Antifungal Activity and Phytochemical Screening of Vernonia amygdalina Extract against Botrytis cinerea Causing Gray Mold Disease on Tomato Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
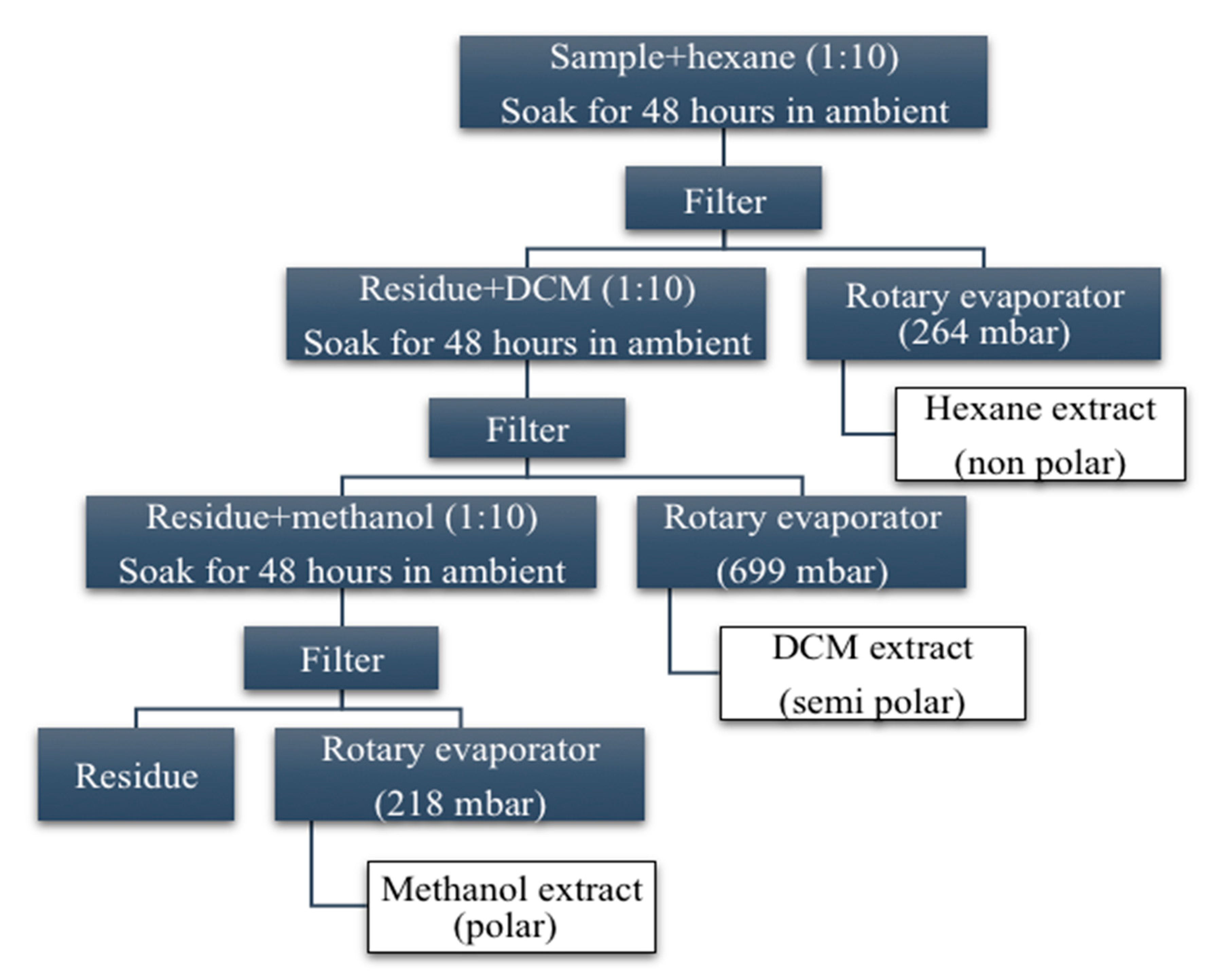
2.2. Preparation of V. amygdalina Crude Extracts
2.3. Preparation of B. cinerea
