1. Introduction
The study areas covered by natural science include physics, chemistry, biology, Earth science, etc. When learning natural science, students can explore the laws behind natural phenomena through observation, logical thinking, and experimentation. Due to the influence of exam-oriented education, teachers often demonstrate an experiment—instead of allowing students to conduct it—or describe the experimental principles and results as an alternative. Therefore, students have not experienced the process of observation and experimentation, which may lead to difficulty in understanding some important concepts. Recently, advanced 3D computer programs have been integrated into traditional chemistry teaching. For example, allowing the observation of chemical bonds and the different reactions that reactants can undergo and cause their properties to change.
Sawyer [
1] pointed out that deep learning usually occurs when what we have learned is connected to real-life situations, which can be achieved by designing a series of open-ended questions, allowing students to make assumptions based on prior knowledge, and verifying the correctness of assumptions through experimentation. Lacking the experimental process and logical reasoning, the knowledge established may not be linked to prior knowledge, resulting in the creation of memory nodes without connection to relevant cognition. Therefore, the memory will gradually decline because the brain capacity is limited while the stimulation decreases over time.
Although the experimental process can effectively assist students in learning science, some scientific concepts are still very abstract due to the difficulty in observation. Taking the lunar phase, movement of stars, and seasonal changes in Earth science as examples, the laws behind these phenomena are not easy to understand over a short period of observation. In science education, texts, pictures, videos, and simulation models are used to explain the causes of natural phenomena. Students can only observe and imagine the situation from a self-centered perspective, which often results in some limitations in learning and may even lead to misconceptions.
Similar learning topics in physics include voltage, current, and magnetic lines of force—which are not visible to the naked eye—and thus, experimentation is needed. Alternative methods of teaching without real experiments include using hydraulic simulators to study the first two electrical quantities and simple metal filings on paper to show the existence of the lines of force in a magnetic field or using 3D programs to show magnetic and electromagnetic fields. However, the complexity and cost of the simulator and software may not be suitable for application in high school teaching. In chemistry, most theories and concepts are derived from experimental results, and some abstract concepts are difficult to understand due to the lack of submicroscopic observation.
Chemistry is a basic natural science studying the properties, composition, structures, and changes of matter, and its research topics involve the relationships between different types of matter as well as between matter and energy. Chemistry is essential for the development of many advanced and applied sciences, and it is also a science that emphasizes both theory and experimentation. Consequently, appropriate experimental courses can not only stimulate students’ learning interest but also cultivate the ability of experimental design, operation and observation. From the process of “learning by doing” [
2], students can observe and think carefully, discover difficulties, design an experiment, verify the principles of chemical reactions using experimental results, and convert theoretical knowledge into problem-solving ability.
Computer-assisted learning (CAL) refers to learning supported by a computer and does not require direct interaction between the learner and a human instructor. CAL can analyze learning status with artificial intelligence (AI) to improve effectiveness, increase classroom participation through computer networks, and make abstract concepts concrete using immersive technology. Augmented reality (AR) is an immersive technology combining virtual objects and information with the real world based on position or image recognition. AR has three major characteristics, including: (1) combining virtual and real worlds, (2) real-time interaction, and (3) operation in three-dimensional environments. It can be used to superimpose virtual objects on the user’s view to enhance interaction with and awareness of surrounding environments [
3].
Freeman [
4] pointed out that the students willing to learn are more engaged in classroom teaching activities and often have better learning outcomes. To improve students’ attitudes towards active learning, teachers can guide them through interesting and meaningful learning activities. Rogers [
5,
6] believed that learning under traditional teaching methods is often one-way knowledge delivery by teachers, so students only receive information passively without much interaction. To provide them with more opportunities for self-initiated investigation, teaching strategy should be changed from teacher-centered to student-oriented, which emphasizes knowledge constructed by active exploration rather than passively delivered by teachers.
