1. Introduction
In a world where the demand for food is constantly increasing due to population growth, and in anticipation of the need to increase food supply, aquatic food products could represent a good compromise between human food needs and preservation of the environment [
1,
2]. Recently, aquaculture has been widely discussed as a way to bridge the gap between demand and supply and to reduce pressure on wild fisheries [
3]. According to the FAO, approximately 300 different species, ranging from fish to shellfish, including bivalve mollusks, crustaceans and algae, can be farmed [
4]. In particular, bivalve mollusks are the second largest category of farmed seafood, accounting for approximately 15% of total global aquaculture production in 2020 [
5,
6]. Currently, clams and oysters are the major species groups, contributing about 38% and 33% of global production, respectively, followed by mussels and scallops with less than 20% each [
7]. Although East Asia is the largest producer, Europe plays a key role in the sector, contributing 28% of the total marketed value [
8]. The importance of bivalve mollusks is not only due to their total commercial value of around USD 30 billion/year, but also to the ecosystem services they can provide, i.e., food supply, water quality maintenance and nutrient remediation [
9,
10]. In addition, as filter feeders that extract naturally occurring food such as plankton from the water, shellfish farming relies entirely on their natural growth cycle without the need for external feeding or medication [
9,
11].
Oysters are farmed worldwide, with China being the main producer, accounting for 86% of world production by weight and 78% by value [
12]. In Europe, although oyster farming was introduced by the Romans in Italy, the most productive country is France, which is the world’s second largest producer [
13]. Two main species, the native flat oyster,
Ostrea edulis, and the non-indigenous Pacific or Japanese oyster,
Crassostrea gigas, are present in European waters [
14], although several factors such as pollution, pathogens, natural seed mortality and extremely cold winters during the eighteenth and nineteenth centuries have severely affected
O. edulis populations throughout Europe [
15]. On the other hand,
C. gigas, thanks to its great tolerance to different environmental conditions, i.e., temperature and salinity, and its great success in the French market, has rapidly gained significant production [
16]. Today, the Pacific oyster is the most produced and consumed species in Europe, while
O. edulis has almost disappeared [
12].
The Pacific oyster can be cultured in three main systems: on-bottom, in the intertidal zone or in deep water; off-bottom, in plastic mesh bags in the intertidal zone; or as suspended culture, in the open sea. On-bottom culture is the closest method to wild oyster culture and simply involves the transfer of seeds from natural areas of the seabed to other areas where population density and growth rate are regulated, and harvesting and predation control are facilitated [
17]. Off-bottom culture can be assessed using a range of surface floating systems (i.e., racks and bags, cages, trays), subject to tidal excursion. In suspended culture, oysters are placed in trays or nets supported by a series of ropes and buoys, in offshore long-line plants. Oysters farmed in this way are not in the intertidal zone at all, but are always in the water, rising and falling with the tide [
18].
Irrespective of the culture system, oyster farming is usually carried out in four stages: seed collection or hatching, pre-fattening, fattening and harvesting. This occurs when they have reached commercial size, which can take 15–18 months to three years. After harvesting, the oysters are selected and packaged [
19,
20].
Although oyster farming is well known in other European countries, such as France or the Netherlands, it is still in its infancy in Italy [
21]. In the last decade, some important examples have emerged, such as the pink oyster of the Scardovari lagoon (Po Delta), the green oyster of the Gulf of Poets in Liguria, the white oyster of the Gargano, the Sardinian oyster and the oysters of Goro (Po Delta), but at the moment, they are rather limited experiences (with a total production not exceeding 500 tons per year) and not yet structured as a competitive sector.
