Molecular Detection of Reticuloendotheliosis Virus 5? Long Terminal Repeat Integration in the Genome of Avipoxvirus Field Strains from Different Avian Species in Egypt
Abstract
:1. Introduction
2. Material and Methods
2.1. Ethical Consideration
2.2. Study Area, Samples Collection, and Preparation
2.3. Standard FWPV
2.4. Embryonated Chicken Eggs (ECEs)
2.5. Virus Isolation
2.6. Molecular Detection of the Isolated Virus
2.6.1. DNA Extraction
2.6.2. Polymerase Chain Reaction
2.7. Sequencing of APV P4b Gene and REV 5′LTR
2.8. Sequencing Data Analysis
3. Results
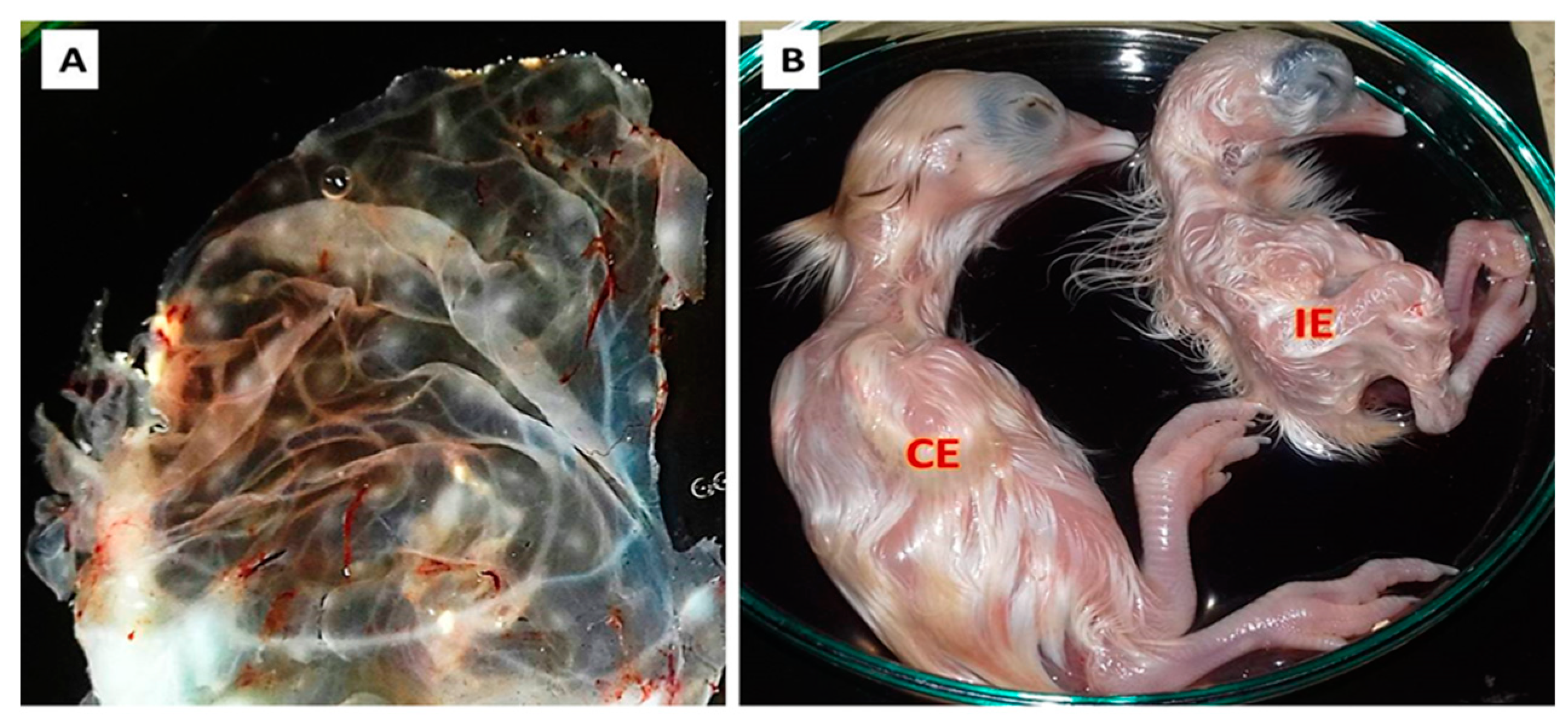