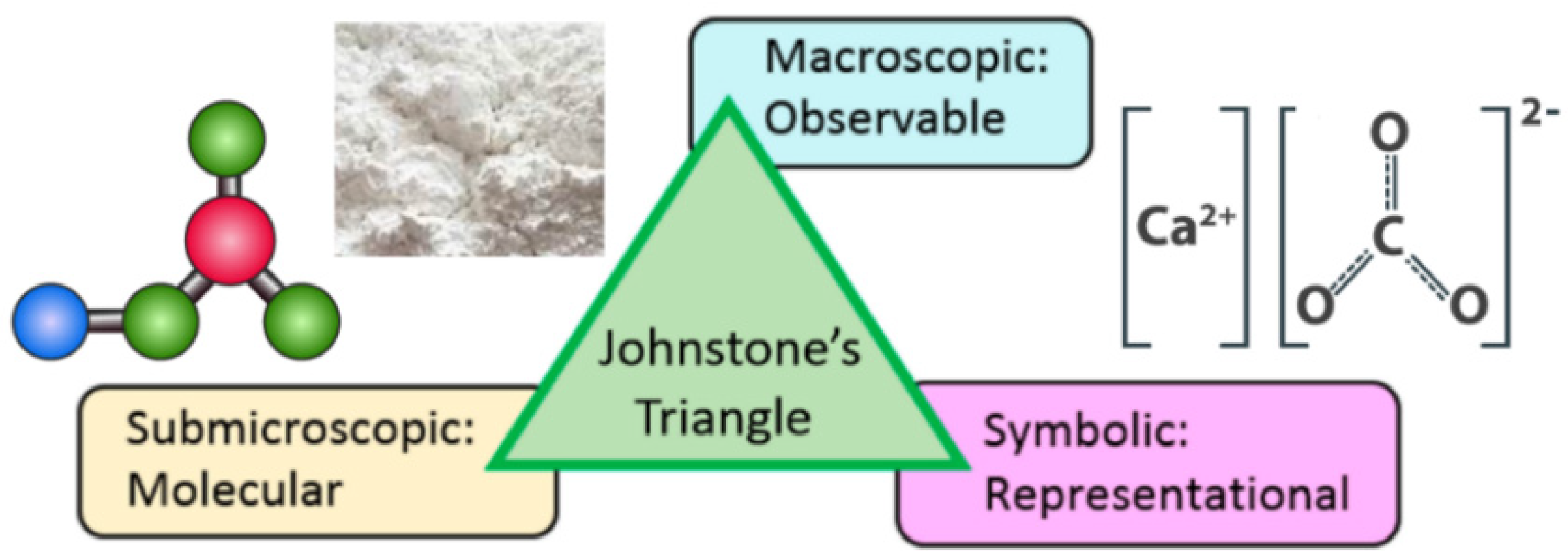
Johnstone [
7] proposed the triangular model to indicate that chemical knowledge can be divided into three levels in learning, and it is often taken into consideration in instructional design (
Figure 1). The three levels are: (1) macroscopic and visual phenomenon, which provides observable chemical phenomena, such as density, smell, color, etc.; (2) submicroscopic presentation, which shows the virtual views with atoms, molecules, ions and their kinetics, interactions, dynamic motions, etc.; (3) symbolic presentation, which describes a reactive event through formulas, equations, mathematical manipulation, and graphs. In traditional teaching, the symbolic level often appears in most chemical instructions, so students must transform the symbols into submicroscopic views. However, the symbolic representation is directly connected to the macroscopic and submicroscopic phenomena, which are required for visualizing a chemical reaction. Without any of them, students may have difficulty understanding some abstract concepts.
To know why a chemical reaction happens, we must understand how the structures of reactants change, such as through electron transfer and chemical bonds, to form the products. The changes of submicroscopic phenomena are usually related to macroscopic phenomena. For example, the reactants may undergo different chemical reactions and change their physical properties. The reason why the abstract concepts are hard to understand is due to the complexity of concepts and the spatial ability of students. Virtual reality and augmented reality are suitable for intervention because they can expand the learning experience and enhance logical thinking by allowing students to visualize the submicroscopic reactions. Herga, Glazar, and Dinevski [
8] pointed out that using virtual experiments to simulate microscopic phenomena is helpful for increasing the cognition of abstract concepts and thus improving learning effectiveness.
To solve the problem caused by exam-oriented education, an AR system is developed in this study to assist high school students in learning chemistry by combining the interactivity, convenience, and 3D visualization of augmented reality. Students can conduct a virtual experiment on smart phones or tablet computers by selecting the reactants and coefficients on AR cards for operation and can enter the submicroscopic world to understand the changes in molecular structures and chemical bonds of reactants in a chemical reaction. AR operation can enhance awareness of essential knowledge and abstract concepts through the explorative process and scaffolding guidance.
The interactive operation of the AR system provides a learning experience different from traditional teaching, and 3D modeling and animation allow learners to understand relevant chemical concepts from active exploration. In addition, the AR system is developed based on the POE strategy [
9], Johnstone’s triangle model [
7], and the multimedia learning theory proposed by Mayer and Moreno [
10,
11]. This study investigated the impact of the AR system on students’ learning effectiveness, learning motivation, and cognitive load through a teaching experiment. A questionnaire survey was also performed to analyze technology acceptance after using the AR system. According to the purpose of this study, the research objectives are described below:
Explore the effectiveness of the AR system on learning material structures and chemical equilibrium for high school students.
Explore the motivation of students when the AR system is used for learning material structures and chemical equilibrium.
Explore the cognitive load of students when the AR system is used for learning material structures and chemical equilibrium.
Analyze students’ technology acceptance after the AR system is used for learning material structures and chemical equilibrium.
2. Theoretical Frameworks
Chemistry is a science with abstract concepts which require the explanation of submicroscopic views for understanding the theory behind macroscopic phenomena. Learning topics in high school chemistry curriculum often involve the study of material structures and properties as well as relevant knowledge in different areas, which often demand transformation of visual observation into abstract thinking.
2.1. Teaching Strategy and Learning Motivation
Rotter [
12] stated that a learner’s behavioral motivation can be inferred from three aspects, i.e., the added value, expectation of goal, and current situation. Atkinson [
13] mentioned in the theory of achievement motivation that two relative psychological effects will occur at the same time when an individual engages in a certain task: “avoiding failure” and “pursuing success”. According to the evaluation of individual experience, the two effects will influence each other and result in the strength of motivation. Weiner’s [
14] attribution theory states that personal past experiences and future needs will affect one’s future behaviors and choices.
