Production and Quality Characteristics of Royal Jelly in Relation to Available Natural Food Resources
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Bee Colonies and Collection of RJ Samples
2.2. Physicochemical Analyses
2.3. Meteorological Measurements
2.4. Statistical Analysis
3. Results and Discussion
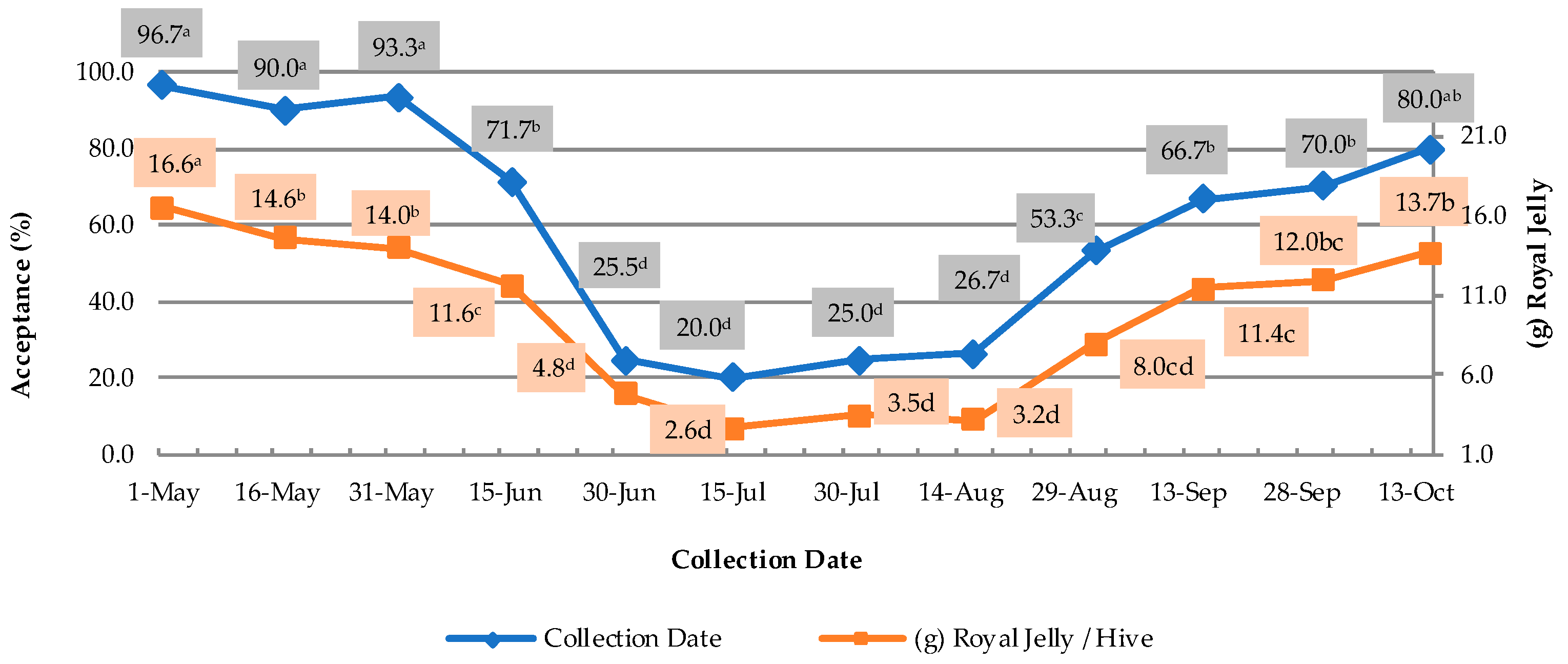
3.1. Production–Acceptance Rate
3.2. Determination of Moisture Content
3.3. Determination of Protein Content (%)
3.4. Determination of Sugars Concentration (%)
3.5. Determination of 10-HDA (%)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, F.L.; Bíliková, K.; Casabianca, H.; Daniele, G.; Salmen Espindola, F.; Feng, M.; Guan, C.; Han, B.; Kraková, T.K.; Li, J.-K.; et al. Standard methods for Apis mellifera royal jelly research. J. Apic. Res. 2019, 58, 1–68. [Google Scholar] [CrossRef]
- Erem, C.; Deger, O.; Ovali, E.; Barlak, Y. The effects of royal jelly on autoimmunity in Graves’ disease. Endocrine 2006, 30, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.A.E.-M. Immunomodulatory effects of royal jelly on aorta CD3, CD68 and eNOS expression in hypercholesterolaemic rats. J. Basic Appl. Zool. 2014, 67, 140–148. [Google Scholar] [CrossRef]
- Pa, Y.; Xu, J.; Chen, C.; Chen, F.; Jin, P.; Zhu, K.; Hu, C.W.; You, M.; Chen, M.; Hu, F. Royal jelly reduces cholesterol levels, ameliorates aβ pathology and enhances neuronal metabolic activities in a rabbit model of alzheimer’s disease. Front. Aging Neurosci. 2018, 10, 50. [Google Scholar] [CrossRef]
- Collazo, N.; Carpena, M.; Nuñez-Estevez, B.; Otero, P.; Simal-Gandara, J.; Prieto, M.A. Health Promoting Properties of Bee Royal Jelly: Food of the Queens. Nutrients 2021, 13, 543. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Singh, A.; Mangal, M.; Mangal, A.K.; Kumar, S. Food adulteration: Sources, health risks, and detection methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 1174–1189. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, A.G.; Marcazzan, G.L.; Caboni, M.F.; Bogdanov, S.; Almeida-Muradian, L.B. Quality and standardisation of Royal Jelly. J. ApiProd. ApiMed. Sci. 2009, 1, 16–21. [Google Scholar] [CrossRef]
- Chen, C.; Chen, S.Y. Changes in protein components and storage stability of Royal Jelly under various conditions. Food Chem. 1995, 54, 195–200. [Google Scholar] [CrossRef]