3.1. Clinical Signs and Postmortem Changes
3.2. Virus Isolation
3.3. Molecular Identification Using Conventional PCR
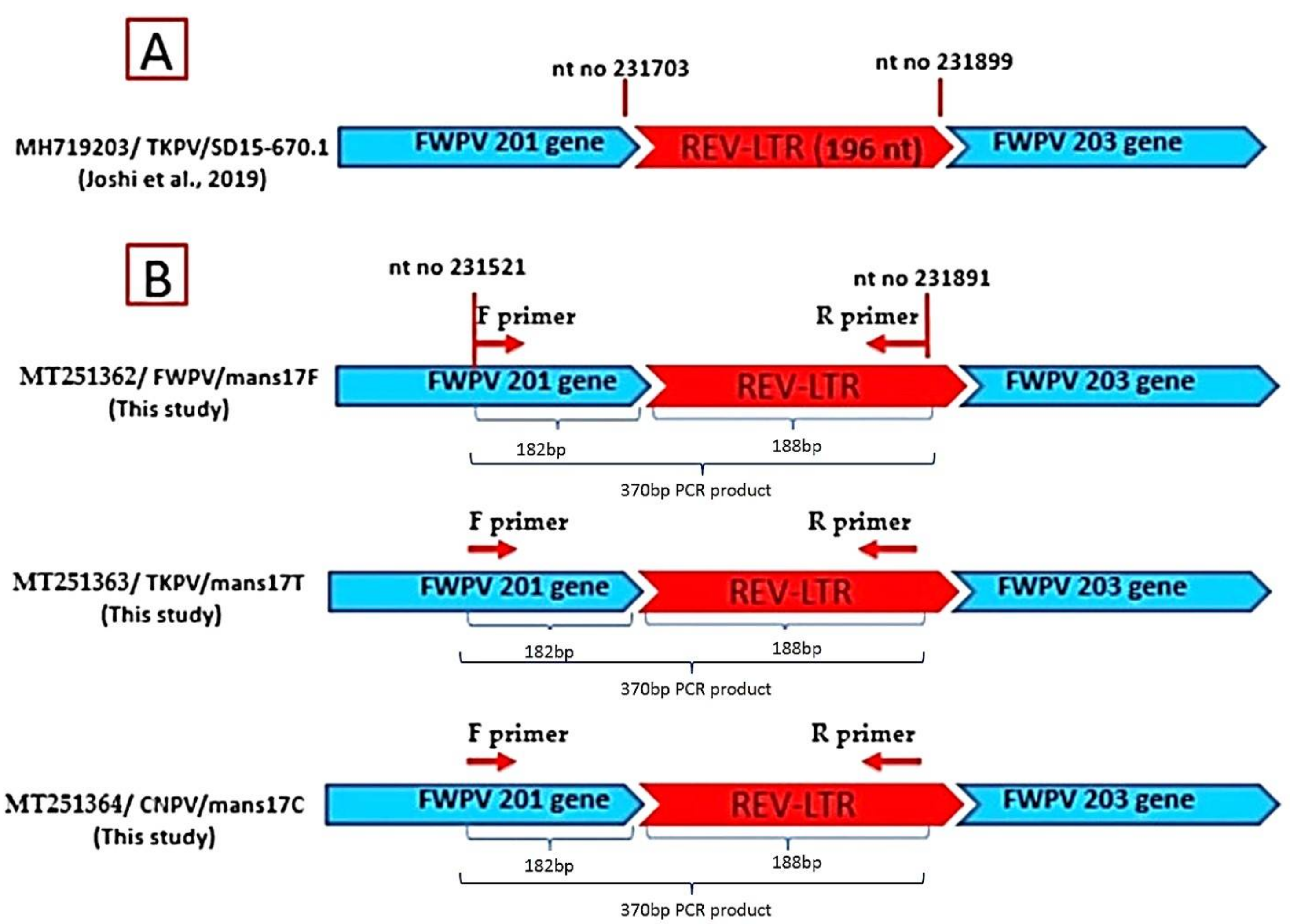
3.4. Sequencing and Phylogenetic Analysis of the APV P4b Gene and REV- 5′LTR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fauquet, C.M. Virus Taxonomy: VIIIth Report of the International Committee on Taxonomy of Viruses; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- CTV. ICTVdB Index of Viruses. International Committee on Taxonomy of Viruses. 2012. Available online: http://ictvonline.org/ (accessed on 15 June 2020).