2.4. In vitro Evaluation for V. amygdalina Antifungal Activity
2.5. Microscopic Observation Using a Scanning Electron Microscope (SEM)
2.6. Antifungal Activities of V. amygdalina by In Vivo Bioassay
2.7. Screening for V. amygdalina Chemical Constituents Using Gas Chromatography-Mass Spectrometry (GCMS) Analysis
2.8. Experimental Design and Statistical Analysis
3. Results
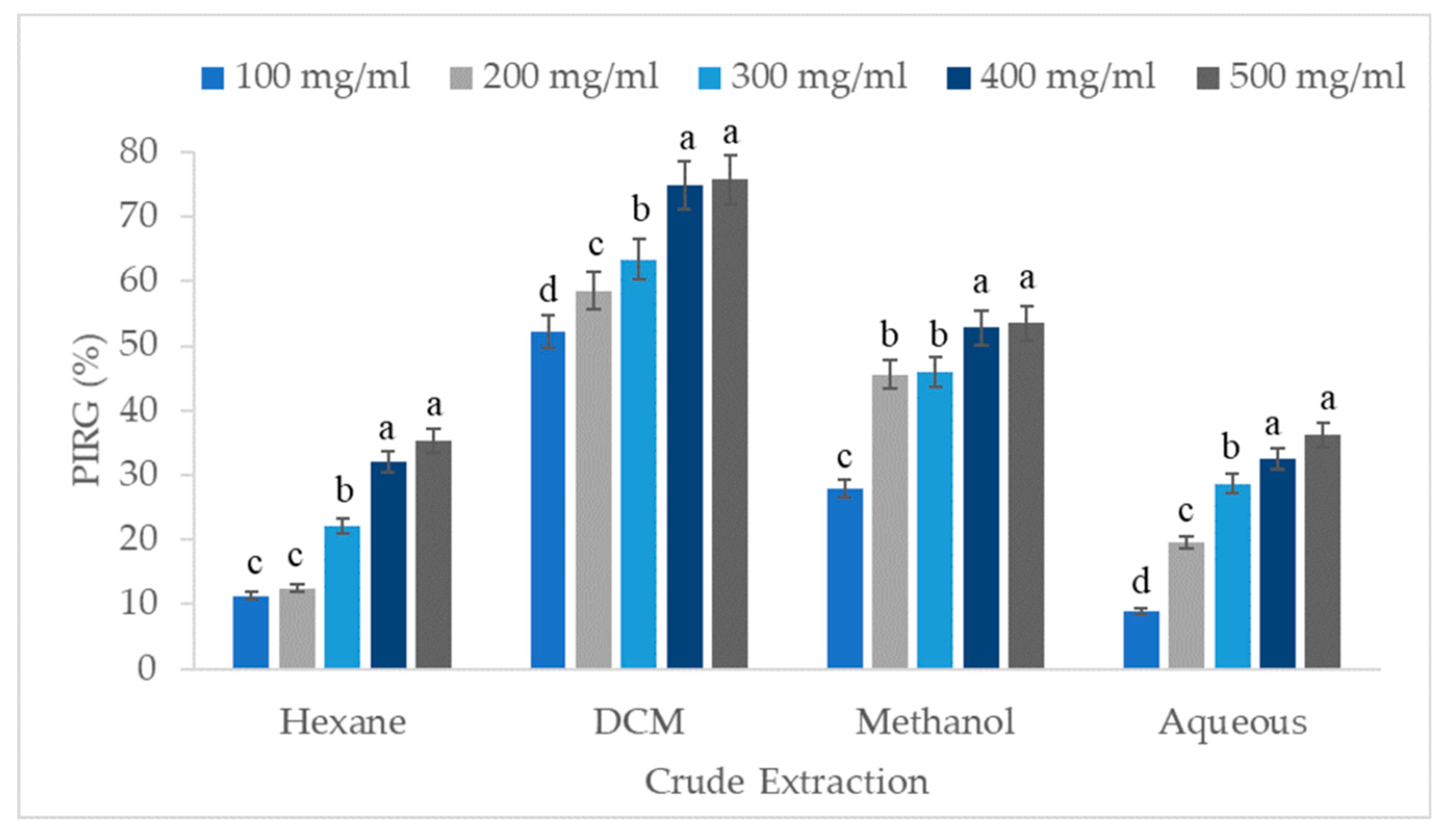
3.1. In Vitro Antifungal Activities of V. amygdalina Crude Extract against B. cinerea
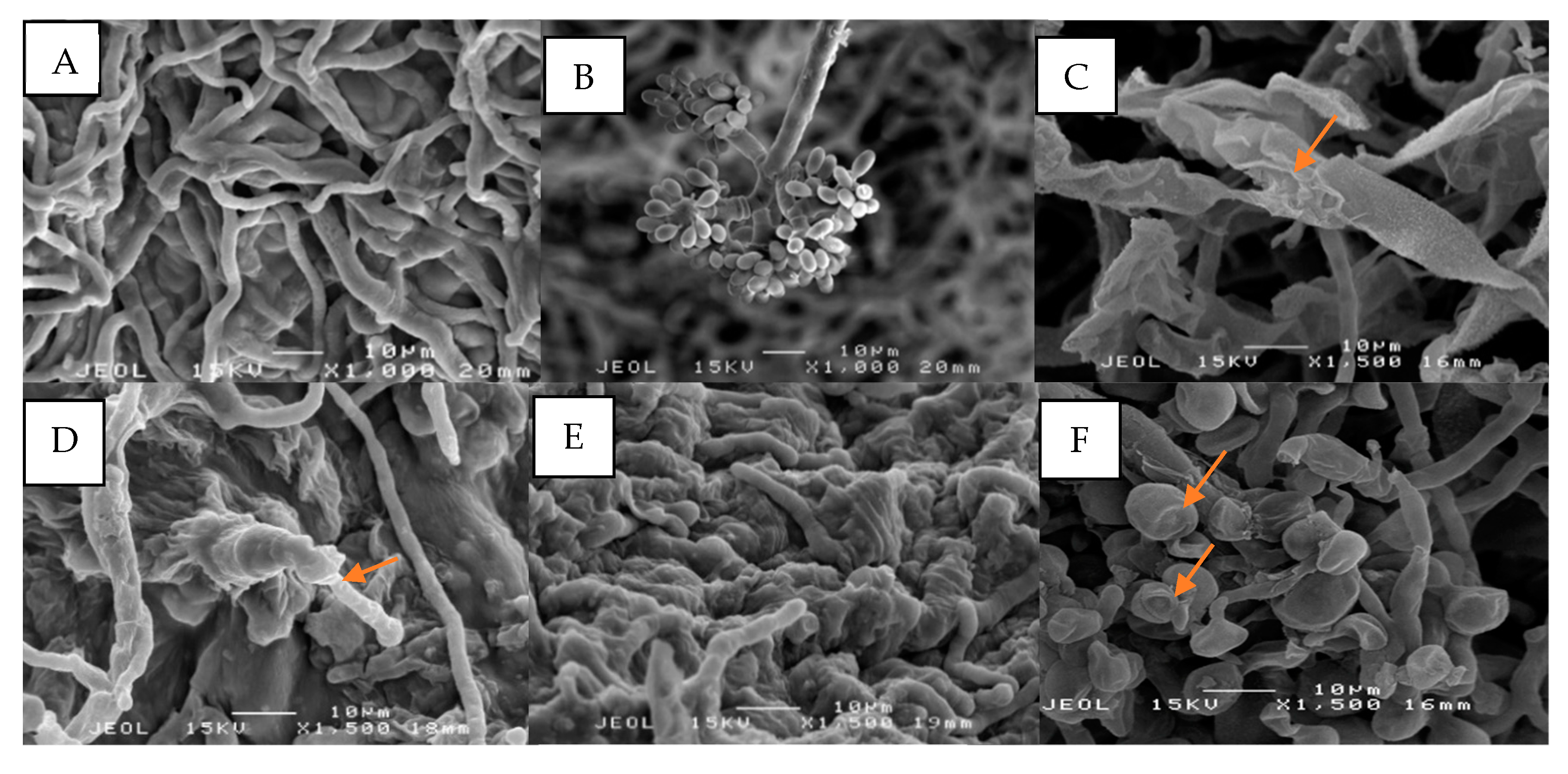
3.2. Effect of V. amygdalina Crude Extract on the Morphology of B. cinerea
