Phycoremediation as a Strategy for the Recovery of Marsh and Wetland with Potential in Colombia
Abstract
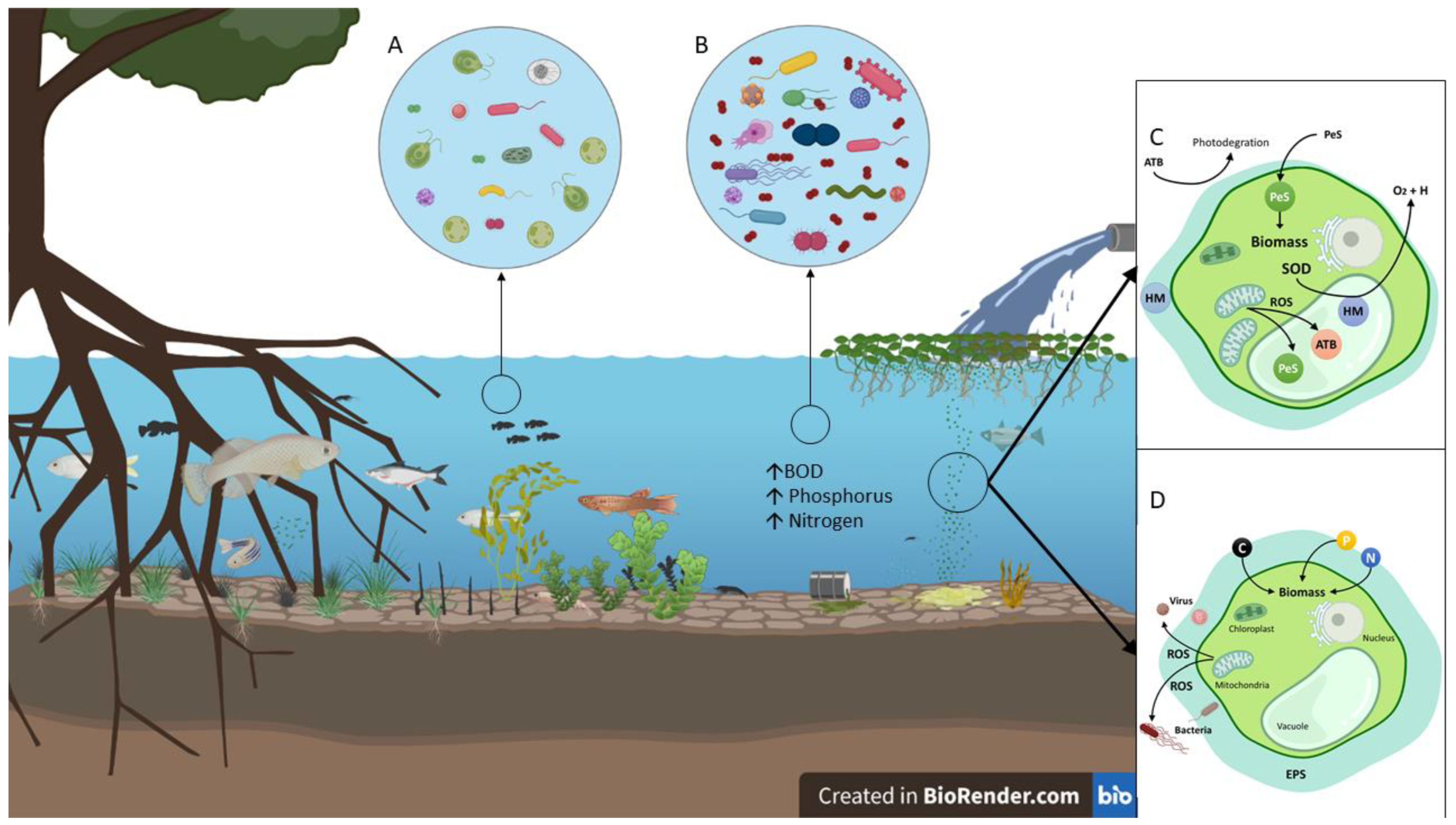
:1. Introduction
2. Phycoremediation
2.1. Phycoremediation In Situ
2.2. Microbial Consortia
2.3. Microalgal Biomass
3. Biodiversity
4. Chemical Biodegradation through Microalgae
4.1. Phycoremediation of Heavy Metals
| Location | Pollution Sources | Type of Assessment | Conclusions | Reference |
|---|---|---|---|---|
| Grande de Santa Marta Marsh-Magdalena | Domestic waste Farming Manufacturing industry | Phosphorus Nitrogen Heat-resistant coliforms Total suspended solids | Eutrophication and microbiological/fecal contamination | [5] |
| Metal (Pb, Cd, and Zn) determination in sediments | High heavy metal levels | [77] | ||
| Determination of organochlorine pesticides (lindane, heptachlor, aldrin, DDE, DDD, and DDT) in sediments | Contamination with agrochemicals | [78] | ||
| Characterization of cyanobacteria and their toxins | Eutrophication and contamination with phycotoxins | [12,13,14,79] | ||
| Mir River Estuary-Pacific Region | Trans-Andean pipeline | Determination of Petroleum Hydrocarbons equivalent of Chrysene (HP) and Polycyclic Aromatic Hydrocarbons (PAH) | contamination with agrochemicals | [80] |
| La Mojana Region (San Antonio Marsh, Machado Marsh, and San Marcos Marsh)-Sucre | Gold mining | Determination of Mercury in sediments, fish, hair, rice crops, and macrophytes | High heavy metal levels | [81] |
| Ayapel Marsh- Córdoba | Municipal and industrial wastewater. | Nitrogen Phosphorus Solids (total, suspended, sedimentable, and dissolved) Total coliforms | Eutrophication and microbiological/fecal contamination | [82] |
| Determination of mercury in fish and marsh inhabitants | High heavy metal levels | [83] | ||
| Not specified | Mercury contamination | [84] | ||
| Cyanobacteria characterization | Eutrophication, high levels of toxic cyanobacteria | [85] | ||
| Mata de Palma Marsh-Cesar | Municipal and industrial wastewater Farming Cattle farming Open-pit mining | Tubificidae and Planorbidae counts- Indicators of highly contaminated waters from organic matter, polysaccharide waters, and hypoxia | eutrophication | [86] |
| Fecal coliforms Total coliforms | Microbiological/fecal contamination | [87] | ||
| La Virgen Marsh-Bolívar | Farming | Organochlorine pesticides DT, DDE, and DDD Heptachlor and methoxychlor | Contamination with agrochemicals | [88] |
| Municipal and industrial wastewater | Total suspended solids Ammonium Phosphorus Fecal coliforms Total coliforms | Eutrophication and microbiological/fecal contamination | [89] | |
| Mallorquín Marsh-Atlantic Ocean | Municipal and industrial wastewater Farming | Metal (Hg, Cd, Cu, Pb, Cr, Ni, and Zn) determination in sediments | High levels of heavy metals | [90] |
| Metal (Hg, Cr, Cd, and Ni) determination in sediments | [91] | |||
| Metal (Zn, Cu, Pb, Cd, and Hg) determination in water | [92] | |||
| Miramar Marsh-Santander | Municipal and industrial wastewater Farming | Total suspended solids Fats and oils Metal determination (Al, Ba, Hg, Pb, and Cd) in water Total coliforms and fecal coliforms | Heavy metal contamination, eutrophication, and microbiological/fecal contamination | [93] |
| Opón and Miramar Marshes-Santander | Municipal and industrial wastewater | Phytoplankton and zooplankton | Eutrophication | [94] |
| Soledad Marsh-Córdoba | Farming Cattle farming Gold mining | Metal (Fe, Mn, Ni, Cr, Al, Hg, Pb, Cd, Cu, and Zn) determination in water, fish, and sediments | Heavy metal contamination | [95] |
| La Quinta Marsh- Bolívar | Municipal and industrial wastewater | Total coliforms and fecal coliforms Metal (Hg, Cr, and Cd) determination in sediments | Heavy metal contamination, eutrophication, and microbiological/fecal contamination | [77] |
| Puerto Caimán Marsh-Atlantic | Municipal and industrial wastewater Cattle farming | Phytoplankton | Eutrophication | [96] |