The aim of this research was to study the development of oyster production in Goro and to compare the environmental impacts of two different farming scenarios, using the life cycle assessment (LCA) methodology. Goro is located in the north-east of the Adriatic Sea and has the most important productive lagoon in Italy for bivalves Manila clams (Ruditapes philippinarum) and mussels (Mytilus galloprovincialis). In Goro, oyster farming is now carried out using suspended culture in offshore long line facilities. In this study, we have considered production in four stages, focusing on two different pre-fattening options, off-shore and within the lagoon. The choice of the alternative scenario in which pre-fattening is carried out in the lagoon is based on the possibility of not only reducing costs and man hours, but also improving the process from an environmental sustainability point of view. In fact, the possibility of reducing the consumption of resources for the construction of the facilities and the reduced consumption of energy, such as fuel to power the boat, can be effective strategies to reduce the environmental impact compared to the current farming method.
LCA is a consolidated and standardized approach that analyses the entire life cycle of a product or system and provides a quantitative assessment of its impacts [
22,
23]. At present, there are numerous studies evaluating the sustainability of aquaculture production [
24,
25] and as many studies showing how shellfish farming is a good choice in terms of environmental impact and also in terms of production [
26,
27]; otherwise, there are only three studies where LCA is applied to oyster farming [
28]. Alvarenga et al. [
29] carried out a cradle-to-grave LCA of oysters produced in southern Brazil, focusing more on the processing of oyster shells for use as raw material in other industrial processes than on the emissions from their cultivation. Fry et al. [
30] reported a cradle-to-grave LCA of intertidal oysters in Scotland, taking into account the traditional bag and trestle method of oyster farming. Tamburini et al. [
19] carried out an LCA of oyster culture in the longline system, the most widely used system in the world, and calculated the impact of oyster culture in Goro, comparing the two upstream stages of purchasing seed from France or producing seed in a local hatchery. On the basis of this previous work, we wanted to take a further step towards optimising oyster farming according to an environmental sustainability criterion, considering the same longline system for oyster farming, but applying the analysis to the possibility of growing oysters inside the lagoon. Sacca di Goro was chosen as a model because it is an important site for the production of bivalves, including oysters. Although oyster farming in this area is well established in the Italian market, there is still much potential for improvements, both from a production and environmental perspective.
2. Materials and Methods
2.1. Description of Case Study
