipients undergoing long-term pharmacological immunosuppressive therapy. Transpl. Proc. 2018, 50. [Google Scholar] [CrossRef]
- Ma, C.; Li, F.; Musharrafieh, R.; Wang, J. Discovery of cyclosporine A and its analogs as broad-spectrum anti-influenza drugs with a high in vitro genetic barrier of drug resistance. Antivir. Res. 2016, 133. [Google Scholar] [CrossRef]
- Hamamoto, I.; Harazaki, K.; Inase, N.; Takaku, H.; Tashiro, M.; Yamamoto, N. Cyclosporin A Inhibits the Propagation of Influenza Virus by Interfering with a Late Event in the Virus Life Cycle. Jpn. J. Infect. Dis. 2013, 66, 276–283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shen, Z.; Tian, Z.; He, H.; Zhang, J.; Li, J.; Wu, Y. Anti-Viral Effects of Cyclosporin A in Neonatal Mice With Rotavirus-Induced Diarrhea. J. Pediatr. Gastroenterol. Nutr. 2014, 60. [Google Scholar] [CrossRef]
- Shen, Z.; He, H.; Wu, Y.; Li, J. Cyclosporin A Inhibits Rotavirus Replication and Restores Interferon-Beta Signaling Pathway In Vitro and In Vivo. PLoS ONE 2013, 8, e71815. [Google Scholar] [CrossRef] [PubMed]
- Shalom, G.; Zisman, D.; Bitterman, H.; Harmanboehm, I.; Greenberg-Dotan, S.; Dreiher, J.; Feldhamer, I.; Moser, H.; Hammerman, A.; Cohen, Y.; et al. Systemic Therapy for Psoriasis and the Risk of Herpes Zoster: A 500 000 Person-year Study. JAMA Dermatol. 2015, 151, 1–7. [Google Scholar] [CrossRef]
- Tsurukawa, S.; Iwanaga, N.; Izumi, Y.; Shirakawa, A.; Kawahara, C.; Shukuwa, T.; Inamoto, M.; Kawakami, A.; Migita, K. Herpes Zoster Meningitis Complicating Combined Tocilizumab and Cyclosporine Therapy for Adult-Onset Still’s Disease. Case Rep. Rheumatol. 2016, 2016, 4232657. [Google Scholar] [CrossRef][Green Version]
- Markowitz, M.; Vaida, F.; Hare, C.; Boden, D.; Mohri, H.; Hecht, F.; Kalayjian, R.; Conrad, A.; Mildvan, D.; Aberg, J.; et al. The Virologic and Immunologic Effects of Cyclosporine as an Adjunct to Antiretroviral Therapy in Patients Treated during Acute and Early HIV-1 Infection. J. Infect. Dis. 2010, 201, 1298–1302. [Google Scholar] [CrossRef]
- Nicolás Ocejo, D.; Ambrosioni, J.; Sued, O.; Brunet, M.; López-Diéguez, M.; Manzardo, C.; Aguero, F.; Plana, M.; Guardo, A.; Mosquera, M.; et al. Cyclosporine A in addition to standard ART during primary HIV-1 infection: Pilot randomized clinical trial. J. Antimicrob. Chemother. 2016, 72. [Google Scholar] [CrossRef]
- Hawley, T.; Spear, M.; Guo, J.; Wu, Y. Inhibition of HIV replication in vitro by clinical immunosuppressants and chemotherapeutic agents. Cell Biosci. 2013, 3, 22. [Google Scholar] [CrossRef]
- Luo, X.; Yang, W.; Gao, G. SUN1 Regulates HIV-1 Nuclear Import in a Manner Dependent on the Interaction between the Viral Capsid and Cellular Cyclophilin A. J. Virol. 2018, 92, JVI00229-18. [Google Scholar] [CrossRef]
- Selyutina, A.; Persaud, M.; Simons, L.; Bulnes-Ramos, A.; Buffone, C.; Martinez-Lopez, A.; Scoca, V.; Di Nunzio, F.; Hiatt, J.; Marson, A.; et al. Cyclophilin A Prevents HIV-1 Restriction in Lymphocytes by Blocking Human TRIM5α Binding to the Viral Core. Cell Rep. 2020, 30, 3766–3777.e6. [Google Scholar] [CrossRef]
- Wall, D.; McMenamin, M.; O’Mahony, D.; Irvine, A.D. Kaposi sarcoma in an patient with atopic dermatitis treated with ciclosporin. BMJ Case Rep. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Imko-Walczuk, B.; Kielbowicz, M.; Malyszko, J.; Malyszko, J.; Barczyk, M.; Debska-Slizien, A.; Mysliwiec, M.; Rutkowski, B. Kaposi Sarcoma in the Genital Area in a Kidney Transplant Patient: A Case Report and Literature Review. Transpl. Proc. 2016, 48, 1843–1848. [Google Scholar] [CrossRef]
- Rudnicka, L.; Gupta, M.; Kassir, M.; Jafferany, M.; Lotti, T.; Sadoughifar, R.; Goldust, M. Priorities for global health community in COVID-19 pandemic. Dermatol. Ther. 2020, e13361. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Fioranelli, M.; Goldust, M.; Lotti, T. Psoriatic arthritis and COVID-19 pandemic: Consequences in medical treatment? Dermatol. Ther. 2020, e13743. [Google Scholar] [CrossRef]