Bandura and Walters [
15] mentioned in the theory of self-efficacy that individuals’ confidence in whether they have completed learning tasks is the key factor affecting their motivation. The factor can be further divided into past achievement performance, substitute experience, verbal persuasion, and emotional stimulation. Pintrich [
16] indicated that learners’ motivation is composed of three components—namely, emotion, expectation, and value—which may affect learning results separately. Keller [
17] integrated the research results of relative motivation theories based on psychology and instructional design to propose the Attention, Relevance, Confidence and Satisfaction (ARCS) motivation model. Miltiadou and Savenye [
18] reported that instructional design using the ARCS model could stimulate students’ motivation to achieve effective learning.
The POE (predict–observe–explain) strategy [
9] requires the prediction and reasoning for an event by students based on their prior knowledge. Students must first predict the outcome, describe what they have observed, and then use experimental results to explain whether the results are consistent with the prediction. Finally, students must resolve the discrepancies between prediction and observation. If there is any conflict, they must clarify their own ideas and repeat the same procedure until consistency is reached. The process of discussion on observation and explanation of the outcomes gives them a more in-depth understanding of the event.
When applying the POE strategy in teaching, the predicted results may conflict with the actual results, and students can learn from such cognitive conflicts. Therefore, the POE strategy is suitable for learning situations where cognitive conflicts often occur. However, the following conditions should be met when applying the POE strategy: (1) providing a situation in which students can reason based on personal understanding rather than random speculation, (2) providing clues or instructions for students to think, and (3) clear and visible observation results. These conditions are required to ensure that the discussion can be connected to prior knowledge to enhance their understanding, because self-knowledge links can facilitate deeper learning.
2.2. Augmented Reality
The prototype of AR emerged about 50 years ago, but commercial AR products did not appear until 2003. With the advance of information and communication technology (ICT), there have been many AR applications in different areas, such as architecture, education, medical care, video games, tourism exhibitions, etc. Augmented reality is similar to virtual reality (VR), and it augments the surroundings by adding virtual elements to the real view using position and/or image recognition. The imposed information does not replace the environmental contexts, so AR is considered an extension of VR into the real world. According to Camba and Contero [
19], AR is a technology to enhance the meaning of an object in the real world by displaying spatial data in the real environment. Caudell and Mizell [
20] explained AR as superimposing virtual materials in real environments for users to experience the effect of coexistence in virtual and real worlds.
According to the real–reality–virtual continuum proposed by Milgram and Kishino [
21], the definition of AR can be understood more clearly as shown in
Figure 2. The left and right sides represent the real environment and the virtual environment, respectively. The intermediate transition process, including augmented reality and augmented virtuality, is called mixed reality (MR), a medium between the virtual and real worlds. Alizadehsalehi, Hadavi, and Huang [
22] reviewed the most relevant articles regarding building information modeling (BIM) and XR (VR, AR, and MR) in the architecture, engineering, and construction (AEC) industry, where XR stands for extended reality, an umbrella term that covers VR, AR, and MR. They identified the outsourcing patterns for such technologies among construction project stakeholders and provided a comprehensive review of using XR to solve a variety of construction project management issues, which can be used as a roadmap for implementing XR in the AEC industry.
Augmented reality is a tool connecting the virtual world by presenting virtual objects and information in the real world. Generally speaking, augmented reality can be categorized as: (1) traditional AR, using markers or images for detection; (2) location-based AR, identifying the position using GPS; and (3) hybrid AR, incorporating markers, images, and GPS data. Azuma [
23] proposed that an AR system has three characteristics: (1) combining real and virtual worlds, (2) interactivity in real time, and (3) being registered in a 3D space. The application of augmented reality is not limited to a specific position, and thus, the degree of freedom is higher. Therefore, the users can adapt to environmental changes more easily by increasing the sensory stimulation in the real world.
The AR system developed in this study is image-based, and it can present the molecular models specified on AR cards and support the functions of dragging and rotation to enhance the understanding of material structures. It can identify the reactants and their amounts to display the corresponding chemical reaction and provide useful information for learners. The diversified learning paths allow them to explore in the submicroscopic world more easily and effectively [
24].
Augmented reality can improve the learning experience and performance by increasing learners’ attention and interaction, and gamification design can also be combined with AR to enhance their learning motivation. The advantages of augmented reality include the ability to concretize abstract concepts and the interactive user interface to allow diverse learning models. Billinghurst et al. [
25] pointed out that augmented reality can be utilized as a teaching tool enabling learners to interact more directly with virtual objects and imposed information in the virtual and real worlds.
When applied in education, AR can be combined with various teaching strategies to lower the operating threshold and allow inexperienced learners to operate smoothly. Kumar, Mantri, and Dutta [
26] introduced AR to the course of embedded systems for learning the knowledge and principles of electronic components and the operating mechanisms behind them. Through AR interaction, the complex concepts can be visualized to help learners understand the operating principles and functions of logic units in an embedded system. Experimental results revealed that AR is more effective if it is used by students without relevant concepts in embedded systems.
