Bacterial Metal Accumulation as a Strategy for Waste Recycling Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media Composition
2.2. Cascaded Approach of Metal Waste Bioconversion
2.3. Electron Microscopy and Elemental Mapping
2.3.1. Cell Preparation for Electron Microscopy
2.3.2. STEM Imaging and EDS Investigations
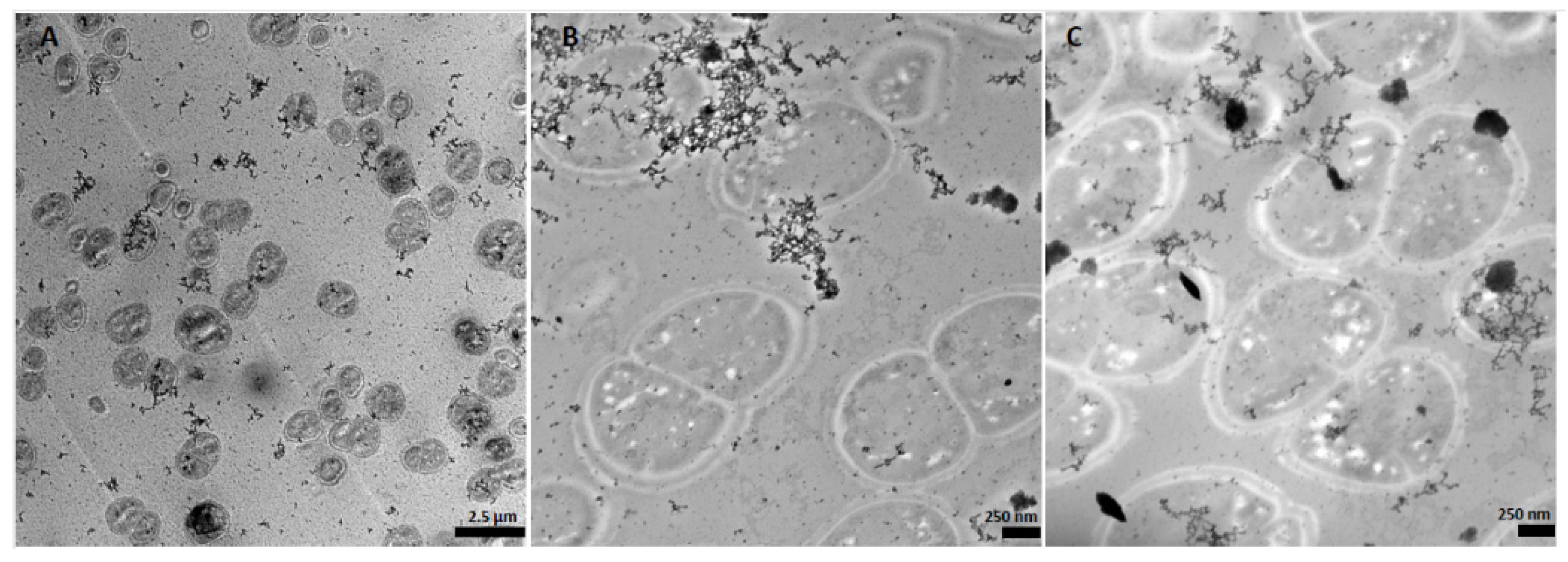
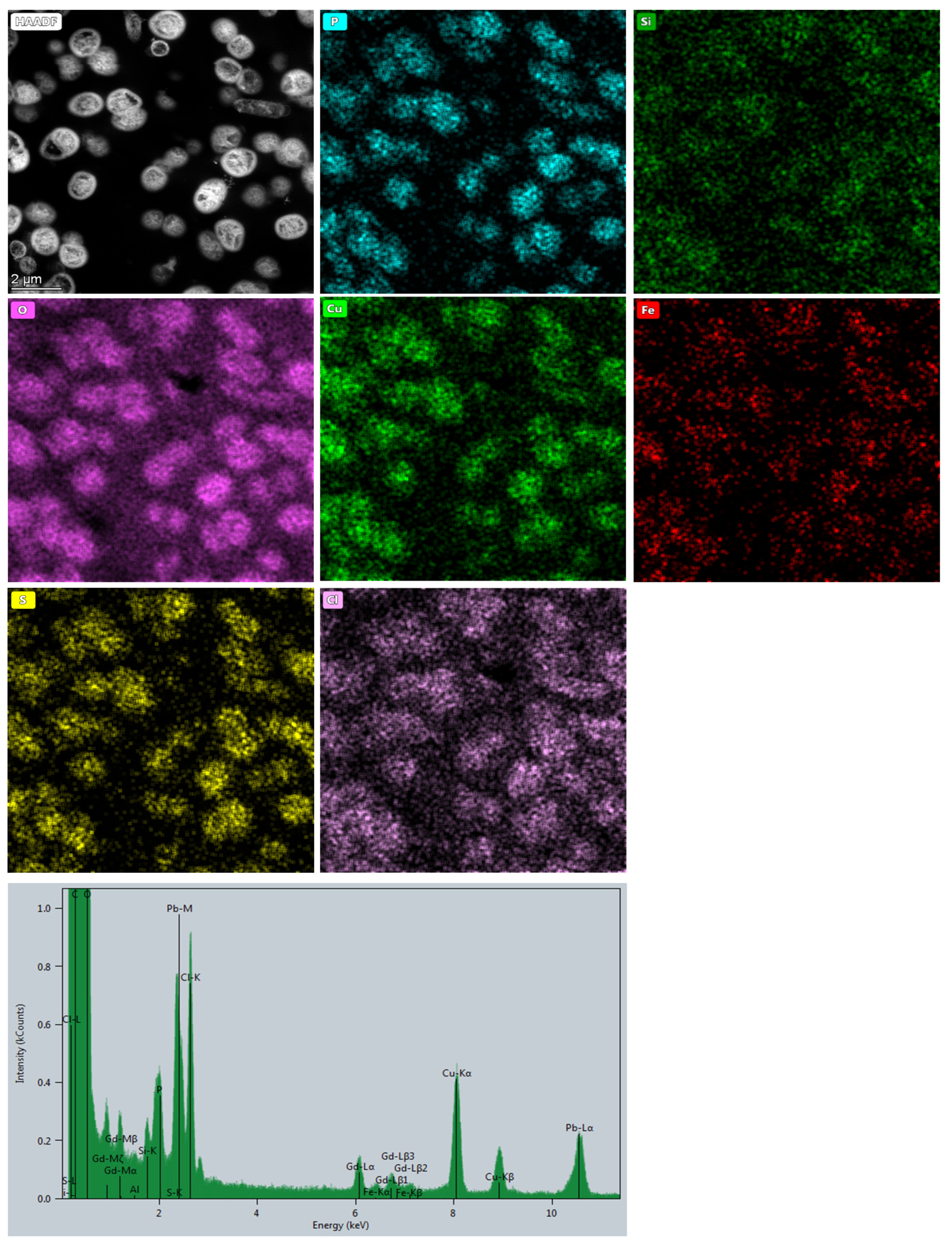
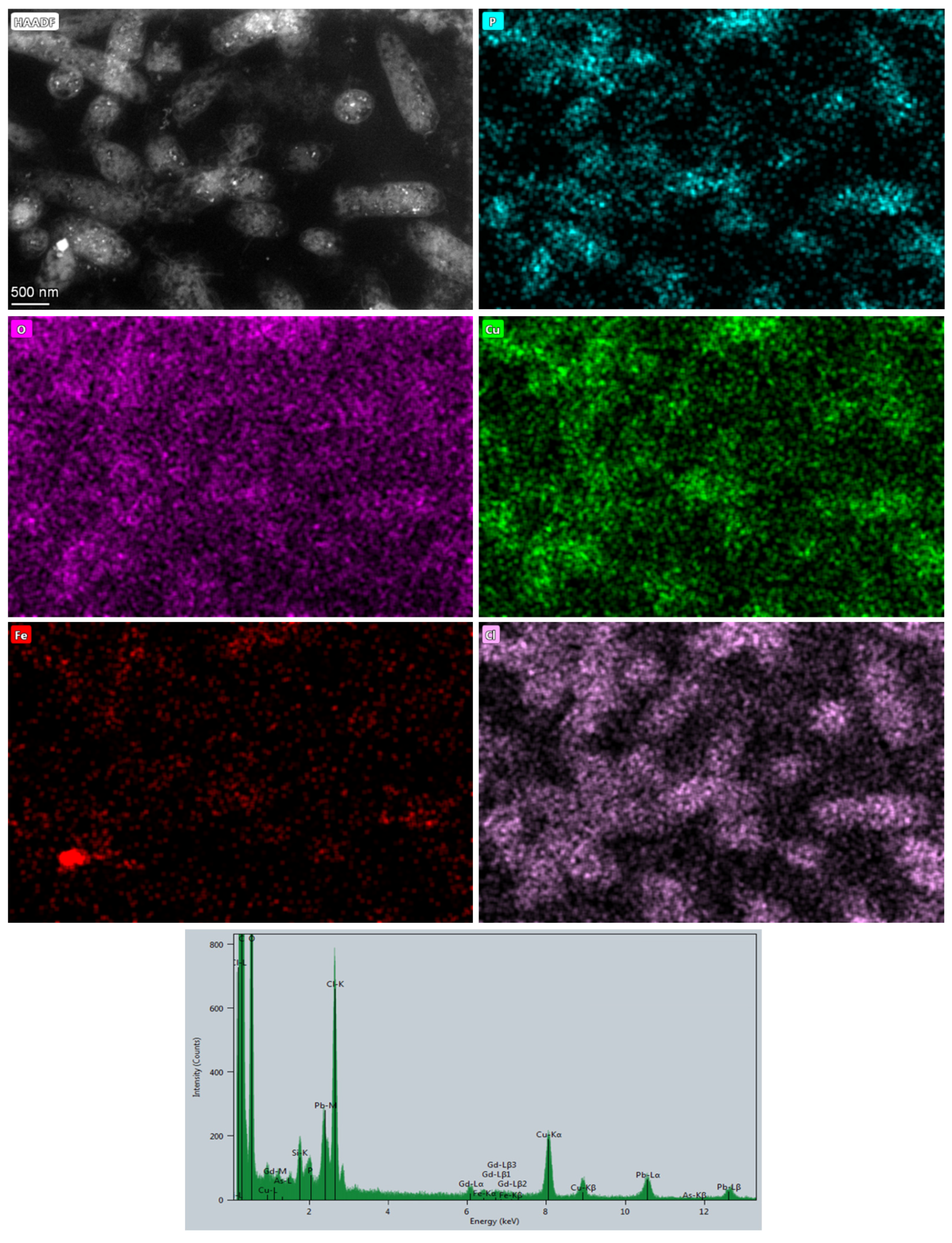
3. Results and Discussion
3.1. Growth on Extremely Acidic Bioleachate Solution
3.2. Microbial Metal Incorporation Abilities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EUROFER. European Steel in Figures. 2022. Available online: https://www.eurofer.eu/publications/brochures-booklets-and-factsheets/european-steel-in-figures-2022 (accessed on 1 July 2022).
- EUROFER. Annual Report. 2020. Available online: https://www.eurofer.eu/publications/archive/annual-report-2020 (accessed on 27 November 2021).
- Russo, F.; Luongo, V.; Mattei, M.R.; Frunzo, L. Mathematical Modeling of Metal Recovery from E-Waste Using a Dark-Fermentation-Leaching Process. Sci. Rep. 2022, 12, 4274. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Wang, X.; Luong, D.X.; Carter, R.A.; Wang, Z.; Tomson, M.B.; Tour, J.M. Rare Earth Elements from Waste. Sci. Adv. 2022, 8, 3132. [Google Scholar] [CrossRef] [PubMed]
- Gabor, A.E.; Davidescu, C.M.; Negrea, A.; Ciopec, M.; Butnariu, M.; Ianasi, C.; Muntean, C.; Negrea, P. Lanthanum Separation from Aqueous Solutions Using Magnesium Silicate Functionalized with Tetrabutylammonium Dihydrogen Phosphate. J. Chem. Eng. Data 2016, 61, 535–542. [Google Scholar] [CrossRef]
- Qiao, J.; Sun, H.; Luo, X.; Zhang, W.; Mathews, S.; Yin, X. EDTA-Assisted Leaching of Pb and Cd from Contaminated Soil. Chemosphere 2017, 167, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Guo, Q.; Yang, J.; Zhang, H.; Wei, R.; Wang, C.; Peters, M.; Zhou, X.; Yang, J. Washing out Heavy Metals from Contaminated Soils from an Iron and Steel Smelting Site. Front. Environ. Sci. Eng. 2015, 9, 634–641. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, T.; Zhang, D.; Jiang, M.; Tan, J.; Li, J.; Lin, Z.; Li, Z. Electro-Enhanced Leaching Method for the Mobilization of Cr(VI) in Contaminated Groundwater Aquifer. Sci. Rep. 2020, 10, 5297. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Mukherjee, S.; Chaudhuri, M.G. Studies on Leaching Characteristics of Electronic Waste for Metal Recovery Using Inorganic and Organic Acids and Base. Waste Manag. Res. 2021, 39, 242–249. [Google Scholar] [CrossRef]
- Kölbl, D.; Memic, A.; Schnideritsch, H.; Wohlmuth, D.; Klösch, G.; Albu, M.; Giester, G.; Bujdoš, M.; Milojevic, T. Thermoacidophilic Bioleaching of Industrial Metallic Steel Waste Product. Front. Microbiol. 2022, 13, 864411. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Y.; Shi, L.; Hussain, R.; Mehmood, K.; Tang, Z.; Zhang, H. Heavy Metals Induced Mitochondrial Dysfunction in Animals: Molecular Mechanism of Toxicity. Toxicology 2022, 469, 153136. [Google Scholar] [CrossRef]
- Gianì, F.; Masto, R.; Trovato, M.A.; Malandrino, P.; Russo, M.; Pellegriti, G.; Vigneri, P.; Vigneri, R. Heavy Metals in the Environment and Thyroid Cancer. Cancers 2021, 13, 4052. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. NPJ Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Lo, W.; Chan, G.Y. Physico-Chemical Treatments for Removal of Recalcitrant Contaminants from Landfill Leachate. J. Hazard. Mater. 2006, 129, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Filote, C.; Roșca, M.; Hlihor, R.M.; Cozma, P.; Simion, I.M.; Apostol, M.; Gavrilescu, M. Sustainable Application of Biosorption and Bioaccumulation of Persistent Pollutants in Wastewater Treatment: Current Practice. Processes 2021, 9, 1696. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Pardo, R.; Herguedas, M.; Barrado, E.; Vega, M. Biosorption of Cadmium, Copper, Lead and Zinc by Inactive Biomass of Pseudomonas putida. Anal. Bioanal. Chem. 2003, 376, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Mahapatra, S.; Das, S. Ca-Alginate as a Support Matrix for Pb(II) Biosorption with Immobilized Biofilm Associated Extracellular Polymeric Substances of Pseudomonas aeruginosa N6P6. J. Chem. Eng. 2017, 328, 556–566. [Google Scholar] [CrossRef]
- Paul, M.L.; Samuel, J.; Chandrasekaran, N.; Mukherjee, A. Comparative Kinetics, Equilibrium, Thermodynamic and Mechanistic Studies on Biosorption of Hexavalent Chromium by Live and Heat Killed Biomass of Acinetobacter junii VITSUKMW2, an Indigenous Chromite Mine Isolate. J. Chem. Eng. 2012, 187, 104–113. [Google Scholar] [CrossRef]
- Li, C.; Yu, Y.; Fang, A.; Feng, D.; Du, M.; Tang, A.; Chen, S.; Li, A. Insight into Biosorption of Heavy Metals by Extracellular Polymer Substances and the Improvement of the Efficacy: A Review. Lett. Appl. Microbiol. 2021, 75, 1064–1073. [Google Scholar] [CrossRef]
- Aryal, M.A. Comprehensive Study on the Bacterial Biosorption of Heavy Metals: Materials, Performances, Mechanisms, and Mathematical Modellings. Rev. Chem. Eng. 2021, 37, 715–754. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Kim, J.; Chang, S.; Chung, W. Bacterial Biosorbents, an Efficient Heavy Metals Green Clean-Up Strategy: Prospects, Challenges, and Opportunities. Microorganisms 2022, 10, 610. [Google Scholar] [CrossRef]
- Mukherjee, A.; Wheaton, G.H.; Blum, P.H.; Kelly, R.M. Uranium Extremophily Is an Adaptive, Rather Than Intrinsic, Feature for Extremely Thermoacidophilic Metallosphaera Species. Proc. Natl. Acad. Sci. USA 2012, 109, 16702–16707. [Google Scholar] [CrossRef]
- Giovanella, P.; Vieira, G.A.L.; Ramos Otero, I.V.; Pais Pellizzer, E.; de Jesus Fontes, B.; Sette, L.D. Metal and Organic Pollutants Bioremediation by Extremophile Microorganisms. J. Hazard. Mater. 2020, 382, 121024. [Google Scholar] [CrossRef]