Sacca di Goro is one of the most important bivalve farming areas in Italy, with an annual production of 15,000–16,000 tons, mainly due to the production of Manila clam (
R. philippinarum) [
31]. This area extends for about 27 km
2 nearby Po River delta, in northern Italy, with an average water depth of 1.5–2.5 m and a sandy seabed (
Figure 1). It is separated from the open sea by a sandy strip, which creates a natural mouth allowing water exchanges. The Sacca di Goro presents high levels of eutrophication, which makes this area particularly suitable for bivalve farming.
The current production of
C. gigas in Goro is about 20 tonnes/year. The cultivation of
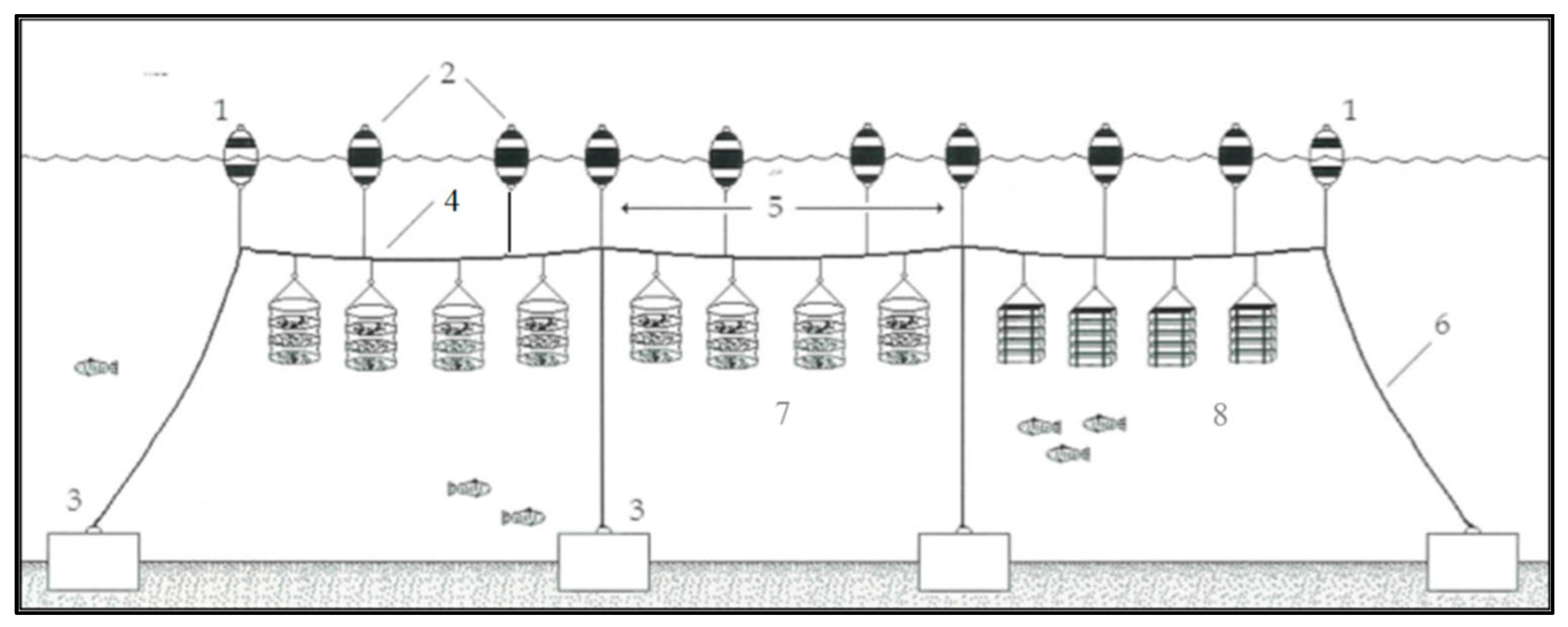
C. gigas is carried out in several phases: seed production in nurseries and hatcheries, pre-fattening, fattening, harvesting and then selection and packaging for the market. During seed production, reproductive organisms are placed in closed tanks with static water. Environmental conditions, such as water temperature and light/dark cycles, are strictly controlled, and the organisms are fed various types of algae, vitamins and minerals. When the organisms are able to adhere to surfaces, they are placed on suspended boxes and allowed to grow at 15–25 °C and 25–32% salinity. When they reach a size of 7–8 mm, they are transferred to the long-line plant where the pre-fattening phase can begin. The long-line plant is an offshore structure located approximately 2.5 miles south of the lagoon mouth and 3 miles off the coast. It consists of a series of parallel beams (800 m) anchored to the seabed by concrete blocks (1200 kg) and kept in suspension by a floating system of buoys (
Figure 2). There are a total of 3 parallel units, each 50 m apart. The oysters grow in vertically stacked multilevel baskets made up of five plastic trays (40 cm in diameter, 10 cm high) and each basket is connected to the buoys. After 4 months, the oysters reach the right size (10–30 mm long) and start the fattening phase until they reach a size of 30–80 mm. After about 8 months, the mature oysters are harvested and selected. Oysters suitable for sale are packed in wooden crates. This production process is defined as Scenario 1.
In the alternative scenario (Scenario 2), prefattening in long-line is replaced with prefattening in lagoon, within the Sacca di Goro. The plant consists of chestnut stakes connected with nylon ropes. Oysters remain suspended on high density polyethylene (HDPE) tubular nets, called lanterns. After prefattening, oysters are transported offshore and conclude their growth as in the Scenario 1. Thus, the two scenarios differ from the prefattening stage.
2.2. General Background of LCA
LCA is a well-established tool for assessing and quantifying water, energy and environmental loads, as well as potential impacts associated with a product or process, in its entire life cycle [
32]. In this study, LCA follows the standardized method, regulated by ISO 14,040 series, which consists of four stages: (1) goal and scope definition; (2) life cycle inventory construction; (3) environmental impact assessment; and (4) interpretation of the results.