Dünser et al. [
27] designed an AR book for learning the concepts of electromagnetism in high schools, which allowed students to interact with virtual objects for observing the working principles. It was discovered that the experimental group (using the AR book) had better learning achievement and memory preservation than the control group (using the traditional teaching method). Weng et al. [
28] combined marker-based AR and biological interpretation in their research, and the experimental results showed that augmented reality could improve learners’ attitudes and cognitive thinking to achieve better learning effectiveness. Zhou et al. [
29] developed virtual microscopes using VR and AR technologies, allowing students to clearly understand the structure and functions of a microscope and familiarize themselves with the related operational skills.
Tarng, Lin, and Ou [
30] combined the AR technology with the operating principle of Daniell cell to develop a virtual experiment for high school chemistry courses. The virtual experiment was conducted on mobile devices by selecting required equipment and materials on AR cards to set up the experimental environment. Students could use the galvanometer to measure the current after mounting the salt bridge on the beakers containing zinc sulfate and copper sulfate solutions. They could see the movement of electrons and the change in molecular structures to understand the concepts of redox reactions. The analytical results showed that the virtual experiment was more effective than the real experiment because students could explore autonomously to enhance cognition by observing the submicroscopic view of redox reactions.
Garzón [
31] provided an overview of AR technology from its first application to the present. He defined three generations of AR in education and identified some major challenges based on the analysis of its evolution. The systematic review by Álvarez-Marín and Velázquez-Iturbide [
32] indicated that there is considerable room for the application of AR in engineering studies, and they suggested a design with higher functional characteristics and interactivity to exploit the full potential of AR. Alizadehsalehi and Yitmen [
33] developed the generic framework of digital twin (DT)-based automated construction progress monitoring through reality capture to extended reality. Their findings validated the positive impact and importance of utilizing technology integration in a logical framework to provide trustable, real-time, transparent, and digital construction progress monitoring. Fombona-Pascual, Fombona and Vicente [
34] identified the real situation of AR development and its potential for 3D visualization of molecules. Their study suggested the application of AR in early education, which has shown potential to facilitate the understanding and visualization of chemical structures.
According to the above literature review, it can be seen that AR applications have the following advantages: (1) interactivity: compared with traditional teaching methods, AR provides learners with opportunities of exploring knowledge actively rather than receiving knowledge passively. Students can be more engaged in learning situations through practical operation and interaction, thereby enhancing their learning motivation. (2) Conceptualization: abstract concepts can be presented in real-time through AR operation such that students are not required to guess the results through imagination, which is helpful for conceptual change and cognition. (3) Immersive learning experience: AR can provide a more attractive and convenient operating environment, not limited by space or time, for students to enhance their learning motivation.
2.3. Cognitive Load
Cognitive load is defined as the amount of working memory resources used when a specific task is placed on an individual’s cognitive system [
35]. If the amount of received information exceeds the capacity of working memory, the learner must be involved in other cognitive processing, which may result in additional cognitive load and a reduction in learning efficiency. To avoid overload in working memory, teaching materials should be presented in an appropriate amount with a clear structure. Too much or insufficient information may distract the learner’s attention and cause increased cognitive load. The difficulty of learning content and prior knowledge are also important factors affecting cognitive load. Because the capacity of working memory is limited and transient, it can easily lead to a decrease in learning effectiveness when the learner is cognitively overloaded. Based on the theory of cognitive load proposed by van Merrienboer and Sweller [
36], cognitive load can be categorized into three types: intrinsic cognitive load, extraneous cognitive load, and germane cognitive load:
Intrinsic cognitive load refers to the difficulty of learning content, which is the basic structure of information obtained by learners to achieve learning goals and has nothing to do with the learning process. Usually, highly relevant and complex content has a higher intrinsic cognitive load. For the case of learning language, it is easier to start learning from a single word because there is no interaction with other words, and therefore, the cognitive load is lower. When learning a sentence, the difficulty increases because it involves combination of several words as well as grammar and thus creates a heavier cognitive load. However, learning with low interaction may still generate high cognitive load due to a large number of simple tasks involved [
37].
Extraneous cognitive load refers to the fact that the same material will produce different cognitive loads due to different presentation methods. A good presentation method can prevent learners from using more cognitive resources for processing unimportant information, thereby improving learning effectiveness. Inappropriate instructional design or ignoring the limited capacity of working memory will lead to additional cognitive overload and cause learning problems. Therefore, improving the presentation of learning materials is a common way for reducing extraneous cognitive load. How instructors modify teaching models, tools, and presentation methods is a major concern of research in reducing extraneous cognitive load. For teaching aids with less interaction, the intrinsic cognitive load is low, and there is room for the learner’s working memory. For the case of high intrinsic cognitive load, the effectiveness of instructional design is extremely important because the capacity of working memory is limited.
Germane cognitive load refers to the use of effective instructional design and strategies to enable learners to focus more on cognitive processes and knowledge construction for reducing cognitive load. The function of germane cognitive load is to promote learning rather than to interfere with learning, but only when the total cognitive load does not exceed the learner’s memory capacity. The introduction of germane cognitive load can effectively improve learning, so it is called the effective cognitive load. Extraneous cognitive load actually includes germane cognitive load, which is not negatively related to learning and can actually be beneficial to learning.