- de Vicente, A.; Avilés, M.; Codina, J.C.; Borrego, J.J.; Romero, P. Resistance to Antibiotics and Heavy Metals of Pseudontonas aeruginosa Isolated from Natural Waters. J. Appl. Bacteriol. 1990, 68, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Kalita, D.; Joshi, S.R. Study on Bioremediation of Lead by Exopolysaccharide Producing Metallophilic Bacterium Isolated from Extreme Habitat. Biotechnol. Rep. 2017, 16, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, G.; Hernáinz, F.; Calero, M.; Martín-Lara, M.A.; Tenorio, G. The Effect of pH on the Biosorption of Cr (III) and Cr (VI) with Olive Stone. J. Chem. Eng. 2009, 148, 473–479. [Google Scholar] [CrossRef]
- Chuan, M.C.; Shu, G.Y.; Liu, J.C. Solubility of Heavy Metals in a Contaminated Soil: Effects of Redox Potential and pH. Water Air Soil Poll. 1996, 90, 543–556. [Google Scholar] [CrossRef]
- Liang, C.; Shen, J. Removal of Yttrium from Rare-Earth Wastewater by Serratia marcescens: Biosorption Optimization and Mechanisms Studies. Sci. Rep. 2022, 12, 4861. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Yun, Y.-S.; Park, J.M. The Past, Present, and Future Trends of Biosorption. Biotechnol. Bioproc. E 2010, 15, 86–102. [Google Scholar] [CrossRef]
- Auernik, K.S.; Kelly, R.M. Physiological Versatility of the Extremely Thermoacidophilic Archaeon Metallosphaera sedula Supported by Transcriptomic Analysis of Heterotrophic, Autotrophic, and Mixotrophic Growth. Appl. Environ. Microbiol. 2010, 76, 931–935. [Google Scholar] [CrossRef]
- Milojevic, T.; Kölbl, D.; Ferrière, L.; Albu, M.; Kish, A.; Flemming, R.L.; Koeberl, C.; Blazevic, A.; Zebec, Z.; Rittmann, S.K.-M.R. Exploring the Microbial Biotransformation of Extraterrestrial Material on Nanometer Scale. Sci. Rep. 2019, 9, 18028. [Google Scholar] [CrossRef]
- Tobita, M.; Yokozeki, M.; Nishikawa, N.; Kawakami, Y. Pyrite Oxidation by Sulfolobus acidocaldarius. Biosci. Biotechnol. Biochem. 1994, 58, 771–772. [Google Scholar] [CrossRef][Green Version]