- Essbauer, S.; Pfeffer, M.; Meyer, H. Zoonotic poxviruses. Veter. Microbiol. 2010, 140, 229–236. [Google Scholar] [CrossRef]
- Weli, S.C.; Tryland, M. Avipoxviruses: Infection biology and their use as vaccine vectors. Virol. J. 2011, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Virus Taxonomy. 2019 Release. International Committee on Taxonomy of Viruses. Available online: talk.ictvonline.org (accessed on 11 July 2020).
- Pledger, A. Avian pox virus infection in a mourning dove. Can. Veter J. La Rev. Veter. Can. 2005, 46, 1143–1145. [Google Scholar]
- Bolte, A.L.; Meurer, J.; Kaleta, E.F. Avian host spectrum of avipoxviruses. Avian Pathol. 1999, 28, 415–432. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, B.W. Avian Viruses: Function and Control; Wingers Publishing: Lake Worth, FL, USA, 1995; pp. 285–311. [Google Scholar]
- Kulich, P.; Roubalova, E.; Dubská, L.Z.; Sychra, O.; Smíd, B.; Literak, I. Avipoxvirus in blackcaps (Sylvia atricapilla). Avian Pathol. 2008, 37, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, C.T. Efficacy of Commercial Canarypox Vaccine for Protecting Hawaii Amakihi from Field Isolates of Avipoxvirus; Hawaii Cooperative Studies Unit Technical Report HCCU-0: Hawaii, HI, USA, 2010. [Google Scholar]
- Fenner, F.; Bachmann, P.A.; Gibbs, E.P.J.; Murphy, F.A.; Studdert, M.J.; White, D.O. Veterinary Virology, 2nd ed.; Academic Press Inc.: Cambridge, MA, USA, 1993; p. 673. [Google Scholar]
- OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 6th ed.; Office International des Epizooties: Paris, France, 2008; pp. 1092–1096. [Google Scholar]
- Thiel, T.; Whiteman, N.K.; Tirapé, A.; Baquero, M.I.; Cedeño, V.; Walsh, T.; Uzcátegui, G.J.; Parker, P.G. Characterization of canarypox-like viruses infecting endemic birds in the galápagos islands. J. Wildl. Dis. 2005, 41, 342–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, C.T.; Wiegand, K.C.; Triglia, D.; Jarvi, S.I. Reversion to virulence and efficacy of an attenuated canarypox vaccine in Hawaii amakihi (hemignathus virens). J. Zoo Wildl. Med. 2012, 43, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Docherty, D.E.; Long, R.I.R.; Flickinger, E.L.; Locke, L.N. Isolation of Poxvirus from Debilitating Cutaneous Lesions on Four Immature Grackles (Quiscalus sp.). Avian Dis. 1991, 35, 244–247. [Google Scholar] [CrossRef] [Green Version]
- Boyle, D.B. Genus avipoxvirus. Poxviruses 2007, 217–251. [Google Scholar] [CrossRef]
- Tulman, E.R.; Afonso, C.L.; Lu, Z.; Zsak, L.; Kutish, G.F.; Rock, D.L. The genome of canarypox virus. J. Virol. 2004, 78, 353–366. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.J.; Schnitzlein, W.M.; McAloose, D.; Pessier, A.P.; Tripathy, D.N. Characterization of an avianpox virus isolated from an Andean condor (Vultur gryphus). Veter. Microbiol. 2003, 96, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Pattison, M.; McMullin, B.; Alexander, D. Poultry Diseases, 6th ed.; Elsevier: Mumbai, India, 2008; pp. 333–339. [Google Scholar]
- Pérez-Tris, J.; Williams, R.A.; Abel-Fernández, E.; Barreiro, J.; Conesa, J.J.; Figuerola, J.; Martinez-Martínez, M.; Ramirez, A.; Benitez, L. A Multiplex PCR for detection of poxvirus and papillomavirus in cutaneous warts from live birds and museum skins. Avian Dis. Dig. 2011, 6, 545–553. [Google Scholar] [CrossRef]
- Manarolla, G.; Pisoni, G.; Sironi, G.; Rampin, T. Molecular biological characterization of avian poxvirus strains isolated from different avian species. Veter. Microbiol. 2010, 140, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasaei, N. Molecular detection and phylogenetic analysis of Avipoxvirus strains isolated from different bird species. Iran. J. Vet. Res. 2014, 15, 40–44. [Google Scholar]