| Grande del Bajo Sinú Marsh-Córdoba | Farming Cattle farming | Organochlorine compounds in fish | Organochlorine contamination | [97] |
| Cyanobacteria evaluation and characterization | Eutrophication, high levels of toxic cyanobacteria | [98] |
4.2. Pesticide Phycoremediation
4.3. Emergent Contaminant Phycoremediation
4.4. Antibiotic Phycoremediation
4.5. Polycyclic Aromatic Hydrocarbons Degradation
5. Biological Contaminant Phycoremediation
5.1. Bacteria Removal
5.2. Viral Inactivation
6. Microalgae in Colombia
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zabala, M.; Hernández Atilano, E. Ciénaga de Ayapel a Través de la Variación Temporal de los Aspectos morfo Funcionales del Fitoplancton y un Indicador de Calidad Ecológica; Universidad de Antioquia, Corporación Académica Ambiental: Medellín, Colombia, 2017. [Google Scholar]
- Schmidt-Mumm, U.; Janauer, G. Seasonal dynamics of the shoreline vegetation in the Zapatosa floodplain lake complex. Colombia. Rev. Biol. Trop. 2014, 62, 1073–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caballero Acosta, J.; Durango, L.C. Aproximaciones para la evaluación ambiental de los complejos cenagosos en el marco de la depresión Momposina. Gest. Ambiente 1998, 1, 27–37. [Google Scholar]
- Moreno, M.; Aguirre, R. State of the art of limnology and flood plain lakes (swamps) in Colombia. Rev. Gestión Ambiente 2009, 12, 85–105. [Google Scholar]
- Vivas, L.J.; Espinosa, L.F.; Parra Henríquez, L.G. Identificación de fuentes terrestres de contaminación y cálculo de las cargas de contaminantes en el área de influencia de la Ciénaga Grande de Santa Marta, caribe colombiano. Bull. Mar. Coast. Res. 2016, 42, 7–30. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [Green Version]
- Granado Lorencio, C.; Serna Hernández, A.; Carvajal, J.D.; Jiménez Segura, L.F.; Gulfo, A.; Alvarez, F. Regionally nested patterns of fish assemblages in floodplain lakes of the Magdalena river (Colombia). Ecol. Evol. 2012, 2, 1296–1303. [Google Scholar] [CrossRef]
- Lehtiniemi, M.; Engström-Öst, J.; Viitasalo, M. Turbidity decreases anti-predator behaviour in pike larvae, Esox lucius. Environ. Biol. Fishes. 2005, 73, 1–8. [Google Scholar] [CrossRef]
- Turner, A.M.; Chislock, M.F. Blinded by the stink: Nutrient enrichment impairs the perception of predation risk by freshwater snails. Ecol. Appl. 2010, 20, 2089–2095. [Google Scholar] [CrossRef]
- Salomón, S.; Rivera-Rondón, C.A.; Zapata, Á.M. Floraciones de cianobacterias en Colombia: Estado del conocimiento y necesidades de investigación ante el cambio global. Rev. Acad. Colomb. Cienc. Exactas Físicas Nat. 2020, 44, 376–391. [Google Scholar] [CrossRef]
- Chislock, M.F.; Doster, E.; Zitomer, R.A.; Wilson, A.E. Eutrophication: Causes, Consequences, and Controls in Aquatic Ecosystems. Nat. Educ. Knowl. 2013, 4, 10. [Google Scholar]
- Mancera, J.E.; Vidal, L.A. Florecimiento de microalgas relacionado con mortandad masiva de peces en el complejo lagunar Ciénaga Grande de Santa Marta, Caribe colombiano. Boletín Investig. Mar. Costeras-Invemar 1994, 23, 103–117. [Google Scholar] [CrossRef]
- Instituto de Investigaciones Marinas y Costeras (INVEMAR) Concepto Técnico Sobre la Mortandad de Peces en la Ciénaga Grande de Santa Marta (sector Tasajera) Magdalena-CPT-CAM-011-15. 2015. Available online: https://alfresco.invemar.org.co/share/s/ANKUYB-PSxiP8UTHa_V8uw (accessed on 23 September 2021).
- Instituto de Investigaciones Marinas y Costeras (INVEMAR) Concepto Técnico Sobre la Mortandad de peces en la Ciénaga Grande de Santa Marta (sector Caño Grande-Pajarales), Magdalena-CPT-CAM-022-15. 2015. Available online: http://cinto.invemar.org.co/alfresco/d/d/workspace/SpacesStore/0d8b0b08-849c-43db-9c0d-3f7ec7c61676/CONCEPTO%20T%C3%89CNICO%20SOBRE%20MORTANDAD%20DE%20PECES%20EN%20LA%20CI%C3%89NAGA%20GRANDE%20DE%20SANTA%20MARTA%20(SECTOR%20CA%C3%91O%20GRANDE%20-%20PAJARALES),%20OCURRIDA%20EN%20NOVIEMBRE%20DE%202015?ticket=TICKET_92c6658f80f850fc6fa724431e054fa0fcf04812 (accessed on 23 September 2021).