- Valdés, J.; Pedroso, I.; Quatrini, R.; Dodson, R.J.; Tettelin, H.; Blake, R.; Eisen, J.A.; Holmes, D.S. Acidithiobacillus ferrooxidans Metabolism: From Genome Sequence to Industrial Applications. BMC Genom. 2008, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Lin, Z.; Pang, S.; Zhang, Y.; Bhatt, P.; Chen, S. Biosurfactant Is a Powerful Tool for the Bioremediation of Heavy Metals from Contaminated Soils. J. Hazard. Mater. 2021, 418, 126253. [Google Scholar] [CrossRef] [PubMed]
- Srinath, T.; Verma, T.; Ramteke, P.W.; Garg, S.K. Chromium (VI) Biosorption and Bioaccumulation by Chromate Resistant Bacteria. Chemosphere 2002, 48, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, J.; Chen, Y.; Xu, X.; Xu, S.; Wang, Y. Tolerance and Biosorption of Copper and Zinc by Pseudomonas putida CZ1 Isolated from Metal-Polluted Soil. Can. J. Microbiol. 2006, 52, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kumar, A.; Kaur, H. Bioremediation of hexavalent Chromium in Wastewater Effluent by Pseudomonas putida (MTCC 102). Int. J. Sci. Knowl. 2014, 1, 7. [Google Scholar]
- Ziagova, M.; Dimitriadis, G.; Aslanidou, D.; Papaioannou, X.; Litopoulou Tzannetaki, E.; Liakopoulou-Kyriakides, M. Comparative Study of Cd(II) and Cr(VI) Biosorption on Staphylococcus xylosus and Pseudomonas sp. in Single and Binary Mixtures. Bioresour. Technol. 2007, 98, 2859–2865. [Google Scholar] [CrossRef]
- Sahmoune, M.N.; Louhab, K.; Boukhiar, A. Studies of Chromium Removal from Tannery Effluents by Dead Streptomyces rimosus. Chem. Prod. Process Model. 2008, 3, 1. [Google Scholar] [CrossRef]
- Sahmoune, M.N. Performance of Streptomyces rimosus Biomass in Biosorption of Heavy Metals from Aqueous Solutions. Microchem. J. 2018, 141, 87–95. [Google Scholar] [CrossRef]
- Manobala, T.; Shukla, S.K.; Rao, T.S.; Kumar, M.D. A New Uranium Bioremediation Approach Using Radio-Tolerant Deinocoocus radiodurans Biofilm. J. Biosci. 2018, 5, 503896. [Google Scholar]
- Gara, S.; Schrimpf, S. Behandlung von Reststoffen und Abfällen in der Eisen- und Stahlindustrie; Bundesministerium für Umwelt, Jugend und Familie: Wien, Austria, 1998.