2.3. Goal and Scope Definition
This study aims to identify environmental advantages and disadvantages generated by the replacement of prefattening from offshore conditions (Scenario 1) to inshore ones, using a dedicated plant inside the lagoon (Scenario 2).
Initially, functional unit and system boundaries were established, and they are the same for both scenarios. The functional unit (FU) chosen for this analysis is 1 kg of oysters (about 12 oysters of commercial size with an average weight of 70–80 g) packaged in a wooden box covered with an HDPE film. System boundaries include all stages in both scenarios, from seed production to packaging (
Table 1). The change of plant did not influence the breeding time; indeed, in both cases, oysters reached commercial size in 12 months. Equipment, technical clothing, materials, electricity, fuel and land uses, as well as the construction of capital goods and transport of equipment from suppliers to consumer, are the factors which are taken into consideration for the environmental assessment.
In both scenarios, the production of all materials from new resources is considered, as the end-of-life management of all materials used during oyster breeding. The consumption of the molluscs and the destiny of the oyster shells waste were excluded as they are not inherent in the oyster farming cycle. Furthermore, although the numerous potentials for reuse of this waste is known, to date, there is no dedicated collection of this waste in the local scenario, and there are no relevant applications at industrial scale [
33].
For both scenarios, a final production of 10 tons/year was considered, with an average loss of product of about 50%, distributed along the entire productive chain. The loss of product was the same since it is independent of the productive system but derives from the presence of pathogens and predation effects [
34,
35], as well as adverse environmental conditions that occurred in the production years considered. In addition, the scenarios share the same boat and long-line plant, even though for different times.
2.4. Life Cycle Inventory
In order to understand the production cycle used in the Goro area, a direct interview was carried out with the farmers of each cooperative. In total, four interviews were held. The primary data, which are the data related to the oyster production process only, were collected through the questionnaire that was submitted to the oyster farming cooperatives, which aimed to map materials, energy, water, and fuel used during each production cycle. The data collected were divided into subgroups (1. seed production, 2. prefattening, 3. fattening, 4. harvesting, selection, and packaging). As there are four cooperatives in the area involved in oyster farming, the dataset used to construct the inventory is an average of the datasets collected for each cooperative. Furthermore, an average inventory of three production cycles, conducted from 2019 to 2021, was considered in the LCA analysis. Secondary data (the production of primary materials, electricity, fuel and the transport of goods) were extrapolated from reference database Ecoinvent ® v.3.7., developed by the Swiss Centre for Life Cycle Inventories (Zurich, Switzerland).
2.4.1. Seed Production
Local nursing and hatching building construction was excluded from the system boundaries because it has been in use for decades and shared with other aquaculture production, so its effect on oyster farming was assumed to be negligible. Oyster seed production required several containers of different capacity and materials, i.e., 200 L HDPE, 400 L HDPE and 500 L glass fibre. Furthermore, the growth of oyster seed requires fresh and salt water, CO
2, and a nutrient solution of mineral salts and vitamins. The main inputs are shown in
Table 2. Tanks and tubs are completely recyclable. In both scenarios, the quantity of oyster seed needed for a production cycle is about 200,000 pieces, and the process lasts 4 months.
2.4.2. Prefattening and Fattening
As already stated, both scenarios share a boat, a long-line plant and technical clothes, which consist of a pair of PVC gloves, a PVC vest and rubber boots per person.
In scenario 1, prefattening and fattening use the same boat, long-line plant and equipment. During the growth cycle, a average of 110 trips are necessary, of which 36% (40 trips) are for prefattening and 64% (70 trips) are for fattening, with a total consumption of about 800 L (1120 kg) of diesel fuel. The long-line plant takes up an area of 800 × 150 m
2 with a sea occupation of about 3 months for prefattening and 12 months for fattening. The long-line structure is composed of concrete blocks, HDPE buoys and nylon ropes, whereas trays and baskets are all made of HDPE. Buoys, baskets, and trays are not recyclable plastic after use because of the accumulation of organic fouling. Prefattening boxes and fattening cassettes are considered separate process, and are not included in the construction of long-line plants, since their use is limited to the growth of oysters (
Table 3).