- Soto Varela, Z.; Pérez Lavalle, L.; Estrada Alvarado, D. Bacteria causing of foodborne diseases: An overview at Colombia. Salud Uninorte 2016, 32, 105–122. [Google Scholar] [CrossRef]
- Liu, X.; He, X.; An, Z.; Sun, W.; Chen, N.; Gao, X.; Li, X.; Zhang, X. Citrobacter freundii infection in red swamp crayfish (Procambarus clarkii) and host immune-related gene expression profiles. Aquaculture 2020, 515, 734499. [Google Scholar] [CrossRef]
- Hossain, M.I.; Neela, F.A.; Hussain, M.A.; Rahman, M.H.; Suzuki, S. Distribution of Pseudomonas aeruginosa in Swamps and it’s Infection to Oreochromis niloticus. J. Bio-Sci. 1970, 14, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Kujawska, N.; Talbierz, S. Microalgae Cultivation Technologies as an Opportunity for Bioenergetic System Development—Advantages and Limitations. Sustainability 2020, 12, 9980. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Mehariya, S.; Bhatia, R.K.; Kumar, M.; Pugazhendhi, A.; Awasthi, M.K.; Atabani, A.E.; Kumar, G.; Kim, W.; Seo, S.-O.; et al. Wastewater based microalgal biorefinery for bioenergy production: Progress and challenges. Sci. Total Environ. 2021, 751, 141599. [Google Scholar] [CrossRef] [PubMed]
- Henkanatte-Gedera, S.M.; Selvaratnam, T.; Karbakhshravari, M.; Myint, M.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Removal of dissolved organic carbon and nutrients from urban wastewaters by Galdieria sulphuraria: Laboratory to field scale demonstration. Algal Res. 2017, 24, 450–456. [Google Scholar] [CrossRef]
- Panda, S.; Mishra, S.; Akcil, A.; Kucuker, M.A. Microalgal potential for nutrient-energy-wastewater nexus: Innovations, current trends and future directions. Energy Environ. 2021, 32, 604–634. [Google Scholar] [CrossRef]
- Salbitani, G.; Carfagna, S. Ammonium Utilization in Microalgae: A Sustainable Method for Wastewater Treatment. Sustainability 2021, 13, 956. [Google Scholar] [CrossRef]
- Di Cicco, M.R.; Iovinella, M.; Palmieri, M.; Lubritto, C.; Ciniglia, C. Extremophilic Microalgae Galdieria Gen. for Urban Wastewater Treatment: Current State, the Case of “POWER” System, and Future Prospects. Plants 2021, 10, 2343. [Google Scholar] [CrossRef]
- Oswald, W.J.; Gotaas, H.B.; Golueke, C.G.; Kellen, W.R.; Gloyna, E.F.; Hermann, E.R. Algae in Waste Treatment [with Discussion]. Sewage Ind. Wastes 1957, 29, 437–457. [Google Scholar]
- Barsanti, L.; Gualtieri, P. Is exploitation of microalgae economically and energetically sustainable? Algal Res. 2018, 31, 107–115. [Google Scholar] [CrossRef]
- González, I.; Ekelhof, A.; Herrero, N.; Siles, J.Á.; Podola, B.; Chica, A.F.; Ángeles Martín, M.; Melkonian, M.; Izquierdo, C.G.; Gómez, J.M. Wastewater nutrient recovery using twin-layer microalgae technology for biofertilizer production. Water Sci. Technol. 2020, 82, 1044–1061. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.-Q.; Fitts, J.P.; Ajo-Franklin, C.M.; Maes, S.; Alvarez-Cohen, L.; Hennebel, T. Recovery of critical metals using biometallurgy. Curr. Opin. Biotechnol. 2015, 33, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Ruan, J.; Zhu, X.; Qian, Y.; Hu, J. A new strain for recovering precious metals from waste printed circuit boards. Waste Manag. 2014, 34, 901–907. [Google Scholar] [CrossRef]
- Ju, X.; Igarashi, K.; Miyashita, S.; Mitsuhashi, H.; Inagaki, K.; Fujii, S.; Sawada, H.; Kuwabara, T.; Minoda, A. Effective and selective recovery of gold and palladium ions from metal wastewater using a sulfothermophilic red alga, Galdieria sulphuraria. Bioresour. Technol. 2016, 211, 759–764. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Su, Y. Revisiting carbon, nitrogen, and phosphorus metabolisms in microalgae for wastewater treatment. Sci. Total Environ. 2021, 762, 144590. [Google Scholar] [CrossRef]
- Hussain, F.; Shah, S.Z.; Ahmad, H.; Abubshait, S.A.; Abubshait, H.A.; Laref, A.; Manikandan, A.; Kusuma, H.S.; Iqbal, M. Microalgae an ecofriendly and sustainable wastewater treatment option: Biomass application in biofuel and bio-fertilizer production. A review. Renew. Sustain. Energy Rev. 2021, 137, 110603. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating micro-algae into wastewater treatment: A review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef]
- De Medeiros, V.P.B.; Pimentel, T.C.; Varandas, R.C.R.; dos Santos, S.A.; de Souza Pedrosa, G.T.; da Costa Sassi, C.F.; da Conceição, M.M.; Magnani, M. Exploiting the use of agro-industrial residues from fruit and vegetables as alternative microalgae culture medium. Food Res. Int. 2020, 137, 109722. [Google Scholar] [CrossRef] [PubMed]
- Hultberg, M.; Bodin, H.; Ardal, E.; Asp, H. Effect of microalgal treatments on pesticides in water. Environ. Technol. 2016, 37, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Sun, Y.; Zhou, Y.; Kumar, M.; Usman, M.; Li, J.; Shao, J.; Wang, L.; Tsang, D.C.W. Bioremediation of water containing pesticides by microalgae: Mechanisms, methods, and prospects for future research. Sci. Total Environ. 2020, 707, 136080. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Wei, L.; Xiong, Q.; Xu, S.; Li, W.; Lv, S.; Lu, Q.; Wan, L.; Wen, Z.; Zhou, W. Use of microalgae based technology for the removal of antibiotics from wastewater: A review. Chemosphere 2020, 238, 124680. [Google Scholar] [CrossRef]
- Ubando, A.T.; Africa, A.D.M.; Maniquiz-Redillas, M.C.; Culaba, A.B.; Chen, W.-H.; Chang, J.-S. Microalgal biosorption of heavy metals: A comprehensive bibliometric review. J. Hazard. Mater. 2021, 402, 123431. [Google Scholar] [CrossRef]
- Ahmad, N.; Mounsef, J.R.; Abou Tayeh, J.; Lteif, R. Bioremediation of Ni, Al and Pb by the living cells of a resistant strain of microalga. Water Sci. Technol. 2020, 82, 851–860. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Kumar, P.S.; Jaikumar, V.; Vardhan, K.H.; SundarRajan, P. Microalgae for biofuel production and removal of heavy metals: A review. Environ. Chem. Lett. 2020, 18, 1905–1923. [Google Scholar] [CrossRef]
- Kaloudas, D.; Pavlova, N.; Penchovsky, R. Phycoremediation of wastewater by microalgae: A review. Environ. Chem. Lett. 2021, 19, 2905–2920. [Google Scholar] [CrossRef]