- Yoshida, N.; Nakasato, M.; Ohmura, N.; Ando, A.; Saiki, H.; Ishii, M.; Igarashi, Y. Acidianus manzaensis sp. nov., a Novel Thermoacidophilic Archaeon Growing Autotrophically by the Oxidation of H2 with the Reduction of Fe3+. Curr. Microbiol. 2006, 53, 406–411. [Google Scholar] [CrossRef]
- Felux, A.-K.; Franchini, P.; Schleheck, D. Permanent Draft Genome Sequence of Sulfoquinovose-Degrading Pseudomonas putida Strain SQ1. Stand. Genom. Sci. 2015, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.W. Bacteriological Studies on the Coagulation of Evaporated Milk. Res. Bull. Iowa Agric. Exp. Stn. 1918, 19, 119–131. [Google Scholar]
- Maleki, H.; Dehnad, A.; Hanifian, S.; Khani, S. Isolation and Molecular Identification of Streptomyces spp. with Antibacterial Activity from Northwest of Iran. Bioimpacts 2013, 3, 129–134. [Google Scholar]
- Schleifer, K.H.; Kloos, W.E. Isolation and Characterization of Staphylococci from Human Skin. I. Amended Descriptions of Staphylococcus epidermidis and Staphylococcus saprophyticus, and Descriptions of Three New Species: Staphylococcus cohnii, Staphylococcus haemolyticus, and Staphylococcus xylosus. Int. J. Syst. Bacteriol. 1975, 25, 50–61. [Google Scholar]
- Yang, Y.; Itoh, T.; Yokobori, S.; Itahashi, S.; Shimada, H.; Satoh, K.; Ohba, H.; Narumi, I.; Yamagishi, A. Deinococcus aerius sp. nov., Isolated from the High Atmosphere. Int. J. Syst. Evol. Microbiol. 2009, 59, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.W.; Murray, R.G.E.; Johnson, J.L.; Stackebrandt, E.; Woese, C.R.; Fox, G.E. Red-Pigmented Micrococci: A Basis for Taxonomy. Int. J. Syst. Bacteriol. 1980, 30, 627–646. [Google Scholar] [CrossRef]
- Kimoto, K.; Aizawa, T.; Urai, M.; Bao Ve, N.; Suzuki, K.; Nakajima, M.; Sunairi, M. Acidocella aluminiidurans sp. nov., an Aluminium-Tolerant Bacterium Isolated from Panicum Repens Grown in a Highly Acidic Swamp in Actual Acid Sulfate Soil Area of Vietnam. Int. J. Syst. Evol. Microbiol. 2010, 60, 764–768. [Google Scholar] [CrossRef]
- Lund, P.A.; De Biase, D.; Liran, O.; Scheler, O.; Mira, N.P.; Cetecioglu, Z.; Fernández, E.N.; Bover-Cid, S.; Hall, R.; Sauer, M.; et al. Understanding How Microorganisms Respond to Acid PH Is Central to Their Control and Successful Exploitation. Front. Microbiol. 2020, 11, 556140. [Google Scholar] [CrossRef]
- Slade, D.; Radman, M. Oxidative Stress Resistance in Deinococcus radiodurans. Microbiol. Mol. Biol. Rev. 2011, 75, 133–191. [Google Scholar] [CrossRef]
- Satoh, K.; Arai, H.; Sanzen, T.; Kawaguchi, Y.; Hayashi, H.; Yokobori, S.; Yamagishi, A.; Oono, Y.; Narumi, I. Draft Genome Sequence of the Radioresistant Bacterium Deinococcus aerius TR0125, Isolated from the High Atmosphere above Japan. Genome Announc. 2018, 6, e00080-18. [Google Scholar] [CrossRef] [PubMed]