- Weli, S.C.; Traavik, T.; Tryland, M.; Coucheron, D.H. Analysis and comparison of the 4b core protein gene of avipoxviruses from wild birds: Evidence for interspecies spatial phylogenetic variation. Arch. Virol. 2004, 149, 2035–2046. [Google Scholar] [CrossRef] [PubMed]
- Lüschow, D.; Hoffmann, T.; Hafez, H.M. Differentiation of avian poxvirus strains on the basis of nucleotide sequences of 4b gene fragment. Avian Dis. 2004, 48, 453–462. [Google Scholar] [CrossRef]
- Puro, K.U.; Ahuja, A.; Joishy, T.; Sen, A.; Ghatak, S.; Shakuntala, I.; Das, S.; Sunjukta, R. Molecular detection of reticuloendotheliosis virus (REV) integration in avian poxvirus in North Eastern India. Proc. Natl. Acad. Sci. India Sect. B: Biol. Sci. 2015, 87, 273–276. [Google Scholar] [CrossRef]
- Singh, P.; Schnitzlein, W.M.; Tripathy, D.N. Reticuloendotheliosis Virus sequences within the genomes of field strains of fowlpox virus display variability. J. Virol. 2003, 77, 5855–5862. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Kim, T.; Tripathy, D.N. Re-emerging fowlpox: Evaluation of isolates from vaccinated flocks. Avian Pathol. 2000, 29, 449–455. [Google Scholar] [CrossRef]
- Walker, M.H.; Rup, B.J.; Rubin, A.S.; Bose, H.R. Specificity in the immunosuppression induced by avian reticuloendotheliosis virus. Infect. Immun. 1983, 40, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Laidlaw, S.M.; Skinner, M.A. Comparison of the genome sequence of FP9, an attenuated, tissue culture-adapted European strain of Fowlpox virus, with those of virulent American and European viruses. J. Gen. Virol. 2004, 85, 305–322. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.M.; Davis, J.R.; Sato, T.; Yasuda, A. Reticuloendotheliosis virus (REV) long terminal repeats incorporated in the genomes of commercial fowl poxvirus vaccines and pigeon poxviruses without indication of the presence of infectious REV. Avian Dis. 2001, 44, 827–841. [Google Scholar] [CrossRef]
- Singh, P.; Schnitzlein, W.M.; Tripathy, D.N. Construction and characterization of a fowlpox virus field isolate whose genome lacks reticuloendotheliosis provirus nucleotide sequences. Avian Dis. 2005, 49, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Joshi, L.R.; Bauermann, F.V.; Hain, K.S.; Kutish, G.F.; Armién, A.G.; Lehman, C.P.; Neiger, R.; Afonso, C.L.; Tripathy, D.N.; Diel, D.G. Detection of Fowlpox virus carrying distinct genome segments of Reticuloendotheliosis virus. Virus Res. 2019, 260, 53–59. [Google Scholar] [CrossRef]
- Meroz, M. Reticuloendotheliosis and ’pullet disease’ in Israel. Veter. Rec. 1992, 130, 107–108. [Google Scholar] [CrossRef]
- Okoye, J.O.A.; Ezema, W.; Agoha, J.N. Naturally occurring clinical reticuloendotheliosis in Turkeys and chickens. Avian Pathol. 1993, 22, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Davidson, I.; Shkoda, I.; Perk, S. Integration of the reticuloendotheliosis virus envelope gene into the poultry fowlpox virus genome is not universal. J. Gen. Virol. 2008, 89, 2456–2460. [Google Scholar] [CrossRef]
- AlFaki, S.H.; Hussien, M.O.; Osman, N.A.; Enan, K.A.; El Hussein, A.R.M. First report on molecular characterization and phylogenetic analysis of Reticuloendotheliosis virus in Sudan. Trop. Anim. Health Prod. 2020, 52, 2073–2078. [Google Scholar] [CrossRef]
- Awad, A.M.; El-Hamid, H.S.A.; Abourawash, A.; Ibrahim, H.H. Detection of reticuloendotheliosis virus as a contaminant of fowl pox vaccines. Poult. Sci. 2010, 89, 2389–2395. [Google Scholar] [CrossRef]
- Hertig, C.; Coupar, B.E.; Gould, A.R.; Boyle, D.B. Field and vaccine strains of fowlpox virus carry integrated sequences from the avian retrovirus, reticuloendotheliosis virus. Virology 1997, 235, 367–376. [Google Scholar] [CrossRef] [Green Version]