3.3. In Vivo Antifungal Activities of V. amygdalina Crude Extract against B. cinerea
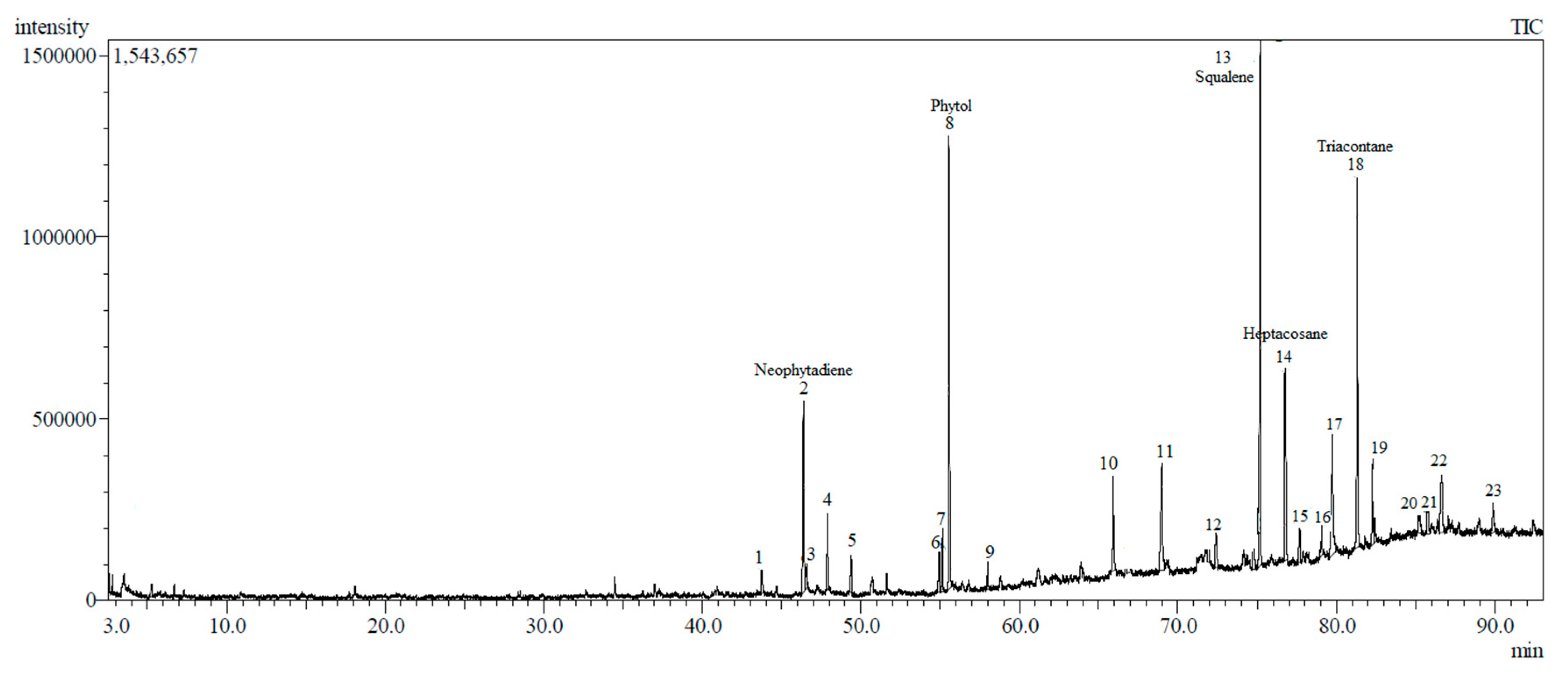
3.4. Phytochemical Screening of DCM Crude Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Department of Agriculture (DOA). Vegetables and Cash Crops Statistic; Department of Agriculture: Kuala Lumpur, Malaysia, 2018.