- Goldust, M.; Hartmann, K.; Abdelmaksoud, A.; Navarini, A.A. Utility and risk of dermatologic medications during the COVID-19 pandemic. Dermatol. Ther. 2020. [Google Scholar] [CrossRef] [PubMed]
- Di Lernia, V.; Goldust, M.; Feliciani, C. Covid-19 infection in psoriasis patients treated with cyclosporin. Dermatol. Ther. 2020, e13739. [Google Scholar] [CrossRef]
- Blicharz, L.; Czuwara, J.; Samochocki, Z.; Goldust, M.; Olszewska, M.; Rudnicka, L. Immunosuppressive treatment for systemic sclerosis-Therapeutic challenges during the COVID-19 pandemic. Dermatol. Ther. 2020, e13619. [Google Scholar] [CrossRef]
- De Wilde, A.; Zevenhoven-Dobbe, J.; Meer, Y.; Thiel, V.; Narayanan, K.; Makino, S.; Snijder, E.; Hemert, M. Cyclosporin A inhibits the replication of diverse coronaviruses. J. Gen. Virol. 2011, 92, 2542–2548. [Google Scholar] [CrossRef]
- Pfefferle, S.; Schopf, J.; Kogl, M.; Friedel, C.C.; Muller, M.A.; Carbajo-Lozoya, J.; Stellberger, T.; Von Dall’Armi, E.; Herzog, P.; Kallies, S.; et al. The SARS-coronavirus-host interactome: Identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog. 2011, 7, e1002331. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sato, Y.; Sasaki, T. Suppression of Coronavirus Replication by Cyclophilin Inhibitors. Viruses 2013, 5, 1250–1260. [Google Scholar] [CrossRef]
- De Wilde, A.; Victor, S.R.; Oudshoorn, D.; Bestebroer, T.; Nieuwkoop, S.; Limpens, R.; Posthuma, C.; Meer, Y.; Bárcena, M.; Haagmans, B.; et al. MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon- treatment. J. Gen. Virol. 2013, 94. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kuok, I.T.; Cheung, M.; Ng, M.; Ng, K.; Hui, K.; Peiris, J.S.; Chan, M.; Nicholls, J. Effect of interferon alpha and cyclosporine treatment separately and in combination on Middle East Respiratory Syndrome Coronavirus (MERS-CoV) replication in a human in-vitro and ex-vivo culture model. Antivir. Res. 2018, 155. [Google Scholar] [CrossRef] [PubMed]
- Camargo, L.F.; Esteves, A.B.; Ulisses, L.R.; Rivelli, G.G.; Mazzali, M. Urinary tract infection in renal transplant recipients: Incidence, risk factors, and impact on graft function. Transpl. Proc. 2014, 46, 1757–1759. [Google Scholar] [CrossRef] [PubMed]
- Sadio, M.; Tourneur, E.; Bens, M.; Goujon, J.-M.; Vandewalle, A.; Chassin, C. Cyclosporine A Induces MicroRNAs Controlling Innate Immunity during Renal Bacterial Infection. J. Innate Immun. 2018, 10, 14–29. [Google Scholar] [CrossRef]
- Tourneur, E.; Ben Mkaddem, S.; Chassin, C.; Bens, M.; Goujon, J.M.; Charles, N.; Pellefigues, C.; Aloulou, M.; Hertig, A.; Monteiro, R.C.; et al. Cyclosporine A impairs nucleotide binding oligomerization domain (Nod1)-mediated innate antibacterial renal defenses in mice and human transplant recipients. PLoS Pathog. 2013, 9, e1003152. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.-M.; Liu, C.-J.; Teng, C.-J.; Lin, Y.-T.; Chang, Y.-S.; Chiang, S.-C.; Tzeng, C.-H.; Chen, T.-J. Impact of pulmonary and extrapulmonary tuberculosis infection in kidney transplantation: A nationwide population-based study in Taiwan. Transpl. Infect. Dis. 2012, 14, 502–509. [Google Scholar] [CrossRef]
- Ozcura, F. Successful treatment of staphylococcus-associated marginal keratitis with topical cyclosporine. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 1049–1050. [Google Scholar] [CrossRef]
- Keles, H.; Pancar Yuksel, E.; Aydin, F.; Senturk, N. Pre-Treatment and Post-Treatment Demodex Densities in Patients under Immunosuppressive Treatments. Medicina 2020, 56, 107. [Google Scholar] [CrossRef]
- Bustos, P.L.; Perrone, A.E.; Milduberger, N.; Postan, M.; Bua, J. Oxidative stress damage in the protozoan parasite Trypanosoma cruzi is inhibited by Cyclosporin A. Parasitology 2015, 142, 1024–1032. [Google Scholar] [CrossRef]