- Bhatt, A.; Arora, P.; Kumar, S. Occurrence, fates and potential treatment approaches for removal of viruses from wastewater: A review with emphasis on SARS-CoV-2. J. Environ. Chem. Eng. 2020, 8, 104429. [Google Scholar] [CrossRef]
- Ayele, A.; Getachew, D.; Kamaraj, M.; Suresh, A. Phycoremediation of Synthetic Dyes: An Effective and Eco-Friendly Algal Technology for the Dye Abatement. J. Chem. 2021, 2021, 9923643. [Google Scholar] [CrossRef]
- Dasan, Y.K.; Lam, M.K.; Yusup, S.; Lim, J.W.; Lee, K.T. Life cycle evaluation of microalgae biofuels production: Effect of cultivation system on energy, carbon emission and cost balance analysis. Sci. Total Environ. 2019, 688, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Phang, S.-M.; Chu, W.-L.; Rabiei, R. Phycoremediation. In The Algae World; Dinabandhu, S., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 357–389. [Google Scholar]
- Mustafa, S.; Bhatti, H.N.; Maqbool, M.; Iqbal, M. Microalgae biosorption, bioaccumulation and biodegradation efficiency for the remediation of wastewater and carbon dioxide mitigation: Prospects, challenges and opportunities. J. Water Process Eng. 2021, 41, 102009. [Google Scholar] [CrossRef]
- Coimbra, R.; Escapa, C.; Vázquez, N.; Noriega-Hevia, G.; Otero, M. Utilization of Non-Living Microalgae Biomass from Two Different Strains for the Adsorptive Removal of Diclofenac from Water. Water 2018, 10, 1401. [Google Scholar] [CrossRef] [Green Version]
- Al-Homaidan, A.A.; Al-Qahtani, H.S.; Al-Ghanayem, A.A.; Ameen, F.; Ibraheem, I.B.M. Potential use of green algae as a biosorbent for hexavalent chromium removal from aqueous solutions. Saudi J. Biol. Sci. 2018, 25, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Sun, W.; Wang, Y. Biosorption of Direct Fast Scarlet 4BS from aqueous solution using the green-tide-causing marine algae Enteromorpha prolifera. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 223, 117347. [Google Scholar] [CrossRef]
- Ali, H. Biodegradation of Synthetic Dyes—A Review. Water Air Soil Pollut. 2010, 213, 251–273. [Google Scholar] [CrossRef]
- Katagi, T. Bioconcentration, Bioaccumulation, and Metabolism of Pesticides in Aquatic Organisms. Rev. Environ. Contam. Toxicol. 2010, 204, 1–132. [Google Scholar] [CrossRef]
- Matamoros, V.; Uggetti, E.; García, J.; Bayona, J.M. Assessment of the mechanisms involved in the removal of emerging contaminants by microalgae from wastewater: A laboratory scale study. J. Hazard. Mater. 2016, 301, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Narala, R.R.; Garg, S.; Sharma, K.K.; Thomas-Hall, S.R.; Deme, M.; Li, Y.; Schenk, P.M. Comparison of Microalgae Cultivation in Photobioreactor, Open Raceway Pond, and a Two-Stage Hybrid System. Front. Energy Res. 2016, 4, 29. [Google Scholar] [CrossRef]
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation techniques–classification based on site of application: Principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016, 32, 180. [Google Scholar] [CrossRef] [Green Version]
- United States Environmental Protection Agency (USEPA). How to Evaluate Alternative Cleanup Technologies for Underground Storage Tank Sites. 2017. Available online: https://www.epa.gov/sites/default/files/2014-03/documents/tum_ch8.pdf (accessed on 18 August 2021).
- Huang, H.; Ye, L. Biological technologies for cHRPs and risk control. In High-Risk Pollutants in Wastewater; Hongqiang, R., Xuxiang, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 209–236. [Google Scholar]
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.; Shi, W.; Cui, F.; Lens, P.N.L.; Tay, J.H. Microalgal-bacterial consortia: From interspecies interactions to biotechnological applications. Renew. Sustain. Energy Rev. 2020, 118, 109563. [Google Scholar] [CrossRef]
- Fuentes, J.; Garbayo, I.; Cuaresma, M.; Montero, Z.; González-del-Valle, M.; Vílchez, C. Impact of Microalgae-Bacteria Interactions on the Production of Algal Biomass and Associated Compounds. Mar. Drugs 2016, 14, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef] [PubMed]
- Buchan, A.; LeCleir, G.R.; Gulvik, C.A.; González, J.M. Master recyclers: Features and functions of bacteria associated with phytoplankton blooms. Nat. Rev. Microbiol. 2014, 12, 686–698. [Google Scholar] [CrossRef]
- Ramanan, R.; Kang, Z.; Kim, B.-H.; Cho, D.-H.; Jin, L.; Oh, H.-M.; Kim, H.-S. Phycosphere bacterial diversity in green algae reveals an apparent similarity across habitats. Algal Res. 2015, 8, 140–144. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.-H.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Algae–bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Hill, R.T.; Zheng, T.; Hu, X.; Wang, B. Effects of bacterial communities on biofuel-producing microalgae: Stimulation, inhibition and harvesting. Crit. Rev. Biotechnol. 2016, 36, 341–352. [Google Scholar] [CrossRef]
- Natrah, F.M.I.; Bossier, P.; Sorgeloos, P.; Yusoff, F.M.; Defoirdt, T. Significance of microalgal-bacterial interactions for aquaculture. Rev. Aquac. 2014, 6, 48–61. [Google Scholar] [CrossRef]
- Al-Jabri, H.; Das, P.; Khan, S.; Thaher, M.; AbdulQuadir, M. Treatment of Wastewaters by Microalgae and the Potential Applications of the Produced Biomass—A Review. Water 2020, 13, 27. [Google Scholar] [CrossRef]
- Shahid, A.; Malik, S.; Zhu, H.; Xu, J.; Nawaz, M.Z.; Nawaz, S.; Asraful Alam, M.; Mehmood, M.A. Cultivating microalgae in wastewater for biomass production, pollutant removal, and atmospheric carbon mitigation; a review. Sci. Total Environ. 2020, 704, 135303. [Google Scholar] [CrossRef]
- Ratha, S.K.; Prasanna, R. Bioprospecting microalgae as potential sources of “Green Energy”—challenges and perspectives (Review). Appl. Biochem. Microbiol. 2012, 48, 109–125. [Google Scholar] [CrossRef]