In scenario 2, prefattening and fattening steps take place in different plants. The prefattening plant takes up an area of 30 m
2 and is made up of wood poles and nylon ropes, which are discarded after their use, and HDPE lanterns (
Table 4). In this phase, there were no boat trips since the plant was close to the facilities.
Fattening takes place in long-line plants, and to reach the fattening plant, 70 boat trips are considered (
Table 5). The impact of trips (<5) needed to transport the pre-fattened oysters to long-lines plant was assumed to be negligible.
2.4.3. Harvesting, Selection and Packaging
After harvesting, during the first selection on a boat, HDPE cassettes are used, which are also used at the end of the prefattening for oyster transport, and they are recycled.
The functional unit corresponds to a 1 kg pack of oysters, which is approximately 12 commercial-sized oysters. Therefore, a wooden box and a polypropylene (PP) film for packaging were included in the inventory. All inputs are shown in
Table 6.
2.5. Life Cycle Impact Assessment (LCIA)
LCIA considers human and environmental health risks, use of resources and consequent environmental damage. Risk potentials are expressed as impact categories.
OpenLCA®1.10.3, an open-source software package developed by GreenDelta (Berlin, Germany), and the reference database Ecoinvent ® v.3.7. were used. The analysis method was ReCiPe™ midpoint (H). To the environmental impacts category, a cut-off of 10−5 on the value was applied; namely, values of impact categories below 10−5 were excluded from the discussion because the relative impacts were reputed negligible. Impact categories selected based on applied cut-off, which are directly correlated with the process, are climate change (GWP100), human toxicity potential (HTP), eutrophication potential (EP), acidification potential (AP), fossil depletion (FD), particulate matter formation (PMF) and land use. These impact categories significantly describe the state of human health, ecosystem and resources depletion. Depending on the effects generated by different impacts, they can be grouped into local/regional (FDP, HTP, MAETP, EP, MDP, PMFP, POFP, AP, WDP and land use) and global impacts (GWP100).
A Monte Carlo simulation was performed to calculate the LCA uncertainty. The use of average inventory data, calculated on three years of sampling, requires the handling of a standard deviation (SD) as a measurement of data quality. SD was used as uncertainty factor, and a Monte Carlo analysis was carried out. In the Monte Carlo analysis, the input and output values are sampled in a dependent manner from the distributions of the unit process for a fixed number of interactions, which, in this study, are equal to 1000, and at a significance level of 95% [
36]. An uncertainty interval is formed by a range of possible outcomes, resulting from the aggregation of 1000 interactions. Considering this uncertainty range and the related parameters, it is possible to calculate the uncertainty of the impact categories generated by the production of 1 kg of fresh oysters.
3. Results and Discussion
The LCIA results for both scenarios are presented in
Table 7, and they are referred to as 1 kg of packed fresh oysters, ready for sale.
In Scenario 1, the fattening phase has the largest contribution to emissions in each impact category (51–68%), except for land use, which is mainly affected by the packaging phase (56%), and MDP, where the contributions of the seed production and fattening phases are about the same (39% and 37%, respectively). The fattening phase has less impact on land use, FDP, HTP, MDP and WDP, but its important contribution to GWP, MAETP, EP, PMFP, POFP and TAP cannot be neglected.
When analysing the contribution of the different processes involved in oyster farming, it’s worth noting that for all impact categories, about 20% or more of the total emissions are caused by the longline. In particular, it generates more than 50% of total FDP and more than 40% of HTP, GWP100, WDP and MAETP. The impact of the longline system is mainly due to its construction; in fact, over 99% is due to the use of plastic materials for ropes (nylon) and buoys (HDPE). The construction of fattening cassettes, which are considered a separate process from longline construction, requires the use of HDPE and steel, so the contribution (in the range of 9–12%) to GWP100, FDP, HTP, and WDP is not negligible, especially for MAETP, where the contribution of feeders is around 18%.