Although both germane and extraneous cognitive loads consume memory resources in learning, they are different in their causes of learning effectiveness. The former helps the construction of schema and improves the learner’s ability in processing information, while the latter can help the learner improve their learning performance through an effective instructional design. As a result, reducing cognitive load via instructional technologies is the most common method used by teachers in addition to rearranging courses and teaching materials. In this study, AR technology is incorporated in instructional design to reduce the cognitive load due to insufficient interaction in traditional teaching.
This study adopts the POE strategy and the AR technology to reduce the generation of cognitive load for improving learning effectiveness, including: (1) classifying teaching materials according to difficulty. In this way, the teaching process can be fine-tuned according to students’ learning statuses. (2) Familiarizing students with AR operation and learning objectives. (3) Providing immediate assistance when students have problems to avoid additional extraneous cognitive load. (4) Enabling students to review the basic concepts when using the AR system to assist schema construction.
AR is an emerging technology currently gaining more influence in education. Together with mobile technology, AR-based materials are used to improve learning. In this sense, augmented reality allows students to better understand certain content with a high visuospatial load by developing and improving their spatial intelligence, as shown in various studies [
38,
39,
40,
41,
42,
43]. As a result, the extrinsic, intrinsic and related cognitive load of certain content is reduced by the application of AR in education.
3. Research Method
In this study, a teaching experiment was conducted to investigate the performance of the AR system on learning high school chemistry. A total of 100 eighth graders were recruited as research samples from a junior high school in Hsinchu, Taiwan, containing 50 students (23 males and 27 females) in the experimental group (using the AR system) and 50 students (28 males and 22 females) in the control group (using the traditional teaching method). The quasi-experimental design was adopted for comparing the differences in learning achievement, learning motivation, and cognitive load between the two groups. A questionnaire survey was also conducted to explore the technology acceptance of using the AR system for learning chemistry. The following describes the research variables, research process, research tools, and the AR system design.
3.1. Research Variables
The learning content of this study was based on the eighth-grade textbook of natural science and technology for high school students, and it was used for designing the AR system to assist students in learning material structure and chemical equilibrium. The impact on students’ learning effectiveness, learning motivation, and cognitive load are explored via analyses of achievement test scores and questionnaire results. The research variables in this study are described in the following (
Figure 3):
The teaching method is the independent variable. The control group used the traditional teaching method (teaching materials and real experiments), and the experimental group used the AR system as an assistant tool for learning chemistry.
The difference in learning effectiveness between the two groups was explored through the analysis of covariance on the achievement test, in which the pre-test score is the covariate and the post-test score is the dependent variable. Learning motivation and cognitive load are compared through the analysis of variance according to questionnaire results.
The teacher, teaching time, teaching strategy, and learning content are the controlled variables and remained the same to reduce interference.
3.2. Research Process
In this study, the research process started with collecting data on the research topic, conducting literature review, and determining research objectives. In order to achieve the research objectives, the AR system was developed based on the learning requirement, and the achievement test and questionnaires were designed to evaluate its performance. Empirical research was conducted, and the results are analyzed in the next section to investigate the learning outcomes and responses of students. Finally, the researchers summarize the findings and provide suggestions for future works.
The learning content in this study includes material structures and chemical equilibrium in high school chemistry.
Figure 4 shows the flowchart of the teaching experiment. The teacher first introduced the learning topics, and then the students took the pre-test on the learning content. After that, both groups conducted learning activities in which the control group utilized the traditional teaching method and the experimental group applied the AR system for learning. Finally, two groups took the post-test and performed the questionnaire survey on cognitive load and learning motivation. In addition, the experimental group had to conduct the questionnaire survey on technology acceptance.
3.3. Research Tools
The main research tools in this study include the achievement test and questionnaires on learning motivation, cognitive load, and technology acceptance, which were designed based on research objectives to evaluate the performance of the AR system. Reliability analysis was performed on questionnaire results to guarantee their validity. The design of research tools is described as follows.
It was composed of ten multiple-choice questions, including conceptual and computational test items, to measure students’ learning achievement before and after the treatment for determining the difference in learning effectiveness between the two groups.
The questionnaire adopted the ARCS model proposed by Keller [
17] to explore the learning motivation of students. It was designed according to the four elements of ARCS (attention, relevance, confidence and satisfaction), and each element contained three questions. The questionnaire used a Likert five-point scale, and the reverted question was scored with opposite weighting. The reliability of questionnaire was measured as Cronbach’s α = 0.842 > 0.8, higher than the acceptable level.
In this study, the subjective method was used for measuring the cognitive load of students after conducting the learning activities. The six dimensions of cognitive load in the NASA-TLS [
44], i.e., psychological needs, physiological needs, time, performance, effort, and setbacks, were modified for designing the questionnaire. Students could quantify the mental load in the cognitive process by reflecting their attitudes according to the questionnaire items. The reliability of questionnaire was measured as Cronbach’s α = 0.900 > 0.8, higher than the acceptable level.
