The Efficacy of the Novel TSPO Ligands 2-Cl-MGV-1 and 2,4-Di-Cl-MGV-1 Compared to the Classical TSPO Ligand PK 11195 to Counteract the Release of Chemokines from LPS-Stimulated BV-2 Microglial Cells
Abstract
:1. Introduction
2. Methods
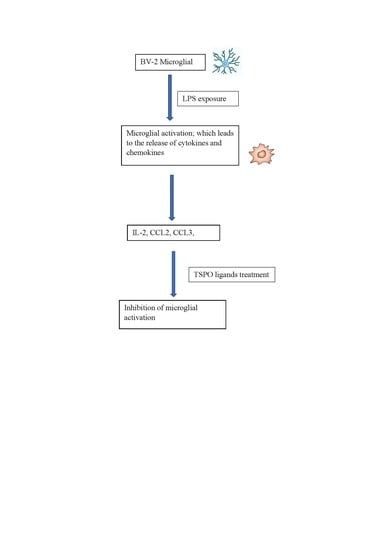
2.1. BV-2 Microglial Cells
2.2. LPS Exposure
2.3. TSPO Ligands Treatment
2.4. Trypan Blue Staining for Cell Counting
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Statistical Analyses
3. Results
3.1. CCL2 (MCP1)
3.2. CCL3 (MIP1α)
3.3. CCL5 (RANTES)
3.4. CCL8 (MCP2)
3.5. IL-2 Cytokine
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, Y.; Park, Y.; Nam, H.; Lee, J.W.; Yu, S.W. Translocator protein (TSPO): The new story of the old protein in neuroinflammation. BMB Rep. 2020, 53, 20–27. [Google Scholar] [CrossRef]
- Monga, S.; Nagler, R.; Amara, R.; Weizman, A.; Gavish, M. Inhibitory Effects of the Two Novel TSPO Ligands 2-Cl-MGV-1 and MGV-1 on LPS-induced Microglial Activation. Cells 2019, 8, 486. [Google Scholar] [CrossRef] [Green Version]
- Monga, S.; Denora, N.; Laquintana, V.; Franco, M.; Marek, I.; Singh, S.; Nagler, R.; Weizman, A.; Gavish, M. The protective effect of the TSPO ligands 2,4-Di-Cl-MGV-1, CB86, and CB204 against LPS-induced M1 pro-inflammatory activation of microglia. Brain Behav. Immun. Heal. 2020, 5, 100083. [Google Scholar] [CrossRef]
- Tremblay, M.È.; Lecours, C.; Samson, L.; Sánchez-Zafra, V.; Sierra, A. From the Cajal alumni Achúcarro and Río-Hortega to the rediscovery of never-resting microglia. Front. Neuroanat. 2015, 9, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef]
- Hoyer, K.K.; Dooms, H.; Barron, L.; Abbas, A.K. Interleukin-2 in the development and control of inflammatory disease. Immunol. Rev. 2008, 226, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Doersch, K.M.; DelloStritto, D.J.; Newell-Rogers, M.K. The contribution of interleukin-2 to effective wound healing. Exp. Biol. Med. 2017, 242, 384–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boff, D.; Crijns, H.; Teixeira, M.M.; Amaral, F.A.; Proost, P. Neutrophils: Beneficial and harmful cells in septic arthritis. Int. J. Mol. Sci. 2018, 19, 468. [Google Scholar] [CrossRef] [Green Version]
- Marcuzzi, E.; Angioni, R.; Molon, B.; Calì, B. Chemokines and chemokine receptors: Orchestrating tumor metastasization. Int. J. Mol. Sci. 2019, 20, 96. [Google Scholar] [CrossRef] [Green Version]
- Raghu, H.; Lepus, C.M.; Wang, Q.; Wong, H.H.; Lingampalli, N.; Oliviero, F.; Punzi, L.; Giori, N.J.; Goodman, S.B.; Chu, C.R.; et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann. Rheum. Dis. 2017, 76, 914–922. [Google Scholar] [CrossRef] [Green Version]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interf. Cytokine Res. 2009, 29, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, A.; Tozaki-Saitoh, H.; Koga, Y.; Tsuda, M.; Inoue, K. Activation of P2X 7 receptors induces CCL3 production in microglial cells through transcription factor NFAT. J. Neurochem. 2009, 108, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Min, K.J.; Jang, J.H.; Kwon, T.K. Inhibitory effects of melatonin on the lipopolysaccharide-induced CC chemokine expression in BV2 murine microglial cells are mediated by suppression of Akt-induced NF-κB and STAT/GAS activity. J. Pineal Res. 2012, 52, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Pharoah, D.S.; Varsani, H.; Tatham, R.W.; Newton, K.R.; de Jager, W.; Prakken, B.J.; Klein, N.; Wedderburn, L.R. Expression of the inflammatory chemokines CCL5, CCL3 and CXCL10 in juvenile idiopathic arthritis, and demonstration of CCL5 production by an atypical subset of CD8+ T cells. Arthritis Res. Ther. 2006, 8, R50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, S.A.; Chang, D.S.; Colvin, R.A.; Byrne, M.H.; McCully, M.L.; Moser, B.; Lira, S.A.; Charo, I.F.; Luster, A.D. Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL-5+TH2 cells. Nat. Immunol. 2011, 12, 167–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azrad, M.; Zeineh, N.; Weizman, A.; Veenman, L.; Gavish, M. The TSPO ligands 2-CL-MGV-1, MGV-1, and PK 11195 differentially suppress the inflammatory response of BV-2 microglial cell to LPS. Int. J. Mol. Sci. 2019, 20, 594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henn, A.; Lund, S.; Hedtjärn, M.; Schrattenholz, A.; Pörzgen, P.; Leist, M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX 2009, 26, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Vainshtein, A.; Veenman, L.; Shterenberg, A.; Singh, S.; Masarwa, A.; Dutta, B.; Tsoglin, E.; Levin, E.; Leschiner, S.; Maniv, I.; et al. Quinazoline-based tricyclic compounds that regulate programmed cell death, induce neuronal differentiation, and are curative in animal models for excitotoxicity and hereditary brain disease. Cell Death Discov. 2015, 1, 15027–15044. [Google Scholar] [CrossRef]
- Laing, K.J.; Secombes, C.J. Chemokines. Dev. Comp. Immunol. 2004, 28, 443–460. [Google Scholar] [CrossRef]
- Hayashi, M.; Luo, Y.; Laning, J.; Strieter, R.M.; Dorf, M.E. Production and function of monocyte chemoattractant protein-1 and other β-chemokines in murine glial cells. J. Neuroimmunol. 1995, 60, 143–150. [Google Scholar] [CrossRef]
- Gunn, M.D.; Nelken, N.A.; Liao, X.; Williams, L.T. Monocyte chemoattractant protein-1 is sufficient for the chemotaxis of monocytes and lymphocytes in transgenic mice but requires an additional stimulus for inflammatory activation. J. Immunol. 1997, 158, 376–383. [Google Scholar] [PubMed]
- Barron, A.M.; Tokunaga, M.; Zhang, M.R.; Ji, B.; Suhara, T.; Higuchi, M. Assessment of neuroinflammation in a mouse model of obesity and β-amyloidosis using PET. J. Neuroinflamm. 2016, 13, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monga, S.; Weizman, A.; Gavish, M. The Efficacy of the Novel TSPO Ligands 2-Cl-MGV-1 and 2,4-Di-Cl-MGV-1 Compared to the Classical TSPO Ligand PK 11195 to Counteract the Release of Chemokines from LPS-Stimulated BV-2 Microglial Cells. Biology 2020, 9, 291. https://doi.org/10.3390/biology9090291
Monga S, Weizman A, Gavish M. The Efficacy of the Novel TSPO Ligands 2-Cl-MGV-1 and 2,4-Di-Cl-MGV-1 Compared to the Classical TSPO Ligand PK 11195 to Counteract the Release of Chemokines from LPS-Stimulated BV-2 Microglial Cells. Biology. 2020; 9(9):291. https://doi.org/10.3390/biology9090291
Chicago/Turabian StyleMonga, Sheelu, Abraham Weizman, and Moshe Gavish. 2020. "The Efficacy of the Novel TSPO Ligands 2-Cl-MGV-1 and 2,4-Di-Cl-MGV-1 Compared to the Classical TSPO Ligand PK 11195 to Counteract the Release of Chemokines from LPS-Stimulated BV-2 Microglial Cells" Biology 9, no. 9: 291. https://doi.org/10.3390/biology9090291
APA StyleMonga, S., Weizman, A., & Gavish, M. (2020). The Efficacy of the Novel TSPO Ligands 2-Cl-MGV-1 and 2,4-Di-Cl-MGV-1 Compared to the Classical TSPO Ligand PK 11195 to Counteract the Release of Chemokines from LPS-Stimulated BV-2 Microglial Cells. Biology, 9(9), 291. https://doi.org/10.3390/biology9090291