- Tato, T.; Beiras, R. The Use of the Marine Microalga Tisochrysis lutea (T-iso) in Standard Toxicity Tests; Comparative Sensitivity with Other Test Species. Front. Mar. Sci. 2019, 6, 488. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Naushad, M.; Iqbal, J.; Bathula, C.; Sharma, G. Production and harvesting of microalgae and an efficient operational approach to biofuel production for a sustainable environment. Fuel 2021, 122543. [Google Scholar] [CrossRef]
- Zhu, L.-D.; Li, Z.-H.; Guo, D.-B.; Huang, F.; Nugroho, Y.; Xia, K. Cultivation of Chlorella sp. with livestock waste compost for lipid production. Bioresour. Technol. 2017, 223, 296–300. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, J.C.; Borghetti, I.A.; Cartas, L.C.; Woiciechowski, A.L.; Soccol, V.T.; Soccol, C.R. Biorefinery integration of microalgae production into cassava processing industry: Potential and perspectives. Bioresour. Technol. 2018, 247, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Miazek, K.; Brozek-Pluska, B. Effect of PHRs and PCPs on Microalgal Growth, Metabolism and Microalgae-Based Bioremediation Processes: A Review. Int. J. Mol. Sci. 2019, 20, 2492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suresh, A.; Kumar, R.P.; Dhanasekar, D.; Thajuddin, N. Biodiversity of Microalgae in Western and Eastern Ghats, India. Pak. J. Biol. Sci. 2012, 15, 919–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, N.K.; Rai, A.K. Biodiversity and biogeography of microalgae: Progress and pitfalls. Environ. Rev. 2011, 19, 1–15. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.-S.S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Metting, F.B. Biodiversity and application of microalgae. J. Ind. Microbiol. Biotechnol. 1996, 17, 477–489. [Google Scholar] [CrossRef]
- Tirado, O.; Manjarrez, G.; Díaz, C. Caracterización ambiental de la Ciénaga de la Quinta localizada en Cartagena de Indias, Colombia, 2009–2010. Rev. UDCA Actual. Divulg. Científica 2011, 14, 2009–2010. [Google Scholar] [CrossRef]
- Espinosa, L.F.; Ramírez, G.; Campos, N.H. Análisis de residuos de organoclorados en los sedimentos de zonas de manglar en la Ciénaga Grande de Santa Marta y la Bahía de Chengue, Caribe colombiano. Bull. Mar. Coast. Res. 2016, 24, 79–94. [Google Scholar] [CrossRef]
- De la Hoz, M. Phytoplankton dynamics in the Ciénaga Grande de Santa Marta, Colombian Caribbean. Boletín Investig. Mar. Costeras-Invemar 2004, 33, 159–179. [Google Scholar]
- Garcés Ordóñez, O.; Espinosa Díaz, L.F. Contaminación por hidrocarburos en sedimentos de manglar del estuario del río Mira, Pacífico colombiano, afectados por derrames de petróleo crudo. Bull. Mar. Coast. Res. 2019, 48, 159–168. [Google Scholar] [CrossRef]
- Marrugo Negrete, J.; Pinedo-Hernández, J.; Paternina–Uribe, R.; Quiroz-Aguas, L.; Pacheco-Florez, S. Distribución espacial y evaluación de la contaminación ambiental por mercurio en la región de la Mojana, Colombia. Rev. MVZ Córdoba 2018, 23, 7062–7075. [Google Scholar] [CrossRef] [Green Version]
- Chalarca Rodríguez, D.; Mejía Ruiz, R.; Aguirre Ramírez, N. Approach to the determination of the impact of the wastewater unloads of the municipality of Ayapel, on the wetland waterquality. Rev. Fac. Ing. Univ. Antioq. 2007, 40, 41–58. [Google Scholar]
- Gracia, L.; Marrugo, J.; Alvis, E. Contaminación por mercurio en humanos y peces en el municipio de Ayapel, Córdoba, Colombia, 2009. Rev. Fac. Nac. Salud Pública 2010, 28, 118–124. [Google Scholar]
- Aponte Estupiñán, C. Ciénaga de Ayapel, afluente hídrico en aprietos. Rev. Científica Perspect. Intel. 2018, 8, 51–62. [Google Scholar]
- Jaramillo, L.; Aguirre, R. Spatio-temporal changes in plankton in the Ciénaga de Ayapel (Córdoba-Colombia), during the period of lower water leve. Caldasia 2012, 34, 213–226. [Google Scholar]
- Nuñez, J.C.; Fragoso Castilla, P.J. Uso de Macroinvertebrados Acuáticos como Bioindicadores de Contaminación del Agua de la Ciénaga Mata de Palma (Colombia). Inf. Tecnol. 2019, 30, 319–330. [Google Scholar] [CrossRef]
- Estupiñán Torres MSC, S.M.; Ávila de Navia MSC, S.L. Calidad sanitaria del agua de la Ciénaga Mata de Palma en el departamento del Cesar, Colombia. Nova 2009, 7, 85. [Google Scholar] [CrossRef]
- Castro, L.Á. Estudio de la contaminación por pesticidas, en ecosistemas costeros en el área de Cartagena, Ciénaga de la Virgen y zona agrícola adyacente (CIOH-IAEA). Boletín Científico CIOH 1998, 18, 15–22. [Google Scholar] [CrossRef]
- Maldonado, W.; Baldiris, I.; Díaz, J. Evaluación de la calidad del agua en la Ciénaga de la Virgen (Cartagena, Colombia). Rev. Científica Guillermo Ockham 2011, 9, 79–87. [Google Scholar]
- Fuentes Gandara, F.; Pinedo Hernández, J.; Marrugo Negrete, J. Metales pesados en especies ícticas de la ciénaga de Mallorquín, Colombia. Espacios 2018, 39, 19. [Google Scholar]
- Portz, L.; Manzolli, R.P.; de Andrade, C.F.F.; Villate Daza, D.A.; Bolivar Bandeira, D.A.; Alcántara-Carrió, J. Assessment of Heavy Metals Pollution (Hg, Cr, Cd, Ni) in the Sediments of Mallorquin Lagoon—Barranquilla, Colombia. J. Coast. Res. 2020, 95, 158. [Google Scholar] [CrossRef]
- Fuentes Gandara, F.; Pinedo Hernández, J.; Gutiérrez, E.; Marrugo Negrete, J.; Díez, S. Heavy metal pollution and toxicity assessment in Mallorquin swamp: A natural protected heritage in the Caribbean Sea, Colombia. Mar. Pollut. Bull. 2021, 167, 112271. [Google Scholar] [CrossRef]
- Amado, J. Evaluación del Estado Actual y Propuesta de Acciones de Recuperación de la Ciénaga Miramar en el Municipio de Barrancabermeja—Santander; Universidad Pontificia Bolivariana: Bucaramanga, Colombia, 2012. [Google Scholar]
- Tapia Larios, C.; Bertel Sevilla, A.; Lambraño, H.; Ricardo Guerra Hernández, M.; Olivero Verbel, J. Caracterización del plancton de las ciénagas del Opón y Miramar. In Perfil Ambiental de la Ciénaga Miramar, Barrancabermeja—Santander; Verbel, J.O., Ed.; Universidad de Cartagena: Cartagena, Colombia, 2015. [Google Scholar]
- Marrugo Negrete, J.L.; Paternina Uribe, R. Evaluación de la Contaminación por Metales Pesados en la Ciénaga la Soledad y Bahía de Cispatá, Cuenca del Bajo Sinú, Departamento de Córdoba; Facultad de Ciencias Básicas, Universidad de Córdoba: Montería, Colombia, 2011. [Google Scholar]