- Milojevic, T.; Treiman, A.H.; Limaye, S.S. Phosphorus in the Clouds of Venus: Potential for Bioavailability. Astrobiology 2021, 21, 1250–1263. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Dopson, M. Life in Acid: PH Homeostasis in Acidophiles. Trends Microbiol. 2007, 15, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Krulwich, T.A.; Sachs, G.; Padan, E. Molecular Aspects of Bacterial PH Sensing and Homeostasis. Nat. Rev. Microbiol. 2011, 9, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Wang, Q.; Lv, M.; Chen, L. Microorganism Remediation Strategies towards Heavy Metals. Chem. Eng. J. 2019, 360, 1553–1563. [Google Scholar] [CrossRef]
- Malik, A. Metal Bioremediation through Growing Cells. Enrivon. Int. 2004, 30, 261–278. [Google Scholar] [CrossRef]
- Kölbl, D.; Pignitter, M.; Somoza, V.; Schimak, M.P.; Strbak, O.; Blazevic, A.; Milojevic, T. Exploring Fingerprints of the Extreme Thermoacidophile Metallosphaera Sedula Grown on Synthetic Martian Regolith Materials as the Sole Energy Sources. Front. Microbiol. 2017, 8, 1918. [Google Scholar] [CrossRef] [PubMed]
- Milojevic, T.; Albu, M.; Kölbl, D.; Kothleitner, G.; Bruner, R.; Morgan, M.L. Chemolithotrophy on the Noachian Martian Breccia NWA 7034 via Experimental Microbial Biotransformation. Commun. Earth Environ. 2021, 2, 39. [Google Scholar] [CrossRef]
- Coupland, C.; Johnson, D.B. Evidence that the Potential for Dissimilatory Ferric Iron Reduction is Widespread among Acidophilic Heterotrophic Bacteria. FEMS Microbiol. Lett. 2008, 279, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K.; Witek-Krowiak, A. State of the Art for the Biosorption Process—A Review. Appl. Biochem. Biotechnol. 2013, 170, 1389–1416. [Google Scholar] [CrossRef]
- Chojnacka, K. Biosorption and Bioaccumulation—The Prospects for Practical Applications. Environ. Int. 2010, 36, 299–307. [Google Scholar] [CrossRef]
- Nie, X.; Dong, F.; Liu, M.; Cheng, W.; Ding, C.; Bian, L.; Sun, S. Bonding Behavior and Mechanism of U(VI) by Chemically Modified Deinococcus Radiodurans. Minerals 2021, 11, 1108. [Google Scholar] [CrossRef]
- Fathollahi, A.; Khasteganan, N.; Coupe, S.J.; Newman, A.P. A Meta-Analysis of Metal Biosorption by Suspended Bacteria from Three Phyla. Chemosphere 2021, 268, 129290. [Google Scholar] [CrossRef]
- Rizki, I.; Okibe, N. Size-Controlled Production of Gold Bionanoparticles Using the Extremely Acidophilic Fe(III)-Reducing Bacterium, Acidocella Aromatica. Minerals 2018, 8, 81. [Google Scholar] [CrossRef]
- Merroun, M.L. Interactions between metals and bacteria: Fundamental and applied research. Biometals 2007, 16, 331–339. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, Minerals and Microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef]
- Wichlacz, P.; Unz, R.F.; Langworthy, T.A. Acidiphilium angustum sp. nov., Acidiphilium facilis sp. nov., and Acidiphilium rubrum sp. nov.: Acidophilic Heterotrophic Bacteria Isolated from Acidic Coal Mine Drainage. Int. J. Syst. Evol. Microbiol. 1986, 36, 197–201. [Google Scholar] [CrossRef]
- Kishimoto, N.; Kosako, Y.; Wakao, N.; Tano, T.; Hiraishi, A. Transfer of Acidiphilium facilis and Acidiphilium aminolytica to the Genus Acidocella gen. nov., and Emendation of the Genus Acidiphilium. Syst. Appl. Microbiol. 1995, 18, 85–91. [Google Scholar] [CrossRef]