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Foster, G.D. The Top10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Tijjani, A.; Ismail, S.I.; Khairulmazmi, A.; Dzolkhifli, O. First report of gray mold rot disease on tomato (Solanum lycopersicum L.) caused by Botrytis cinerea in Malaysia. J. Plant Pathol. 2018, 101, 207. [Google Scholar] [CrossRef]
- Leyronas, C.; Duffaud, M.; Parès, L.; Jeannequin, B.; Nicot, P.C. Flow of Botrytis cinerea inoculum between lettuce crop and soil. Plant Pathol. 2015, 64, 701–708. [Google Scholar] [CrossRef]
- Shridhar, B.P.; Sharma, M.; Gupta, S.K.; Sharma, S.K. New generation fungicides for the management of buckeye rot of tomato. Indian Phytopathol. 2018, 71, 621–625. [Google Scholar] [CrossRef]
- Ma, T.; Luo, J.; Tian, C.; Sun, X.; Quan, M.; Zheng, C.; Kang, L.; Zhan, J. Influence of technical processing units on chemical composition and antimicrobial activity of carrot (Daucus carrot L.) juice essential oil. Food Chem. 2015, 170, 394–400. [Google Scholar] [CrossRef]
- Compean, K.L.; Ynalvez, R.A. Antimicrobial activity of plant secondary metabolites: A review. Res. J. Med. Plant. 2014, 8, 204–213. [Google Scholar]
- Soylu, E.M.; Kurt, Ş.; Soylu, S. In vitro and in vivo antifungal activities of the essential oils of various plants against tomato grey mould disease agent Botrytis cinerea. Int. J. Food. Microbiol. 2010, 143, 183–189. [Google Scholar] [CrossRef]
- Mohammadi, S.; Aroiee, H.; Aminifard, M.H.; Jahanbakhsh, V. In vitro and in vivo antifungal activates of the essential oils of various plants against strawberry grey mould disease agent Botrytis cinerea. Arch. Phytopathol. Plant Prot. 2012, 45, 2474–2484. [Google Scholar] [CrossRef]
- Vitoratos, A.; Bilalis, D.; Karkanis, A.; Efthimiadou, A. Antifungal activity of plant essential oils against Botrytis cinerea, Penicillium italicum and Penicillium digitatum. Not. Bot. Horti Agrobot. 2013, 41, 86–92. [Google Scholar] [CrossRef]
- De Bona, G.S.; Adrian, M.; Negrel, J.; Chiltz, A.; Klinguer, A.; Poinssot, B.; Héloir, M.; Angelini, E.; Vincenzi, S.; Bertazzon, N. Dual mode of action of grape cane extracts against Botrytis cinerea. J. Agric. Food Chem. 2019, 67, 5512–5520. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahmana, N.H.; Mudalipa, S.K.A.; Olalerea, O.A. Phytochemical and pharmacological properties of Vernonia amygdalina: A review. J. Chem. Eng. Ind. Biotech. 2017, 2, 96. [Google Scholar]
- Kadiri, O.; Olawoye, B. Vernonia amygdalina: An underutilized vegetable with nutraceutical Potentials—A Review. Turk. J. Agric. Food Sci. Technol. 2016, 4, 763–768. [Google Scholar] [CrossRef]
- Toyang, N.J.; Verpoorte, R. A Review of the Medicinal Potentials of Plants of the Genus Vernonia (Asteraceae). J. Ethnopharmacol. 2013, 146, 681–723. [Google Scholar] [CrossRef]
- Akowuah, G.A.; May, L.L.Y.; Chin, J.H. Toxicological evaluation of Vernonia amygdalina methanol leaves extracts in rats. Orient. Pharm. Exp. Med. 2015, 15, 365–369. [Google Scholar] [CrossRef]
- John, W.C.; Anyanwu, N.C.J.; Ayisa, T. Evaluation of the Effects of the Extract of Vernonia amygdalina on Fungi Associated with Infected Tomatoes (Lycopersicon esculentum) in Jos North Local Government Area, Plateau State, Nigeria. Annu. Res. Rev. Biol. 2016, 9, 1. [Google Scholar] [CrossRef]
- Okey, E.N.; Akwaji, P.I.; Akpan, J.B.; Umana, E.J.; Bassey, G.A. In vitro control of tomato (Solanum lycopersicon L.) fruit rot caused by fungi using two plant extracts. Int. Lett. Nat. Sci. 2016, 52, 19–27. [Google Scholar] [CrossRef]
- Ilondu, E.M. Phytochemical composition and efficacy of ethanolic leaf extracts of some Vernonia species against two phytopathogenic fungi. J. Biopestic. 2013, 6, 165–172. [Google Scholar]
- Haron, F.F.; Sijam, K.; Omar, D.; Rahmani, M. Bioassay-guided isolation of antifungal plumericin from Allamanda species (Apocynaceae). J. Biol. Sci. 2013, 13, 158–162. [Google Scholar]
- Javed, S.; Javaid, A.; Anwar, W.; Majeed, R.A.; Akhtar, R.; Naqvi, S.F. First report of Botrytis bunch rot of grapes caused by Botrytis cinerea in Pakistan. Plant Dis. 2017, 101, 1036. [Google Scholar] [CrossRef]
- Heckman, C.; Kanagasundaram, S.; Cayer, M.; Paige, J. Preparation of cultured cells for scanning electron microscope. Protoc. Exch. 2007. Available online: https://protocols.scienceexchange.com/protocols/preparation-of-cultured-cells-for-scanning-electron-microscope (accessed on 25 July 2020).