- Blanco-Muñoz, E.; De la Parra-Guerra, A.C.; García-Alzate, C.; Villarreal-Blanco, E. Análisis físico-químico y fitoplanctónico de la ciénaga Puerto Caimán, vertiente Caribe, Colombia. Intropica 2020, 15, 114–125. [Google Scholar] [CrossRef]
- Lans, C.E.; Díaz, P.B.; Paez, M.M. Compuestos organoclorados residuales en dos especies ícticas de la Ciénaga Grande del Bajo Sinú, Córdoba, Colombia. Rev. MVZ Cordoba 2011, 16, 2402–2409. [Google Scholar] [CrossRef] [Green Version]
- Mogollón, M.; Aycardi, M.P.; Galeano, J.; Villalobos, J.; Arango, C. Variación espacio-temporal de las cianoprocariotas del antiguo delta del río Sinú, Córdoba, Colombia. Intropica 2014, 9, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Jacinto, V.; García-Barrera, T.; Gómez-Ariza, J.L.; Garbayo-Nores, I.; Vílchez-Lobato, C. Elucidation of the defence mechanism in microalgae Chlorella sorokiniana under mercury exposure. Identification of Hg–phytochelatins. Chem. Biol. Interact. 2015, 238, 82–90. [Google Scholar] [CrossRef]
- Sivasubramanian, V.; Subramanian, V.V.; Muthukumaran, M. Bioremediation of chromesludge from an electroplating industry using the micro alga Desmococcus olivaceus—A pilot study. Algal Biomass 2010, 1, 104–128. [Google Scholar]
- Yen, H.W.; Chen, P.W.; Hsu, C.Y.; Lee, L. The use of autotrophic Chlorella vulgaris in chromium (VI) reduction under different reduction conditions. J. Taiwan Inst. Chem. Eng. 2017, 74, 1–6. [Google Scholar] [CrossRef]
- Sjahrul, M.; Arifin, D. Phytoremediation of Cd2+ by Marine Phytoplanktons, Tetracelmis chuii and Chaetoceros calcitrans. Int. J. Chem. 2012, 4, 69–74. [Google Scholar] [CrossRef]
- Ferreira, L.S.; Rodrigues, M.S.; de Carvalho, J.C.M.; Lodi, A.; Finocchio, E.; Perego, P.; Converti, A. Adsorption of Ni2+, Zn2+ and Pb2+ onto dry biomass of Arthrospira (Spirulina) platensis and Chlorella vulgaris. I. Single metal systems. Chem. Eng. J. 2011, 173, 326–333. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chang, H.W.; Kao, P.C.; Pan, J.L.; Chang, J.S. Biosorption of cadmium by CO 2-fixing microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 2012, 105, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Shanab, S.; Essa, A.; Shalaby, E. Bioremoval capacity of three heavy metals by some microalgae species (Egyptian isolates). Plant Signal. Behav. 2012, 7, 392–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Behl, R.K.; Narula, N. Effect of phosphate-solubilizing strains of Azotobacter Chroococcum on yield traits and their survival in the rhizosphere of wheat genotypes under field conditions. Acta Agron. Hung. 2001, 49, 141–149. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Monllor-Alcaraz, L.S.; Postigo, C.; Uggetti, E.; López de Alda, M.; Díez-Montero, R.; García, J. Microalgae-based bioremediation of water contaminated by pesticides in peri-urban agricultural areas. Environ. Pollut. 2020, 265, 114579. [Google Scholar] [CrossRef]
- Priyadarshani, I.; Sahu, D.; Rath, B. Microalgal bioremediation: Current practices and perspectives. J. Biochem. Tech. 2011, 3, 299–304. [Google Scholar]
- Ardal, E. Phycoremediation of Pesticides Using Microalgae; Faculty of Landscape Architecture, Horticulture and Crop Production Science, Swedish University of Agricultural Sciences: Alnarp, Sweden, 2014. [Google Scholar]
- Singhal, M.; Jadhav, S.V.; Sonone, S.S.; Sankhla, M.S. Microalgae Based Sustainable Bioremediation of Water Contaminated by Pesticides. Biointerface Res. Appl. Chem. 2021, 12, 149–169. [Google Scholar] [CrossRef]
- Rempel, A.; Gutkoski, J.P.; Nazari, M.T.; Biolchi, G.N.; Cavanhi, V.A.F.; Treichel, H.; Colla, L.M. Current advances in microalgae-based bioremediation and other technologies for emerging contaminants treatment. Sci. Total Environ. 2021, 772, 144918. [Google Scholar] [CrossRef]
- Avila, R.; Peris, A.; Eljarrat, E.; Vicent, T.; Blánquez, P. Biodegradation of hydrophobic pesticides by microalgae: Transformation products and impact on algae biochemical methane potential. Sci. Total Environ. 2021, 754, 142114. [Google Scholar] [CrossRef] [PubMed]
- Nanda, M.; Kumar, V.; Fatima, N.; Pruthi, V.; Verma, M.; Chauhan, P.K.; Vlaskin, M.S.; Grigorenko, A.V. Detoxification mechanism of organophosphorus pesticide via carboxylestrase pathway that triggers de novo TAG biosynthesis in oleaginous microalgae. Aquat. Toxicol. 2019, 209, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Nicodemus, T.J.; DiRusso, C.C.; Wilson, M.; Black, P.N. Reactive Oxygen Species (ROS) mediated degradation of organophosphate pesticides by the green microalgae Coccomyxa subellipsoidea. Bioresour. Technol. Rep. 2020, 11, 100461. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Ralph, P.J. Microalgal bioremediation of emerging contaminants—Opportunities and challenges. Water Res. 2019, 164, 114921. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Hu, L.X.; Liu, Y.S.; Zhao, J.L.; He, L.Y.; Ying, G.G. Microalgae-based technology for antibiotics removal: From mechanisms to application of innovational hybrid systems. Environ. Int. 2021, 155, 106594. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, Y.; Wang, Z.; Torres, O.L.; Guo, R.; Chen, J. Investigation of the removal mechanism of antibiotic ceftazidime by green algae and subsequent microbic impact assessment. Sci. Rep. 2017, 7, 4168. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Ge, F.; Zhou, W.; Tao, N.; Liang, Z.; Zhu, L. Subcellular distribution of fluoranthene in Chlorella vulgaris with the presence of cetyltrimethylammonium chloride. Chemosphere 2013, 90, 929–935. [Google Scholar] [CrossRef]
- Echeveste, P.; Agustí, S.; Dachs, J. Cell size dependent toxicity thresholds of polycyclic aromatic hydrocarbons to natural and cultured phytoplankton populations. Environ. Pollut. 2010, 158, 299–307. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Sartoros, C.; Yerushalmi, L.; Béron, P.; Guiot, S.R. Effects of surfactant and temperature on biotransformation kinetics of anthracene and pyrene. Chemosphere 2005, 61, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Semple, K.T.; Cain, R.B.; Schmidt, S. Biodegradation of aromatic compounds by microalgae. FEMS Microbiol. Lett. 1999, 170, 291–300. [Google Scholar] [CrossRef]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [CrossRef] [Green Version]