- Kishimoto, N.; Kosako, Y.; Tano, T. Acidiphilium aminolytica sp. nov.: An Acidophilic Chemoorganotrophic Bacterium Isolated from Acidic Mineral Environment. Curr. Microbiol. 1993, 27, 131–136. [Google Scholar] [CrossRef] [PubMed]

| Strain | Medium Components (w/v) | T (°C) | pH |
|---|---|---|---|
| P. putida | 0.5% peptone; 0.3% meat extract | 28 | 7.0 |
| B. coagulans | 0.5% peptone; 0.3% meat extract | 40 | 7.0 |
| S. xylosus | 0.5% peptone; 0.3% meat extract; 0.3% yeast extract | 37 | 7.2 |
| D. aerius | 1% tryptone; 0.6% meat extract; 0.2% glucose | 28 | 7.0 |
| D. radiodurans | 1% tryptone; 0.6% meat extract; 0.2% glucose | 28 | 7.0 |
| A. aluminiidurans | 0.2% (NH4)2SO4; 0.01 KCl; 0.05% K2HPO4; 0.05% MgSO4 × 7H2O; 0.03 yeast extract; 0.1% glucose; 0.1% trypticase soy broth | 37 | 4.0 |
| S. rimosus | 0.4% glucose; 0.4% yeast extract; 1% malt extract; 0.2% CaCO3 | 28 | 7.2 |
| mg/L | mg/L | ||
|---|---|---|---|
| Mg | 778 | Ni | 0.185 |
| Ca | 618 | Co | 0.17 |
| Fe | 145 | Ti | 0.169 |
| Zn | 111 | V | 0.144 |
| Mn | 85.5 | Cd | 0.143 |
| Al | 36.6 | Mo | 0.068 |
| Sr | 0.906 | As | 0.037 |
| Cu | 0.757 | Pb | 0.021 |
| Cr | 0.695 | Ba | 0.003 |
| Strain | 0 h | 24 h | 48 h |
|---|---|---|---|
| B. coagulans | 7.98 × 106 ± 1.18 × 106 | 4.69 × 106 ± 1.27 × 106 | 8.03 × 105 ± 1.31 × 105 |
| S. xylosus | 1.17 × 107 ± 4.35 × 105 | 5.60 × 106 ± 8.57 × 105 | 2.50 × 104 ± 5.00 × 103 |
| P. putida | 9.92 × 106 ± 4.17 × 105 | 7.09 × 106 ± 5.28 × 105 | 1.54 × 106 ± 3.80 × 105 |
| D. radiodurans | 2.76 × 106 ± 2.38 × 105 | 3.15 × 106 ± 1.14 × 106 | 3.36 × 106 ± 4.71 × 105 |
| D. aerius | 6.96 × 105 ± 9.98 × 104 | 1.01 × 106 ± 6.40 × 105 | 5.60 × 106 ± 6.00 × 105 |
| A. aluminiidurans | 1.06 × 106 ± 1.24 × 105 | 4.00 × 106 ± 4.80 × 105 | 3.20 × 107 ± 6.40 × 105 |
| S. rimosus | N.D. * | N.D. * | N.D. * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kölbi, D.; Memic, A.; Schnideritsch, H.; Wohlmuth, D.; Klösch, G.; Albu, M.; Milojevic, T. Bacterial Metal Accumulation as a Strategy for Waste Recycling Management. Resources 2023, 12, 144. https://doi.org/10.3390/resources12120144
Kölbi D, Memic A, Schnideritsch H, Wohlmuth D, Klösch G, Albu M, Milojevic T. Bacterial Metal Accumulation as a Strategy for Waste Recycling Management. Resources. 2023; 12(12):144. https://doi.org/10.3390/resources12120144
Chicago/Turabian StyleKölbi, Denise, Alma Memic, Holger Schnideritsch, Dominik Wohlmuth, Gerald Klösch, Mihaela Albu, and Tetyana Milojevic. 2023. "Bacterial Metal Accumulation as a Strategy for Waste Recycling Management" Resources 12, no. 12: 144. https://doi.org/10.3390/resources12120144
APA StyleKölbi, D., Memic, A., Schnideritsch, H., Wohlmuth, D., Klösch, G., Albu, M., & Milojevic, T. (2023). Bacterial Metal Accumulation as a Strategy for Waste Recycling Management. Resources, 12(12), 144. https://doi.org/10.3390/resources12120144