Another important component is the construction, use and maintenance of the boat, as well as diesel fuel consumption. Diesel consumption is the main source of particulate matter (PM10) and non-methane volatile organic compounds (NMVOC) and is one of the main contributors to GWP100, EP and AP.
NMVOCs, due to their oxidation to ozone by sunlight in the troposphere [
37], are the main contributors to POFP, and of the total amount produced during the oyster farm (1.01 × 10
−2), 49% is due to diesel combustion. PMFP is the main indicator of how much a process or product determines the formation of particulate matter, expressed as PM10 eq. These pollutants are recognised as toxic agents harmful to human health due to their dispersion in the air and their ability to absorb toxic metals and acidic sulphur species on the surface [
38]. The emission potential of these pollutants in the current scenario of oyster farming is 3.04 × 10
−3 kg PM10 eq, of which 50% comes from diesel combustion, while the emissions generated by the use and maintenance of the boat are minimal.
Another significant contribution to pollutant emissions and depletion of biotic and abiotic resources comes from oyster seed production. In this study, local seed production is considered, and the LCI includes the energy and material inputs for the construction of the seed growing facility. Although the impact generated by the construction of the facility has been neglected, the production of feed for the growth of the oyster seed is an important contributor. In all categories, the impact due to seed production is around 10–20%, except for 39% of the contribution in the MDP. Therefore, the recovery and recycling of the feed solution, in order to obtain a complete depletion of all nutrients, could represent an interesting opportunity to reduce the MDP while optimising the use of resources.
Overall, considering the whole cycle of oyster farming in Scenario 1, the use of wooden boxes for final packaging is an important aspect to be considered, as the use of wood for their construction determines an important impact on the sustainability of the process. While it is understandable that the wooden box is attractive from a marketing point of view, the environmental impact should not be overlooked. The production of the wooden boxes in which the oysters are packaged accounts for 80% of the land use potential. The land use impact category includes the environmental impacts of the actual use of land for other purposes, including those not directly related to oyster farming. There is general agreement that such conversion, fragmentation or degradation of natural and semi-natural ecosystems for human purposes is a major cause of declining habitat and wildlife diversity [
39]. Therefore, in addition to the use of marine and lagoon areas, the indirect effect caused by the production of raw materials, especially for the construction of wooden cassettes, must also be taken into account.
Finally, we can affirm that in Scenario 1, the main hotspots are the use of boats and fuel and the use of longlines during the fattening and pre-fattening phases.
In Scenario 2, prefattening takes place in the lagoon, while seed production, fattening and packaging are the same as in Scenario 1. The use of a different facility for pre-fattening reduces the use of longlines by approximately 23%, resulting in a reduction in environmental impact by approximately 11% in each impact category, in particular MAETP. The LCA has shown that the impact of the construction and use of the prefattening plant is negligible compared to the longline, mainly due to the avoidance of the use of buoys and concrete blocks in this phase.
In addition, pre-fattening within the lagoon means a significant reduction in the need to use boats. Proximity to the farm brings both economic and environmental benefits. The number of trips can be reduced by around 36% over the entire oyster farming cycle, resulting in lower fuel consumption. Diesel combustion is not only responsible for a portion of CO2 emissions, but is also the main cause of PMFP, POFP, AP and EP, and its low consumption leads to a reduction in its impact equal to 22–23% in all these categories. Prefattening reduces the working hours of farmers, which also has a positive effect on the social impacts generated by this activity.
Prefattening in lagoons, however, does not have a significant impact on land use because, as mentioned above, it is mainly affected by activities indirectly related to oyster farming and by the production of raw materials needed for the construction of facilities and materials.