A questionnaire survey was conducted in this study to explore the technology acceptance of using the AR system for learning chemistry. The technology acceptance model proposed by Venkatesh and Bala [
45] was revised for designing the questionnaire to explore students’ technology acceptance on the AR system, including three dimensions—namely, behavioral intention, perceived usefulness, and perceived ease of use. The questionnaire adopted a seven-point Likert scale, and it was revised according to the comments of two chemistry teachers to guarantee its validity. The reliability of questionnaire was measured as Cronbach’s α = 0.944 > 0.8, higher than the acceptable level.
3.4. AR System Design
The AR system was developed using Unity and Vuforia engines, and the interactive user interface was designed with the C# programming language and the relative APIs. The animation of chemical reactions was created using Cinema 4D, and the AR cards (
Figure 5) were designed by Illustrator, a widely used graphics editor. The learning content of the AR system is divided into two parts, i.e., learning mode and testing mode (
Figure 6). The former can be used to study the learning content of chemical reactions, and the latter can present the animation of chemical reactions according to the operation of AR cards. The AR system can provide a direct and convenient learning experience to increase the flexibility for learning important concepts in chemical reactions. The learning content and design process of the AR system are shown in
Figure 7.
In this study, Cinema4D (
Figure 8) is used for creating the 3D models of material structures and the animation of chemical reactions. Cinema 4D is popular 3D modeling, animation, simulation, and rendering software. The colors of molecular models used in this study are matched with the CPK international atomic color map by Corey and Pauling [
46], allowing learners to identify the atoms of different elements more easily. The material structures were created by following the models on MOLVIEW [
47]. The main steps for animating a chemical reaction include presenting products, separating chemical bonds, rearranging elements, creating new chemical bonds, and displaying macroscopic views—e.g., solid precipitation and gas evolution. It is worth noticing that most deformation effects such as particle effects, environment rendering, distortion, rotation, and expansion created in Cinema4D cannot be transformed in the FBX format, so the animation of chemical reactions in the AR system was developed in Unity using C# coding.
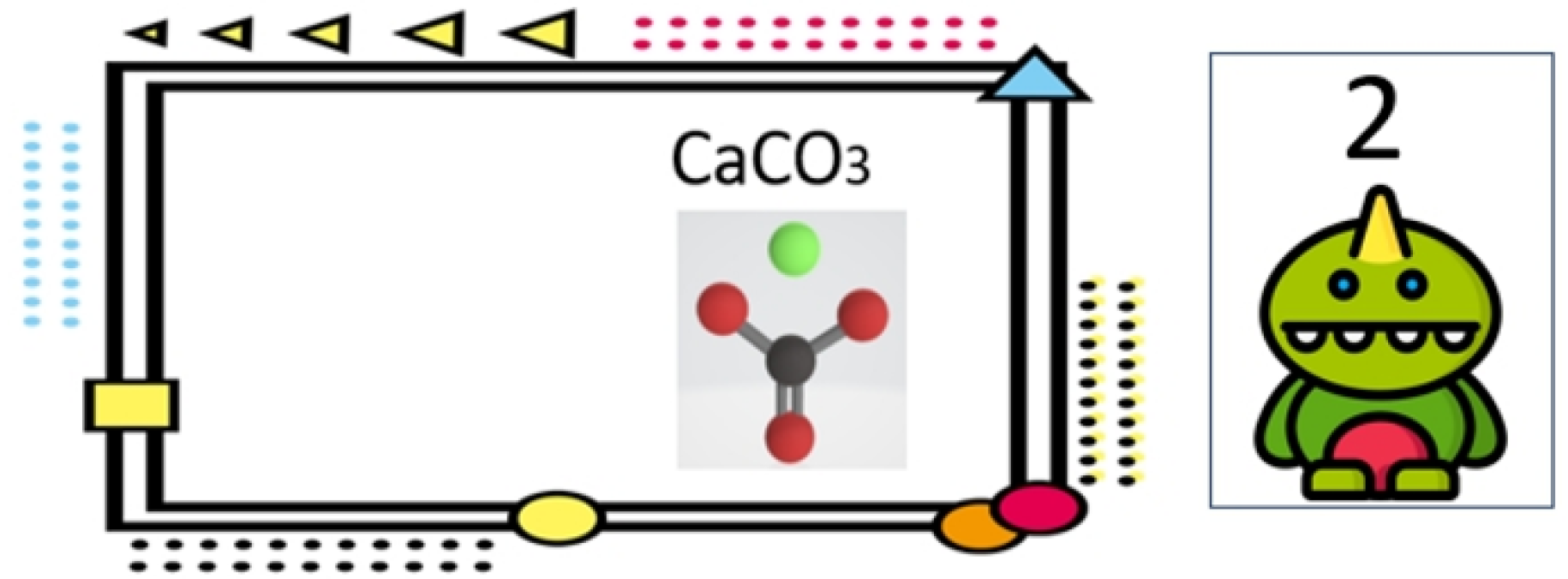
As mentioned before, the material cards and coefficient cards are identified individually to determine the reactants and their amounts in a chemical reaction. Therefore, it is necessary to calculate the distance between material cards when a coefficient card is identified to decide which material card it belongs to. Then, the coefficient can be written to the compute engine.