- El-Sheekh, M.M.; Ghareib, M.; EL-Souod, G.A. Biodegradation of Phenolic and Polycyclic Aromatic Compounds by Some Algae and Cyanobacteria. J. Bioremediat. Biodegrad. 2012, 3. [Google Scholar] [CrossRef]
- Warshawsky, D.; Cody, T.; Radike, M.; Reilman, R.; Schumann, B.; LaDow, K.; Schneider, J. Biotransformation of benzo[a]pyrene and other polycyclic aromatic hydrocarbons and heterocyclic analogs by several green algae and other algal species under gold and white light. Chem. Biol. Interact. 1995, 97, 131–148. [Google Scholar] [CrossRef]
- Cerniglia, C.E.; Gibson, D.T.; Van Baalen, C. Naphthalene Metabolism by Diatoms Isolated from the Kachemak Bay Region of Alaska. Microbiology 1982, 128, 987–990. [Google Scholar] [CrossRef] [Green Version]
- Cerniglia, C.E.; Van Baalen, C.; Gibson, D.T. Metabolism of Naphthalene by the Cyanobacterium Oscillatoria sp., Strain JCM. Microbiology 1980, 116, 485–494. [Google Scholar] [CrossRef] [Green Version]
- Tomar, R.S.; Jajoo, A. Enzymatic pathway involved in the degradation of fluoranthene by microalgae Chlorella vulgaris. Ecotoxicology 2021, 30, 268–276. [Google Scholar] [CrossRef]
- Semple, K.T.; Cain, R.B. Biodegradation of phenols by the alga Ochromonas danica. Appl. Environ. Microbiol. 1996, 62, 1265–1273. [Google Scholar] [CrossRef] [Green Version]
- Al-Gheethi, A.A.; Mohamed, R.M.; Jais, N.M.; Efaq, A.N.; Halid, A.A.; Wurochekke, A.A.; Amir-Hashim, M.K. Influence of pathogenic bacterial activity on growth of Scenedesmus sp. and removal of nutrients from public market wastewater. J. Water Health 2017, 15, 741–756. [Google Scholar] [CrossRef] [Green Version]
- Molinuevo-Salces, B.; Riaño, B.; Hernández, D.; García-González, M.C. Microalgae and wastewater treatment: Advantages and disadvantages. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Springer: Singapore, 2019; pp. 505–533. ISBN 9789811322648. [Google Scholar]
- Chai, W.S.; Tan, W.G.; Halimatul Munawaroh, H.S.; Gupta, V.K.; Ho, S.H.; Show, P.L. Multifaceted roles of microalgae in the application of wastewater biotreatment: A review. Environ. Pollut. 2021, 269, 116236. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Ansari, F.A.; Shriwastav, A.; Sahoo, N.K.; Rawat, I.; Bux, F. Dual role of Chlorella sorokiniana and Scenedesmus obliquus for comprehensive wastewater treatment and biomass production for bio-fuels. J. Clean. Prod. 2016, 115, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Sebastian, S.; Nair, K.V.K. Total removal of coliforms and E. coli from domestic sewage by high-rate pond mass culture of Scenedesmus obliquus. Environ. Pollut. Ser. A Ecol. Biol. 1984, 34, 197–206. [Google Scholar] [CrossRef]
- Delanka-Pedige, H.M.K.; Munasinghe-Arachchige, S.P.; Cornelius, J.; Henkanatte-Gedera, S.M.; Tchinda, D.; Zhang, Y.; Nirmalakhandan, N. Pathogen reduction in an algal-based wastewater treatment system employing Galdieria sulphuraria. Algal Res. 2019, 39, 101423. [Google Scholar] [CrossRef]
- Verbyla, M.E.; Mihelcic, J.R. A review of virus removal in wastewater treatment pond systems. Water Res. 2015, 71, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Talyshinsky, M.M.; Souprun, Y.Y.; Huleihel, M.M. Anti-viral activity of red microalgal polysaccharides against retroviruses. Cancer Cell Int. 2002, 2, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kremer, B.P. Marine Algae in Pharmaceutical Science. Phycologia 1980, 19, 168–169. [Google Scholar] [CrossRef]
- Delanka-Pedige, H.M.K.; Munasinghe-Arachchige, S.P.; Zhang, Y.; Nirmalakhandan, N. Bacteria and virus reduction in secondary treatment: Potential for minimizing post disinfectant demand. Water Res. 2020, 177, 115802. [Google Scholar] [CrossRef] [PubMed]
- Delanka-Pedige, H.M.K.; Cheng, X.; Munasinghe-Arachchige, S.P.; Abeysiriwardana-Arachchige, I.S.A.; Xu, J.; Nirmalakhandan, N.; Zhang, Y. Metagenomic insights into virus removal performance of an algal-based wastewater treatment system utilizing Galdieria sulphuraria. Algal Res. 2020, 47, 101865. [Google Scholar] [CrossRef]
- Gustafsson, O.; Manukyan, L.; Gustafsson, S.; Tummala, G.K.; Zaman, S.; Begum, A.; Alfasane, M.A.; Siddique-e-Rabbani, K.; Mihranyan, A. Scalable and Sustainable Total Pathogen Removal Filter Paper for Point-of-Use Drinking Water Purification in Bangladesh. ACS Sustain. Chem. Eng. 2019, 7, 14373–14383. [Google Scholar] [CrossRef]
- Templeton, M.R.; Andrews, R.C.; Hofmann, R. Particle-Associated Viruses in Water: Impacts on Disinfection Processes. Crit. Rev. Environ. Sci. Technol. 2008, 38, 137–164. [Google Scholar] [CrossRef] [Green Version]
- Miki, T.; Jacquet, S. Complex interactions in the microbial world: Underexplored key links between viruses, bacteria and protozoan grazers in aquatic environments. Aquat. Microb. Ecol. 2008, 51, 195–208. [Google Scholar] [CrossRef]
- Flynn, K.J.; Kenny, P.; Mitra, A. Minimising losses to predation during microalgae cultivation. J. Appl. Phycol. 2017, 29, 1829–1840. [Google Scholar] [CrossRef] [Green Version]
- Vacca Jimeno, V.A.; Angulo Mercado, E.R.; Puentes Ballesteros, D.M.; Torres Yépez, J.G.; Plaza Vega, M.E. Uso de la micro-alga Chlorella sp. viva en suspensión en la decoloración del agua residual de una empresa textil/Using the microalgae Chlorella sp. live suspended in decoloration wastewater from a textile factory. Prospectiva 2017, 15, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Pérez Silva, K.R.; Vega Bolaños, A.M.; Hernández Rodríguez, L.C.; Parra Ospina, D.A.; Ballen Segura, M.Á. Uso de Scenedesmus para la remoción de metales pesados y nutrientes de aguas residuales para la industria textil. Ing Solidar. 2016, 12, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Nuñez, D.; Serrano, J.; Otalora, L.; Rerez, R. Microalgas presentes en Rio Negro entre los Municipios de Utica y Sasaima, Cundinamarca y su utilidad en medicina Microalgae. Línea Vida 2018, 2, 1–11. [Google Scholar] [CrossRef]