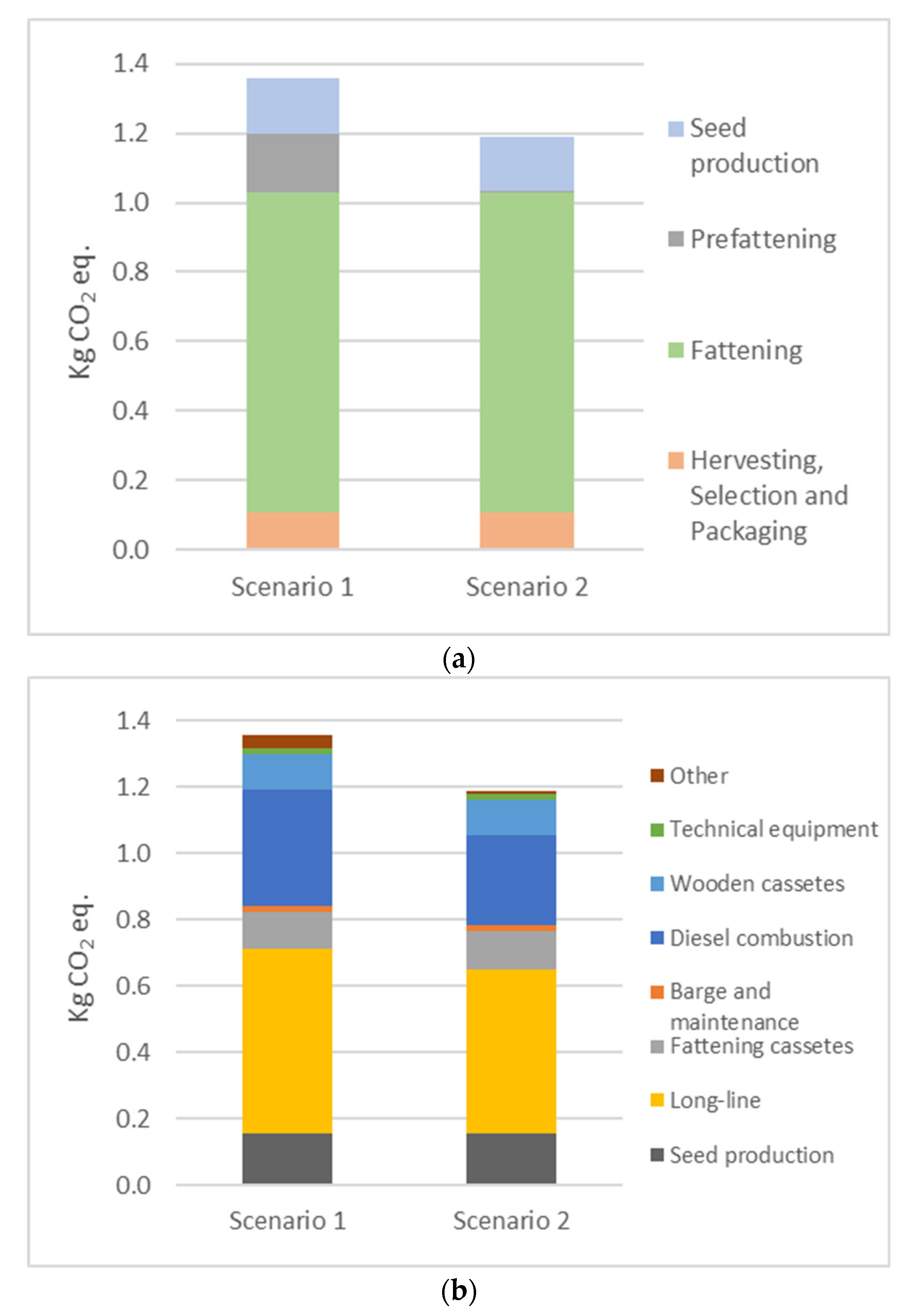
Thus, with the exception of land use, the environmental impact of prefattening in lagoons is lower than that of prefattening in the open sea, with an average reduction of around 9% in all impact categories. In particular, if only GWP100, EP, PMFP, POFP and AP are considered, the reduction is greater, at around 10–14% (
Figure 3).
In addition, a Monte Carlo (MC) simulation was performed to verify the significance of the reduction and the level of uncertainty in the LCI data. For this analysis, the Monte Carlo method was run with 1000 interactions at a 95% level of significance, and the results are shown in
Table 8. The standard deviation (SD) is the normalised indicator of the dispersion of the data around the mean, and the coefficient of variation (CV) indicates how large the standard deviation (SD) is.