- García, M.; Narang, N.; Reed, W.M.; Fadly, A. Molecular characterization of reticuloendotheliosis virus insertions in the genome of field and vaccine strains of fowl poxvirus. Avian Dis. 2003, 47, 343–354. [Google Scholar] [CrossRef]
- Kim, T.; Tripathy, D.N. Reticuloendotheliosis virus integration in the fowl poxvirus genome: Not a recent event. Avian Dis. 2001, 45, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Tadese, T.; Reed, W.M. Detection of specific reticuloendotheliosis virus sequence and protein from REV-integrated fowlpox virus strains. J. Virol. Methods 2003, 110, 99–104. [Google Scholar] [CrossRef]
- Fadly, A.M.; Witter, R.L.; Smith, E.J.; Silva, R.F.; Reed, W.M.; Hoerr, F.J.; Putnam, M.R. An outbreak of lymphomas in commercial broiler breeder chickens vaccinated with a fowlpox vaccine contaminated with reticuloendotheliosis virus. Avian Pathol. 1996, 25, 35–47. [Google Scholar] [CrossRef]
- Rajasekaran, R. Molecular detection of integrated reticuloendothelial virus genes in fowlpox virus field isolates and live vaccines of poultry. Indian J. Anim. Sci. 2019, 89. [Google Scholar]
- Lebdah, M.A.; Hassanin, O.A.; Ali, A.M. Avipoxvirus in Egypt and African continent: A review. Zagazig Veter. J. 2019, 47, 364–377. [Google Scholar] [CrossRef] [Green Version]
- Tadese, T.; Fitzgerald, S.; Reed, W.M. Detection and differentiation of re-emerging fowlpox virus (FWPV) strains carrying integrated reticuloendotheliosis virus (FWPV-REV) by real-time PCR. Veter. Microbiol. 2008, 127, 39–49. [Google Scholar] [CrossRef]
- Mosad, S. Conventional and molecular detection of avipoxviruses from chickens, pigeons and turkeys. Mansoura Veter. Med. J. 2019, 20, 85–91. [Google Scholar] [CrossRef]
- Ali, A.M.; Fathy, A.; Eid, A. Detection of Avian Poxvirus in an Egyptian Goose. Glob. Anim. Sci. J.-GASJ 2014, 1, 65–68. [Google Scholar]
- Abdallah, F.; Hassanin, O. Detection and molecular characterization of avipoxviruses isolated from different avian species in Egypt. Virus Genes 2012, 46, 63–70. [Google Scholar] [CrossRef]
- El-Mahdy, S.S.; Awaad, M.H.H.; Soliman, Y. Molecular identification of local field isolated fowl pox virus strain from Giza governorate of Egypt. Veter. World 2014, 7, 66–71. [Google Scholar] [CrossRef]
- Cannon, R.M. Livestock Disease Surveys: A Field Manual for Veterinarians; Cannon, R.M., Roe, R.T., Eds.; Australian Bureau of Animal Health; A.G.P.S.: Canberra, Australia, 1982.
- Li, K.; Gao, H.; Gao, L.; Qi, X.; Qin, L.; Gao, Y.; Xu, Y.; Wang, X. Development of TaqMan real-time PCR assay for detection and quantitation of reticuloendotheliosis virus. J. Virol. Methods 2012, 179, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Diallo, I.S.; Taylor, J.; Gibson, J.; Hoad, J.; De Jong, A.; Hewitson, G.; Corney, B.G.; Rodwell, B.J. Diagnosis of a naturally occurring dual infection of layer chickens with fowlpox virus and gallid herpesvirus 1 (infectious laryngotracheitis virus). Avian Pathol. 2010, 39, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, L.H.; Lee, K.H. Application of the polymerase chain reaction for the diagnosis of fowl poxvirus infection. J. Virol. Methods 1997, 63, 113–119. [Google Scholar] [CrossRef]
- Biswas, S.K.; Jana, C.; Chand, K.; Rehman, W.; Mondal, B. Detection of fowl poxvirus integrated with reticuloendotheliosis virus sequences from an outbreak in backyard chickens in India. Veter. Ital. 2011, 47, 147–153. [Google Scholar]
- OIE. Fowl Pox. In Manual of Diagnostc Tests and Vaccines for Terrestrial Animals; Office International des Epizooties: Paris, France, 2016; Available online: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.03.10_FOWLPOX.pdf (accessed on 22 May 2020).