- Kulkarni, M.; Karafova, A.; Kamysz, W.; Schenkman, S.; Pelle, R.; McGwire, B. Secreted Trypanosome Cyclophilin Inactivates Lytic Insect Defense Peptides and Induces Parasite Calcineurin Activation and Infectivity. J. Biol. Chem. 2013, 288. [Google Scholar] [CrossRef]
- Vorberg, L.; Coors, A. Impact of an immunosuppressive human pharmaceutical on the interaction of a bacterial parasite and its invertebrate host. Aquat. Toxicol. 2018, 206. [Google Scholar] [CrossRef]
- Nicolao, M.C.; Denegri, G.M.; Carcamo, J.G.; Cumino, A.C. P-glycoprotein expression and pharmacological modulation in larval stages of Echinococcus granulosus. Parasitol. Int. 2014, 63, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Azouzi, S.; Morandat, S.; Kirat, K. The Potent Antimalarial Peptide Cyclosporin A Induces the Aggregation and Permeabilization of Sphingomyelin-Rich Membranes. Langmuir ACS J. Surf. Colloids 2011, 27, 9465–9472. [Google Scholar] [CrossRef] [PubMed]
- Yau, W.-L.; Pescher, P.; MacDonald, A.; Hem, S.; Zander, D.; Retzlaff, S.; Blisnick, T.; Rotureau, B.; Rosenqvist, H.; Wiese, M.; et al. The Leishmania donovani chaperone cyclophilin 40 is essential for intracellular infection independent of its stage-specific phosphorylation status. Mol. Microbiol. 2014, 93, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-W.; Li, J.; Chen, H.; He, J.-L.; Chen, Q.-W.; Zhang, J.-H.; Zhou, Q.; Chen, J.-P. Evaluation of in vitro antileishmanial efficacy of cyclosporin A and its non-immunosuppressive derivative, dihydrocyclosporin A. Parasites Vectors 2020, 13. [Google Scholar] [CrossRef] [PubMed]

| Possible Positive Effect of Cyclosporine on Disease Course | Conflicting Results | Possible Negative Effect of Cyclosporine on Disease Course |
|---|---|---|
| Hepatitis C [15,16,17] Influenza virus infection [28] Rotavirus infection [29,30] Human Immunodeficiency Virus infection [26,33,34] Coronavirus infection [39,40,41,42,43] | Hepatitis B [10,11,12,13,14] Hepatitis D [12,20] Herpes simplex infection [26,27] Herpes Zoster Virus infection [35,36] | Hepatitis E [21] Cytomegalovirus infection [19,20,22] Human Papilloma Virus infection [29,30] Human Herpesvirus-8 (Kaposi Sarcoma virus) infection [42,43] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glowacka, P.; Rudnicka, L.; Warszawik-Hendzel, O.; Sikora, M.; Goldust, M.; Gajda, P.; Stochmal, A.; Blicharz, L.; Rakowska, A.; Olszewska, M. The Antiviral Properties of Cyclosporine. Focus on Coronavirus, Hepatitis C Virus, Influenza Virus, and Human Immunodeficiency Virus Infections. Biology 2020, 9, 192. https://doi.org/10.3390/biology9080192
Glowacka P, Rudnicka L, Warszawik-Hendzel O, Sikora M, Goldust M, Gajda P, Stochmal A, Blicharz L, Rakowska A, Olszewska M. The Antiviral Properties of Cyclosporine. Focus on Coronavirus, Hepatitis C Virus, Influenza Virus, and Human Immunodeficiency Virus Infections. Biology. 2020; 9(8):192. https://doi.org/10.3390/biology9080192
Chicago/Turabian StyleGlowacka, Paulina, Lidia Rudnicka, Olga Warszawik-Hendzel, Mariusz Sikora, Mohamad Goldust, Patrycja Gajda, Anna Stochmal, Leszek Blicharz, Adriana Rakowska, and Malgorzata Olszewska. 2020. "The Antiviral Properties of Cyclosporine. Focus on Coronavirus, Hepatitis C Virus, Influenza Virus, and Human Immunodeficiency Virus Infections" Biology 9, no. 8: 192. https://doi.org/10.3390/biology9080192
APA StyleGlowacka, P., Rudnicka, L., Warszawik-Hendzel, O., Sikora, M., Goldust, M., Gajda, P., Stochmal, A., Blicharz, L., Rakowska, A., & Olszewska, M. (2020). The Antiviral Properties of Cyclosporine. Focus on Coronavirus, Hepatitis C Virus, Influenza Virus, and Human Immunodeficiency Virus Infections. Biology, 9(8), 192. https://doi.org/10.3390/biology9080192