- Wang, C.; Zhang, J.; Chen, H.; Fan, Y.; Shi, Z. Antifungal activity of eugenol against Botrytis cinerea. Trop. Plant Pathol. 2010, 35, 137–143. [Google Scholar] [CrossRef]
- Rosero-Hernández, E.D.; Moraga, J.; Collado, I.G.; Echeverri, F. Natural Compounds That Modulate the Development of the Fungus Botrytis cinerea and Protect Solanum lycopersicum. Plants 2019, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Cotoras, M.; Mendoza, L.; Muñoz, A.; Yáñez, K.; Castro, P.; Aguirre, M. Fungitoxicity against Botrytis cinerea of a flavonoid isolated from Pseudognaphalium robustum. Molecules 2011, 16, 3885–3895. [Google Scholar] [CrossRef]
- Righini, H.; Baraldi, E.; García Fernández, Y.; Martel Quintana, A.; Roberti, R. Different Antifungal Activity of Anabaena sp., Ecklonia sp., and Jania sp. against Botrytis cinerea. Mar. Drugs 2019, 17, 299. [Google Scholar] [CrossRef] [PubMed]
- Rauha, J.P.; Remes, S.; Heinonen, M.; Hopia, A.; Ka¨hko¨nen, M.; Kujala, T.; Pihlaja, K.; Vuorela, H.; Vuorela, P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and extraction: A review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Krzyśko-Łupicka, T.; Walkowiak, W.; Białoń, M. Comparison of the Fungistatic Activity of Selected Essential Oils Relative to Fusarium graminearum Isolates. Molecules 2019, 24, 311. [Google Scholar]
- Zhu, Z.; Tian, S. Resistant responses of tomato fruit treated with exogenous methyl jasmonate to Botrytis cinerea infection. Sci. Hortic. 2012, 142, 38–43. [Google Scholar] [CrossRef]
- Hua, C.; Li, Y.; Wang, X.; Kai, K.; Su, M.; Zhang, D.; Liu, Y. The effect of low and high molecular weight chitosan on the control of gray mold (Botrytis cinerea) on kiwifruit and host response. Sci. Hortic. 2019, 246, 700–709. [Google Scholar] [CrossRef]
- Barkai-Golan, R. Postharvest Diseases of Fruits and Vegetables. Development and Control; Elsevier Science B.V.: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Fillinger, S.; Elad, Y. Botrytis-the Fungus, the Pathogen and Its Management in Agricultural Systems; Springer: New York, NY, USA, 2016. [Google Scholar]
- Evans, E. Systemic fungicides in practice. Pestic. Sci. 1971, 2, 192–196. [Google Scholar] [CrossRef]
- Hammerschlag, R.S.; Sisler, H.D. Benomyl and methyl-2-benzimidazolecarbamate (MBC): Biochemical, cytological and chemical aspects of toxicity to Ustilago maydis and Saccharomyces cerevisiae. Pestic. Biochem. Physiol. 1973, 3, 42–54. [Google Scholar] [CrossRef]
- Reddy, L.H.; Couvreur, P. Squalene: A natural triterpene for use in disease management and therapy. Adv. Drug Deliv. Rev. 2009, 61, 1412–1426. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Grande, M.A.; Gorinstein, S.; Espitia-Rangel, E.; Dávila-Ortiz, G.; Martínez-Ayala, A.L. Plant sources, extraction methods, and uses of squalene. Int. J. Agron. 2018. [Google Scholar] [CrossRef]
- Gnamusch, E.; Ryder, N.S.; Paltauf, F. Effect of squalene on the structure and function of fungal membranes. J. Dermatol. Treat. 1992, 3, 9–13. [Google Scholar] [CrossRef]