- Tripathy, D.; Reed, W. Pox. In Diseases of Poultry, 10th ed.; Calnek, B.W., Barnes, H., Beard, C., McDougald, L., Eds.; Iowa State Press: Ames, IA, USA, 1997; pp. 643–659. [Google Scholar]
- Forrester, D.J.; Spalding, M.G. Parasites and Diseases of Wild Birds in Florida; University Press of Florida: Gainesville, FL, USA, 2003; p. 1132. [Google Scholar]
- Williams, R.A.; Duch, C.E.; Pérez-Tris, J.; Benitez, L. Polymerase chain reaction detection of avipox and avian papillomavirus in naturally infected wild birds: Comparisons of blood, swab and tissue samples. Avian Pathol. 2014, 43, 130–134. [Google Scholar] [CrossRef]
- Malik, Y.S.; Raj Kumar, S.; Mahendra, P.Y. Recent Advances in Animal Virology; Springer: Singapore, 2019; p. 152. [Google Scholar]
- Literak, I.; Kulich, P.; Robesova, B.; Adamík, P.; Roubalova, E. Avipoxvirus in great tits (Parus major). Eur. J. Wildl. Res. 2009, 56, 529–534. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, C.H. Avian pox. In Diseases of Poultry; Hofstad, M.S., Calnek, B.W., Helmboldi, C.F., Keid, W.M., Yoder, H.W., Eds.; Iowa State University Press: Ames, IA, USA, 1978; pp. 597–609. [Google Scholar]
- Murphy, F.A.; Gibbs, E.P.J.; Horzinek, M.C.; Studdert, M.J. Poxviridae. In Veterinary Virology, 3rd ed.; Gibbs, E.P.J., Horzineck, M.C., Studdert, M.J., Eds.; Academic Press: San Diego, CA, USA, 1999; pp. 61–81, 277–291. [Google Scholar]
- Haydar, M.R.; Ara, M.S.; Soma, S.S.; Rahman, M.M.; Rahman, M.B.; Rahman, M.T. Isolation and molecular detection of turkeypox virus from turkey for the first time in Bangladesh. Bangladesh J. Veter. Med. 2017, 15, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, D.N. Pox. In Diseases in Poultry, 9th ed.; Calnek, B.W., Ed.; Iowa State Press: Ames, IA, USA, 1984; pp. 583–596. [Google Scholar]
- Jarmin, S.; Manvell, R.; Gough, R.E.; Laidlaw, S.M.; Skinner, M.A. Avipoxvirus phylogenetics: Identification of a PCR length polymorphism that discriminates between the two major clades. J. Gen. Virol. 2006, 87, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Siddique, A.B.; Hossain, F.; Zinnah, M. Determination of host specificity of pigeon pox and fowl pox viruses isolated from a field outbreak. Bulg. J. Vet. Med. 2011, 14, 209–214. [Google Scholar]
- Masola, S.N.; Mzula, A.; Kasanga, C.J.; Wambura, P.N. Integration of reticuloendotheliosis virus in most of tanzanian fowl pox virus isolates is not attributed to imported commercial fowl pox vaccines. Br. Biotechnol. J. 2014, 4, 659–669. [Google Scholar] [CrossRef]
- Robinson, F.R.; Twiehaus, M.J. Historical note: Isolation of the avian reticuloendothelial virus (Strain T). Avian Dis. 1974, 18, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.; Howell, J. Suspected reticuloendotheliosis in turkeys with cutaneous lesions reminiscent of fowl pox. Can. Veter. J. La Rev. Veter. Can. 1979, 20, 161–164. [Google Scholar]
- Wang, J.; Meers, J.; Spradbrow, P.B.; Robinson, W.F. Evaluation of immune effects of fowlpox vaccine strains and field isolates. Veter. Microbiol. 2006, 116, 106–119. [Google Scholar] [CrossRef]
- Witter, R.; Fadly, A. Diseases of poultry, chapter 15: Subchapter—Reticuloendotheliosis. In Diseases of Poultry; U.S. Department of Agriculture (USDA): Washington, DC, USA, 2003; pp. 517–536. [Google Scholar]