The MC method suggests that the highest level of uncertainty is for HTP and MDP in both scenarios and for MAETP only in Scenario 1. The variability of the data set for the other impact categories is lower in both scenarios, especially for GWP100. Although all impact categories are important indicators of the potential environmental impact of a process or product, climate change is certainly one of the most representative [
40]. The amount of greenhouse gases in terms of CO
2 eq produced in Scenario 1 is 1.36 ± 0.046 kg CO
2 eq, while in Scenario 2, it is 1.19 ± 0.044 kg CO
2 eq. Therefore, the reduction in fuel consumption and the use of the long line plant leads to a significant reduction in GWP100, corresponding to about 12%. For the other impact categories, although there is an overall improvement in Scenario 2, the CV% values are too high compared to the difference in the mean impact values. In particular, after identifying hotspots in the oyster production process, a sensitivity analysis was carried out to see what improvements could be made to achieve a significant reduction in all impacts. Looking at the EP, PMFP and POFP indicators, the largest contribution comes from the use of fossil fuels. In Scenario 2, biodiesel consumption would need to be further reduced by at least 50% to achieve a significant reduction in these impact categories, in addition to a 10% reduction in GWP100. For the other impact categories, a total reduction in biodiesel is required to achieve a significant reduction in impacts, so it would be more appropriate to focus on other hotspots, such as replacing virgin plastic materials with more environmentally sustainable materials to increase the sustainability of the current oyster farming process and make this improvement statistically significant.
4. Conclusions
Oyster farming is a widespread activity throughout the world, especially in East Asia and Northern Europe. In Italy, the sector is still developing, but it has shown a growing interest among farmers and consumers. In fact, given the lack of sustainability analyses of the Italian production system and the incomparability of the processes currently used in Italy with those used abroad, it is important to accompany the development of the oyster farming sector with in-depth knowledge of the environmental impact. In particular, the Sacca di Goro is one of the most promising sites for Italian oyster farming, where oyster farming, with the exception of oyster seed production, is carried out entirely offshore. In order to reduce the environmental impact of oyster farming and to optimize the use of resources, fattening and pre-fattening phases play a crucial role. The strategy of moving pre-fattening from the open sea to the lagoon has been shown to improve the sustainability of the entire oyster farming process. In fact, all impact categories calculated in the LCA analysis showed a reduction in environmental impact, mainly due to the reduced use of longline and the reduced fuel consumption for boat trips. Furthermore, the reduction in the use of longlines is important not only in terms of not depleting primary materials, but also in terms of reducing the use of plastics. In fact, even though microplastics pollution is not calculated in an LCA, this aspect should have considered in a wider evaluation. Prefattening in lagoons also significantly reduces fuel consumption, with a direct effect on GWP reduction. Finally, environmental impact could be further reduced by progressively replacing plastic items derived from virgin-oil-derived materials, inherently unsustainable, with recycled ones, as well as evaluating the introduction of biodegradable plastics. Furthermore, this study has potential for improvement, as shown by the results of the uncertainty analysis. In fact, although LCA analysis is more effective for industrial processes than for activities in the development phase, the inventory is quite extensive, as it has been constructed on the basis of production information from four cooperatives, the only ones involved in oyster farming in the Sacca di Goro. It follows that an expansion of the sector, taking into account the limitations inherent to its industrialization, would lead to an increase in the data set and an even more accurate analysis.
In conclusion, oyster farming certainly represents a valuable opportunity for Goro and for Italy to introduce new species of commercial interest. Oyster farming is currently mostly linked to France, and so a targeted promotion of Italian oysters is needed to spread a new perception of the Italian product. Several factors provide potential opportunities to create an Italian oyster culture, with the advantage of diversification in a mono-economy context based on mussel or clam farming, as seen in Sacca di Goro, integrated with an overall territorial promotion perspective.