Figure 9 shows the identification of two material cards and two coefficient cards for performing a chemical reaction.
The animation of decomposition and composition of molecules was designed by Cinema4D, and the effects such as bubbles, precipitation, and the change of solution color were developed by Unity. The left picture in
Figure 10 shows the result of the chemical reaction:
, a complete reaction without reactants left; the right picture shows the chemical reaction
with
as the remaining reactant. The user can see the bubble of carbon dioxide during the reaction as well as the precipitation of calcium chloride.
There are several chemical reactions for learners to select and conduct the virtual experiment. After selecting a chemical reaction, the corresponding material cards and coefficient cards must be scanned in sequence. If a material card does not match the chemical reaction, the coefficient cards cannot be scanned to start the virtual experiment. When the material cards are all correctly selected, the user can choose the most suitable coefficients cards to achieve the simplest integer ratio among the reactants and products for the chemical reaction based on his or her own prediction.
The AR system is composed of “learning mode” and “testing mode”. The former displays the information and models of materials, and the latter provides the interactive user interface for virtual experiments. When the user enters the AR system, the main menu shows the two modes for selection according to the learning objective. After entering “learning mode”, the user can study the learning content through the built-in menu and scan the AR cards to display the 3D structures and properties of materials. After learning the basic knowledge, the user can return to the main menu and enter “testing mode” to conduct the virtual experiments. The user can select the AR cards to undergo a chemical reaction, and the step corresponds to the prediction in the POE strategy.
After scanning the AR cards, the user can click on the experiment button to write the data into the compute engine, and then trigger the animation for displaying the submicroscopic presentation of the chemical reaction according to the reactants and their coefficients. After observing the animation, the user can propose a better combination of coefficients to repeat the experiment until the simplest integer ratio has been achieved, which can be used as the explanation step of the POE strategy. After that, the user can select another chemical reaction to perform the virtual experiment again (
Figure 11).
3.5. Data Analysis
In this study, the pre-test and post-test scores—as well as the questionnaire results of learning motivation, cognitive load, and technology acceptance after the treatment—are analyzed by statistical methods, which include descriptive statistics, analysis of variance (ANOVA), analysis of covariance (ANCOVA), dependent sample t-test, and independent sample t-test as described below:
Descriptive statistics are mainly used to examine the mean, standard deviation and quantity of important variables in the population under observation, which can provide the most intuitive and summarized characteristics of a data set.
The analysis of variance is mainly used for the significance analysis of questionnaire results, including the difference in learning motivation and cognitive load between the two groups and technology acceptance by different genders.
In this study, the pre-test score is used as the covariate to examine whether a significant difference exists in the post-test scores of the two groups.
It is utilized to analyze whether there is a significant difference in the test scores for students before and after the treatment.
It is utilized to analyze the significance of questionnaire results by evaluating the difference of students’ responses between the two groups.
5. Practical Implications
Compared with virtual reality, augmented reality is more often used as a teaching tool because of its high portability, easy operation, and lower time or space constraints. The main objectives of applying AR in education are improving learning outcomes as well as learning interest and motivation. It provides more diverse teaching methods, which means that students have more learning paths to choose from. This study integrated the abstract concepts of chemical reactions and the mathematical calculation in chemical equations into the AR system, and investigated if students could enhance the cognition and construction of abstract concepts, for example, the law of conservation of mass, through AR operation and observation. The research results show that the AR system was effective in improving the learning effectiveness, especially for the low-achieving students.
Whether students are willing to learn has a great impact on their performance, so it is an important issue to stimulate their motivation through timely guidance and suitable teaching strategies. According to the research results, the learning motivation of the two groups has not reached a significant level, but the average score of the experimental group is slightly higher than that of the control group. The independent sample t-test results reveal no significant difference in most questions except for the question “I need good luck to receive a good grade in this course” (p = 0.008 < 0.01), indicating the AR system is effective for increasing students’ confidence in learning chemistry.
Using the right teaching tools or strategies can reduce the cognitive load and enhance the learning performance. According to the research results, the cognitive load of the two groups has not reached a significant level, but the average score of the experimental group is slightly higher than that of the control group. The independent sample t-test results reveal no significant difference in most questions except for the question “I have a sense of achievement after the learning process” (p = 0.049 < 0.05), indicating the AR system is helpful for reducing the cognitive load by improving the learning performance.
Compared with traditional teaching tools such as simulation software and e-books, AR is an emerging technology based on position and/or image recognition. This study used a questionnaire which included the dimensions of behavioral intention, perceived usefulness, and perceived ease of use to evaluate the technology acceptance of students on the AR system. According to the research results, the scores of all questions are higher than 5 (average = 5.40), and the dimensional average scores are 5.59, 5.41, and 5.22, respectively. According to the questionnaire results, it is suggested that a clearer and easier user interface should be provided to improve students’ acceptance of the AR system and enhance the incentives of its application. A further investigation reveals no significant difference in technology acceptance between male and female students or between high- and low-achievement students. It can be inferred that the AR system is suitable for most students regardless of their genders or learning achievement.