- Payne, L.N. Retrovirus-induced disease in poultry. Poult. Sci. 1998, 77, 1204–1212. [Google Scholar] [CrossRef]



| Host | Chicken | Pigeon | Turkey | Canary |
|---|---|---|---|---|
| Breed(s) | White leghorn | Egyptian Swift | Bronze Turkey | white canary and yellow canary |
| No. of collected samples | 10 | 10 | 10 | 10 (6 white canaries, 4 yellow canaries) |
| Vaccination | 4/10 vaccinated 6/10 not vaccinated | Not vaccinated | Not vaccinated | Not vaccinated |
| Morbidity rate | 70–100% | 80–100% | 80–100% | 75–100% |
| Mortality rate | 0–25% | 15–30% | 0–20% | 20–50% |
| Pox lesions distribution | Legs, wings, comb, wattles, and head, especially around the eye | on the head and wings | on the head, under the wings, and vent areas | All over the body, especially on the head and wings |
| Primer | Sequence (5′– 3′) Direction | Target | Product Size (bp) | Reference |
|---|---|---|---|---|
| APV P4b forward | CAGCAGGTGCTAAACAACAA | APV-P4b gene | 578 | [53] |
| APV P4b reverse | CGGTAGCTTAACGCCGAATA | |||
| REV 5′LTR forward | ACCTATGCCTCTTATTCCAC | REV-5′LTR | 370 | [54] |
| REV 5′LTR reverse | CTGATGCTTGCCTTCAAC |
| Virus Species | Host | Isolate | P4b Gene Accession Number | REV-5′LTR Accession Number |
|---|---|---|---|---|
| FWPV | Chicken | mans17F | MT219995 | MT251362 |
| PGPV | Pigeon | mans17P | MT219996 | ------------- |
| TKPV | Turkey | mans17T | MT219997 | MT251363 |
| CNPV | Canary | mans17C | MT219998 | MT251364 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosad, S.M.; El-Tholoth, M.; El-Kenawy, A.A.; Abdel-Hafez, L.J.M.; El-Gohary, F.A.; El-Sharkawy, H.; Elsayed, M.M.; Saleh, A.A.; Elmahallawy, E.K. Molecular Detection of Reticuloendotheliosis Virus 5? Long Terminal Repeat Integration in the Genome of Avipoxvirus Field Strains from Different Avian Species in Egypt. Biology 2020, 9, 257. https://doi.org/10.3390/biology9090257
Mosad SM, El-Tholoth M, El-Kenawy AA, Abdel-Hafez LJM, El-Gohary FA, El-Sharkawy H, Elsayed MM, Saleh AA, Elmahallawy EK. Molecular Detection of Reticuloendotheliosis Virus 5? Long Terminal Repeat Integration in the Genome of Avipoxvirus Field Strains from Different Avian Species in Egypt. Biology. 2020; 9(9):257. https://doi.org/10.3390/biology9090257
Chicago/Turabian StyleMosad, Samah M., Mohamed El-Tholoth, Ali A. El-Kenawy, Lina Jamil M. Abdel-Hafez, Fatma A. El-Gohary, Hanem El-Sharkawy, Mona Mohieldin Elsayed, Ayman A. Saleh, and Ehab Kotb Elmahallawy. 2020. "Molecular Detection of Reticuloendotheliosis Virus 5? Long Terminal Repeat Integration in the Genome of Avipoxvirus Field Strains from Different Avian Species in Egypt" Biology 9, no. 9: 257. https://doi.org/10.3390/biology9090257
APA StyleMosad, S. M., El-Tholoth, M., El-Kenawy, A. A., Abdel-Hafez, L. J. M., El-Gohary, F. A., El-Sharkawy, H., Elsayed, M. M., Saleh, A. A., & Elmahallawy, E. K. (2020). Molecular Detection of Reticuloendotheliosis Virus 5? Long Terminal Repeat Integration in the Genome of Avipoxvirus Field Strains from Different Avian Species in Egypt. Biology, 9(9), 257. https://doi.org/10.3390/biology9090257