- Ardila, L.; Godoy, R.; Montenegro, L. Sorption Capacity Measurement of Chlorella vulgaris and Scenedesmus acutus to Re-move Chromium from Tannery Waste Water. IOP Conf. Ser. Earth Environ. Sci. 2017, 83, 012031. [Google Scholar] [CrossRef] [Green Version]
- Romero-Morales, M.A.; Ortiz-Villota, M.T.; Meza-Rodríguez, L.D. La biorremediación con microalgas (Spirulina máxima, Spirulina platensis y Chlorella vulgaris) como alternativa para tratar la eutrofización de la laguna de Ubaque, Colombia. Rev. Investig. Desarro. E Innovación 2018, 9, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Sandoval Herrera, J.A.; Malo Malo, B.O.; Cartagena Arévalo, J.C.; Rubio Fernández, D. Evaluación a nivel laboratorio de la capacidad de remoción de materia orgánica de Chlorella vulgaris en las aguas residuales de la PTAR Salitre. Rev. Mutis 2018, 8, 34–42. [Google Scholar] [CrossRef]
- Angulo, M.E.; Castellar, O.G.; Cely, B.M.M.; Ibáñez, S.L.; Prasca, M.L. Decoloración de aguas residuales de una industria de pinturas por la microalga Chlorella sp. Rev. MVZ Córdoba 2017, 5706–5717. [Google Scholar] [CrossRef] [Green Version]
- Gómez Santos, J.A.; Rodríguez González, L.G. Obtención de biomasa de microalgas en aguas residuales para la producción de biocombustibles. Renov. Rev. Estud. Interdiscip. Ciencias Soc. Tecnol. E Innovación 2019, 3, 21–36. [Google Scholar]
- Mora-Salguero, D.; Vives Florez, M.J.; Husserl Orjuela, J.; Fernández-Niño, M.; González Barrios, A.F. Evaluation of the phenol degradation capacity of microalgae-bacteria consortia from the bay of Cartagena, Colombia. TecnoLógicas 2019, 22, 149–158. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Bashan, Y. Bacterias promotoras de crecimiento de microalgas: Una nueva aproximación en el tratamiento de aguas residuales. Rev. Colomb. Biotecnol. 2003, 5, 85–90. [Google Scholar]
- Leani, K.; García, Q.; Patricia, D.; Zúñiga, R.; Elena, M.; Duque, G.; Amir, J.; Rojas, A. Artículo de Investigación Evaluación de la remoción de nitrógeno y materia orgánica a través de humedales artificiales de flujo subsuperficial, acoplados a reacto-res de lecho fijo con microalgas en la Institución Universitaria Colegio Mayor de Antioquia. Rev. Ing. Región 2021, 25, 82–94. [Google Scholar] [CrossRef]
- Torres, D.D.; Cáceres Sepúlveda, S.; Roa, A.L.; Suárez Gelvez, J.H.; Urbina Suárez, N.A. Utilización de microalgas de la divi-sión Chlorophyta en el tratamiento biológico de drenajes ácidos de minas de carbón. Rev. Colomb. Biotecnol. 2017, 19, 95–104. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M.; Morrison, L.; Rindi, F.; Miranda, S.V.; Mathieson, A.C.; Parker, B.C.; Langangen, A.; John, D.M.; Bárbara, I.; et al. AlgaeBase: An On-line Resource for Algae. Cryptogam. Algol. 2014, 35, 105–115. [Google Scholar] [CrossRef]
- Ortiz-Moreno, M.L.; Sandoval-Parra, K.X.; Solarte-murillo, L.V. Chlorella, um potencial biofertilizante? Orinoquia 2019, 23, 71–78. [Google Scholar] [CrossRef]
- Ramírez-Merlano, J.; Mira-López, T.; Cruz-Casallas, P. Effect of light intensity on reproductive efficiency of cladoceran Moina sp., under laboratory conditions. Orinoquia 2013, 17, 117–182. [Google Scholar] [CrossRef] [Green Version]
- Jaramillo-campaña, H.A.; Calpa, N.C.; Gómez-nieves, V.Y. Evaluación del periodo de llenado y evacuación intestinal de Brachionus calyciflorus alimentado con Chlorella sp. Orinoquia 2019, 23, 41–47. [Google Scholar] [CrossRef]
- Tejeda-Benítez, L.; Henao-Argumedo, D.; Alvear-Alayón, M.; Castillo-Saldarriaga, C.R. Caracterización y perfil lipídico de aceites de microalgas. Rev. Fac. Ing. 2015, 24, 43. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Moreno, M.L.; Cortés-Castillo, C.E.; Sánchez-Villarraga, J.; Padilla, J.; Otero-Paternina, A.M. Evaluación del crecimiento de la mi-croalga chlorella sorokiniana en diferentes medios de cultivo en condiciones autotróficas y mixotróficas. Orinoquia 2013, 17, 2. [Google Scholar]
- Malagón-Micán, M.; Suárez-Chaparro, M. Influencia de la concentración inicial de Chlorella vulgaris y CO2 en la producción de lípidos. Rev. Lasallista Investig. 2000, 17, 59–69. [Google Scholar] [CrossRef]
- Gallego-Cartagena, E.; Castillo-Ramírez, M.; Martínez-burgos, W. Effect of stressful conditions on the carotenogenic activity of a Colombian strain of Dunaliella salina. Saudi J. Biol. Sci. 2019, 26, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Duque, S.R.; Núñez-Avellaneda, M. Microalgas acuáticas de la Amazonía colombiana. Biota Colomb. 2000, 1, 216. [Google Scholar]
| Name | Metal | Reference |
|---|---|---|
| Chaetoceros calcitrans | Cadmium | [102] |
| Chlorella vulgaris | Nickel, zinc, lead | [103] |
| Scenedesmus obliquus | Cadmium | [104] |
| Pithophora lanceolatum | Lead, mercury, cadmium | [105] |
| Spirogyra hialina | Mercury, cadmium, lead, arsenic, and cobalt | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranguren Díaz, Y.; Monterroza Martínez, E.; Carillo García, L.; Serrano, M.C.; Machado Sierra, E. Phycoremediation as a Strategy for the Recovery of Marsh and Wetland with Potential in Colombia. Resources 2022, 11, 15. https://doi.org/10.3390/resources11020015
Aranguren Díaz Y, Monterroza Martínez E, Carillo García L, Serrano MC, Machado Sierra E. Phycoremediation as a Strategy for the Recovery of Marsh and Wetland with Potential in Colombia. Resources. 2022; 11(2):15. https://doi.org/10.3390/resources11020015
Chicago/Turabian StyleAranguren Díaz, Yani, Edy Monterroza Martínez, Laura Carillo García, María C. Serrano, and Elwi Machado Sierra. 2022. "Phycoremediation as a Strategy for the Recovery of Marsh and Wetland with Potential in Colombia" Resources 11, no. 2: 15. https://doi.org/10.3390/resources11020015
APA StyleAranguren Díaz, Y., Monterroza Martínez, E., Carillo García, L., Serrano, M. C., & Machado Sierra, E. (2022). Phycoremediation as a Strategy for the Recovery of Marsh and Wetland with Potential in Colombia. Resources, 11(2), 15. https://doi.org/10.3390/resources11020015