- Kanelis, D.; Tananaki, C.; Liolios, V.; Dimou, M.; Goras, G.; Rodopoulou, M.A.; Karazafiris, E.; Thrasyvoulou, A. A suggestion for royal jelly specifications. Arh. Hig. Rada Toksikol. 2015, 66, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Daniele, G.; Casabianca, H. Sugar composition of French royal jelly for comparison with commercial and artificial sugar samples. Food Chem. 2012, 134, 1025–1029. [Google Scholar] [CrossRef]
- Pina, A.; Begou, O.; Kanelis, D.; Gika, H.; Kalogiannis, S.; Tananaki, C.; Theodoridis, G.; Zotou, A. Targeted profiling of hydrophilic constituents of royal jelly by hydrophilic interaction liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2018, 153, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Virgiliou, C.; Kanelis, D.; Pina, A.; Gika, H.; Tananaki, C.; Zotou, A.; Theodoridis, G. A targeted approach for studying the effect of sugar bee feeding on the metabolic profile of Royal Jelly. J. Chromatogr. A 2020, 1616, 460783. [Google Scholar] [CrossRef]
- Dimou, M.; Tananaki, C.; Goras, G.; Kanelis, D.; Thrasyvoulou, A. The effect of pollen supplements on the determination of the geographical origin of royal jelly. J. Apic. Res. 2014, 53, 124–128. [Google Scholar] [CrossRef]
- Mazzei, P.; Piccolo, A.; Brescia, M.; Caprio, E. Assessment of geographical origin and production period of royal jelly by NMR metabolomics. Chem. Biol. Technol. Agric. 2020, 7, 24. [Google Scholar] [CrossRef]
- Yan, S.; Wang, X.; Sun, M.; Wang, W.; Wu, L.; Xue, X. Investigation of the lipidomic profile of royal jelly from different botanical origins using UHPLC-IM-Q-TOF-MS and GC-MS. LWT 2022, 169, 113894. [Google Scholar] [CrossRef]
- Bernués, A.; Olaizola, A.; Corcoran, K. Labelling information demanded by European consumers and relationships with purchasing motives, quality and safety of meat. Meat Sci. 2003, 65, 1095–1106. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.; Zhang, W.; Cui, X.; Wang, H.; Xu, B. Comparison of the nutrient composition of royal jelly and worker jelly of honey bees (Apis mellifera). Apidologie 2016, 47, 48–56. [Google Scholar] [CrossRef]
- Rehman, N.U.; Anjum, S.I.; Qureshi, N.A.; Khan, M.H.; Albasher, G.; Kaleem, M.; Kamal, A. The effect of larval age, and wet and dry grafting, on the rearing of queen bees using the Doolittle grafting method. Entomol. Res. 2024, 54, e12700. [Google Scholar] [CrossRef]
- Taber, S.; Polle, H.K. Rearing and mating of queen and drone honey bees in winter. Am. Bee J. 1974, 114, 18–19. [Google Scholar]