AR and VR technologies can be used to design teaching tools in science education, and suitable instructional design can be adopted for different learning units by taking the application environments into consideration. For example, VR can be applied in dangerous experiments, and AR is selected when it is necessary to observe invisible phenomena to concretize abstract concepts. In this study, the goal of the AR system is to assist high school students in learning chemistry. Students can use AR cards to conduct virtual chemistry experiments and obtain the simplest integer ratio in a chemical equation. This is helpful for understanding the concept that a chemical reaction changes the composition of reactants and the process obeys the law of conservation of mass.
The AR system can be installed on mobile phones or tablet computers, so it is highly portable, and its application is not limited by time or space. There are two modes for selection on the AR system according to the learning objective. The learning mode mainly presents the material models on the AR cards through markerless operation and it provides the functions of dragging and rotation to increase interactivity. The testing mode displays the composition of materials and animation of a reaction process according to the reactants and their coefficients for students to observe the experimental results. The diversified learning paths allow learners to understand the changes in molecular structures during a chemical reaction. This is helpful for establishing the connection between the macroscopic and submicroscopic knowledge and the cognition of basic concepts.
6. Conclusions and Future Works
Material structures and chemical equilibrium are important learning units in high school chemistry, but students often have difficulty understanding the abstract concepts of chemical reactions due to the lack of submicroscopic view and complex mathematical calculation in symbolic representation. AR can improve the learning experience of students by increasing their attention and interaction. The abstract concepts can be presented in real time through AR interface to provide a learning experience different from traditional teaching methods, and the 3D modeling and animation allow learners to understand relevant chemical concepts from active exploration.
In this study, an AR system was developed as an assistant tool in learning material structures and chemical equilibrium for high school students. They can use the AR system to conduct virtual experiments, in which the submicroscopic view of a chemical reaction will be displayed according to the chemical equation specified by the AR cards. Students can change the reactants and their coefficients for observing the experimental results to obtain the simplest integer ratio in a chemical equation, which is helpful for understanding the conservation of mass after a chemical reaction.
6.1. Concluding Remarks
A teaching experiment was conducted to investigate students’ learning effectiveness, learning motivation, cognitive load, and technology acceptance after using the AR system for learning chemistry, and the analytical results are listed as follows:
The AR system was more effective than the traditional teaching method, especially for the low-achievement students.
The learning motivation of using the AR system was slightly higher than that of the traditional teaching method (no significant difference). A further exploration revealed that the AR system was helpful for increasing students’ confidence in learning.
The cognitive load of using the AR system was slightly lower than that of the traditional teaching method (no significant difference). A further exploration showed that the AR system improved the sense of achievement after learning.
The AR system was acceptable for high school students regardless of their gender or learning achievement, with the overall acceptance equal to 5.4 out of 7.
According to the observation during the learning process, the experimental group had higher interaction and more feedback than the control group because the AR system provided learners with opportunities to explore knowledge actively rather than receive knowledge passively. As a result, they were more engaged in learning situations through AR operation to enhance their learning motivation. The application of augmented reality is not limited by space or time, and it provides a convenient user interface for autonomous exploration, which is helpful for conceptual change and cognition. Students can download the AR system for learning chemistry and practice after school, so it is a useful tool for learning the abstract concepts of chemical reactions.
6.2. Limitations and Future Works
In this study, some detailed information about chemical reactions were not included to avoid excessive cognitive load for high school students. The submicroscopic animation was concise for easy observation, mainly focusing on the concepts of atomic rearrangement and the law of conservation of mass. The teaching time and the sample size were small, so it can not represent the performance of all high school students, nor can it be deduced to the effects of other learning units. Based on the design and findings of this research, three directions for the future works are suggested:
According to the questionnaire results on technology acceptance, there is room for improvement in the operation and interface design for the AR system. During the experiment, it was found that students spent some time becoming familiar with the operational process. Basically, the recognition rate of AR cards depends on the light source, image resolution, and lens quality. Therefore, it is important to control the light source to ensure successful recognition of multiple AR cards at the same time when performing the virtual experiment. The animation of chemical reactions is presented according to the coefficients of reactants and products, which are calculated by the compute engine. Currently, each chemical equation has its own compute engine and thus requires more computation time and memory space to present the animation. In the future, the performance of the AR system can be improved by using an integrated compute engine.
In this study, the AR system covered only the learning units of “material structures” and “chemical equilibrium” in high school chemistry. The teaching time and the sample size were small for the empirical research. Therefore, it is suggested that the teaching time be extended and that the sample size be increased to obtain more accurate results. There are some learning units with abstract concepts and difficult theoretical knowledge, e.g., “periodic law of elements” and “oxidation-reduction reaction”. It is suggested to include more learning units in the AR system to help students in learning chemistry.
In this study, the control group used the traditional teaching method, and the experimental group used the AR system for learning chemistry, both combining with the POE strategy. In the future, the strategy of flipped classroom can be adopted to increase students’ engagement by having them complete the assigned tasks at home and discuss the results during class time. In addition, students can also conduct cooperative learning through peer discussion, and summarize the exploration results to share with their classmates. The role of teachers is to provide timely explanation and correction when students have questions or misconceptions. The cooperative learning can also be conducted in the form of competition between groups to inspire knowledge exploration, for which the AR system can be used as a tool for verifying experimental results.