- Haque, E.; Irfan, S.; Kamil, M.; Sheikh, S.; Hasan, A.; Ahmad, A.; Lakshmi, V.; Nazir, A.; Mir, S.S. Terpenoids with antifungal activity trigger mitochondrial dysfunction in Saccharomyces cerevisiae. Microbiology 2016, 85, 436–443. [Google Scholar] [CrossRef]
- Yoshihiro, I.; Toshiko, H.; Shiraishi, A.; Hirose, K.; Hamashima, H.; Kobayashi, S. Biphasic effects of geranylgeraniol, teprenone and phytol on the growth of Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 1770–1774. [Google Scholar]
- Bhattacharjee, I.; Ghosh, A.; Chowdhury, N.; Chatterjee, S.K.; Chandra, G.; Laskar, S. n-Alkane profile of Argemone mexicana leaves. Z. Naturforsch. C 2010, 65, 533–536. [Google Scholar] [CrossRef]
- Eltahir, A.S.; AbuEReish, B.I. Microscopical Studies on the leaf and petiole of Vernonia amygadlina Del. Adv. Appl. Sci. Res. 2011, 2, 398–406. [Google Scholar]
- Yin, Y.; Bi, Y.; Chen, S.; Li, Y.; Wang, Y.; Ge, Y.; Ding, B.; Li, Y.; Zhang, Z. Chemical composition and antifungal activity of cuticular wax isolated from Asian pear fruit (cv. Pingguoli). Sci. Hortic. 2011, 129, 577–582. [Google Scholar] [CrossRef]
- Kumbum, S.; Sivarao, S. Antibacterial, antioxidant activity and GC-MS analysis of Eupatorium odoratum. Asian J. Pharm. Clin. Res. 2012, 5, 12. [Google Scholar]
- Freiesleben, S.; Jäger, A. Correlation between plant secondary metabolites and their antifungal mechanisms-a review. Med. Aromat. Plants 2014, 3, 1–6. [Google Scholar]
- Ahmed, S.M.; Abdelgaleil, S.A. Antifungal activity of extracts and sesquiterpene lactones from Magnolia grandiflora L. (Magnoliaceae). Int. J. Agric. Biol. 2005, 7, 638–642. [Google Scholar]
- Tabet Zatla, A.; Dib, M.E.A.; Djabou, N.; Ilias, F.; Costa, J.; Muselli, A. Antifungal activities of essential oils and hydrosol extracts of Daucus carota subsp. sativus for the control of fungal pathogens, in particular gray rot of strawberry during storage. J. Essent. Oil Res. 2017, 29, 391–399. [Google Scholar] [CrossRef]
- Howard, C.B.; Johnson, W.K.; Pervin, S.; Izevbigie, E.B. Recent perspectives on the anticancer properties of aqueous extracts of Nigerian Vernonia amygdalina. Bot. Targets Ther. 2015, 5, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene lactones from Artemisia genus: Biological activities and methods of analysis. J. Anal. Methods Chem. 2015. [Google Scholar] [CrossRef]
- Pusztahelyi, T.; Holb, I.J.; Pócsi, I. Secondary metabolites in fungus-plant interactions. Front. Plant Sci. 2015, 6, 573. [Google Scholar] [CrossRef]
| Factors | PIRG (%) |
|---|---|
| Crude extracts (CE) | |
| Hexane | 22.65 ± 2.34d x |
| Dichloromethane | 64.94 ± 2.19a |
| Methanol | 45.15 ± 2.28b |
| Aqueous | 25.18 ± 2.32c |
| Concentration levels (mg/mL) (CL) | |
| 100 | 25.07 ± 4.47d |
| 200 | 34.04 ± 4.93c |
| 300 | 40.04 ± 4.25b |
| 400 | 48.05 ± 4.61a |
| 500 | 50.18 ± 4.29a |
| Significance | |
| CE × CL | ** |
| Treatment | Disease Incidence (%) |
|---|---|
| Negative control | 100 ± 0.00a X |
| Benomyl | 68.75 ± 2.69b |
| DCM 300 mg/mL | 60.41 ± 2.69c |
| DCM 400 mg/mL | 50.0 ± 3.40d |
| DCM 500 mg/mL | 46.88 ± 3.13d |
| Treatment | DSI (%) |
|---|---|
| Negative control | 27.28 ± 0.29a X |
| Benomyl | 10.84 ± 0.69b |
| DCM 300 mg/mL | 4.50 ± 0.53c |
| DCM 400 mg/mL | 2.27 ± 0.12d |
| DCM 500 mg/mL | 2.19 ± 0.05d |
| Peak | Retention Index ** | Compound Name | Chemical Group | Area (%) |
|---|---|---|---|---|
| 1 | 1763 | Loliolide | Monoterpenoid hydroxylactones | 0.76 |
| 2 | 1839 | Neophytadiene | Sesquiterpene | 6.28 |
| 3 | 1841 | Phytone | Terpene ketone | 0.90 |
| 4 | 1860 | 2-Hexadecen-1-ol | Acyclic diterpene | 0.79 |
| 5 | 1882 | 3,7,11,15-tetramethyl-2-hexadecen-1-ol | Acyclic diterpene | 1.46 |
| 6 | 1966 | Hexadecanoic acid | Fatty acid | 1.06 |
| 7 | 2089 | 9,12-Octadecadienoic acid | Fatty acid | 1.19 |
| 8 | 2119 | Phytol | Diterpene alcohol | 15.05 |
| 9 | 2147 | Linolenic acid | Fatty acid | 1.86 |
| 10 | 2499 | l-caryophyllene | Bicyclic sesquiterpene | 3.35 |
| 11 | 2577 | γ-Elemene | Sesquiterpene | 5.72 |
| 12 | 2711 | 1,3,7-Nonatriene-1 | Monoterpene | 1.35 |
| 13 | 2830 | Squalene | Triterpene | 16.92 |
| 14 | 2892 | Heptacosane | Hydrocarbon lipid | 7.14 |
| 15 | 2577 | Geranyl linalool | Monoterpenoid | 1.12 |
| 16 | 2993 | Tetratriacontane | Hydrocarbon lipid | 1.02 |
| 17 | 3021 | Unknown | - | 6.41 |
| 18 | 3097 | Triacontane | Hydrocarbon lipid | 11.31 |
| 19 | 3139 | α-Tocopherol | Vitamin E | 6.04 |
| 20 | 3272 | Stigmasterol | Stigmastane | 0.98 |
| 21 | 3290 | α-spinasterol acetate | Stigmastane | 1.37 |
| 22 | 3295 | Chondrillasterol | Triterpene (sterol) | 2.90 |
| 23 | 3472 | α-tocopherol acetate | Vitamin E | 1.46 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusoff, S.F.; Haron, F.F.; Tengku Muda Mohamed, M.; Asib, N.; Sakimin, S.Z.; Abu Kassim, F.; Ismail, S.I. Antifungal Activity and Phytochemical Screening of Vernonia amygdalina Extract against Botrytis cinerea Causing Gray Mold Disease on Tomato Fruits. Biology 2020, 9, 286. https://doi.org/10.3390/biology9090286
Yusoff SF, Haron FF, Tengku Muda Mohamed M, Asib N, Sakimin SZ, Abu Kassim F, Ismail SI. Antifungal Activity and Phytochemical Screening of Vernonia amygdalina Extract against Botrytis cinerea Causing Gray Mold Disease on Tomato Fruits. Biology. 2020; 9(9):286. https://doi.org/10.3390/biology9090286
Chicago/Turabian StyleYusoff, Siti Fairuz, Farah Farhanah Haron, Mahmud Tengku Muda Mohamed, Norhayu Asib, Siti Zaharah Sakimin, Faizah Abu Kassim, and Siti Izera Ismail. 2020. "Antifungal Activity and Phytochemical Screening of Vernonia amygdalina Extract against Botrytis cinerea Causing Gray Mold Disease on Tomato Fruits" Biology 9, no. 9: 286. https://doi.org/10.3390/biology9090286
APA StyleYusoff, S. F., Haron, F. F., Tengku Muda Mohamed, M., Asib, N., Sakimin, S. Z., Abu Kassim, F., & Ismail, S. I. (2020). Antifungal Activity and Phytochemical Screening of Vernonia amygdalina Extract against Botrytis cinerea Causing Gray Mold Disease on Tomato Fruits. Biology, 9(9), 286. https://doi.org/10.3390/biology9090286