- Wang, Y.; Ma, L.; Wang, H.; Liu, Z.; Chi, X.; Xu, B. Effects of sucrose feeding on the quality of royal jelly produced by honeybee Apis mellifera L. Insects 2023, 14, 742. [Google Scholar] [CrossRef]
- Kanelis, D.; Tananaki, C.; Liolios, V.; Rodopoulou, M.A.; Goras, G.; Argena, N.; Thrasyvoulou, A. Investigating the effect of supplementary feeding on carbohydrate composition and quantity of royal jelly. Open J. Appl. Sci. 2018, 8, 141–149. [Google Scholar] [CrossRef]
- Hussain, A.R.E.; Abied, M.K.; Abo Laban, G.F.; Badwy, A. Effect of Different Seasons on the Royal Jelly production Under Nasr City Conditions—Cairo-Egypt. Egypt. Acad. J. Biol. Sci. A Entomol. 2020, 13, 197–205. [Google Scholar] [CrossRef]
- Fahad Raza, M.; Anwar, M.; Husain, A.; Rizwan, M.; Li, Z.; Nie, H.; Hlaváč, P.; Ali, M.A.; Rady, A.; Su, S. Differential gene expression analysis following olfactory learning in honeybee (Apis mellifera L.). PLoS ONE 2022, 17, e0262441. [Google Scholar] [CrossRef]
- Portman, Z.M.; Orr, M.C.; Griswold, T. A review and updated classification of pollen gathering behavior in bees (Hymenoptera, Apoidea). J. Hymen. Res. 2019, 71, 171–208. [Google Scholar] [CrossRef]
- Stanley, R.G.; Linskens, H.F. Storage. In Pollen Biology, Biochemistry, Management, 1st ed.; Stanley, R.G., Linskens, H.F., Eds.; Springer: Berlin/Heidelberg, Germany, 1974; pp. 56–66. [Google Scholar]
- Yang, K.; Wu, D.; Ye, X.; Liu, D.; Chen, J.; Sun, P. Characterization of chemical composition of bee pollen in China. J. Agric. Food Chem. 2013, 61, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Liolios, V.; Tananaki, C.; Dimou, M.; Kanelis, D.; Goras, G.; Karazafiris, E.; Thrasyvoulou, A. Ranking pollen from bee plants according to their protein contribution to honey bees. J. Apic. Res. 2015, 54, 582–592. [Google Scholar] [CrossRef]
- Cao, L.F.; Zheng, H.Q.; Pirk, C.W.; Hu, F.L.; Xu, Z.W. High Royal Jelly-Producing Honeybees (Apis mellifera ligustica) (Hymenoptera: Apidae) in China. J. Econ. Entomol. 2016, 109, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Popescu, O.; Mǎrghitaş, L.A.; Dezmirean, D.S. A study about physicochemical composition of fresh and lyophilized royal jelly. Zooteh. Si Biotehnol. 2008, 41, 328–332. [Google Scholar]
- Nabas, Z.; Haddadin, M.S.; Haddadin, J.; Nazer, I.K. Chemical Composition of Royal Jelly and effects of synbiotic with two different locally isolated probiotic strains on antioxidant activities. Polish J. Food Nutr. Sci. 2014, 64, 171–180. [Google Scholar] [CrossRef]
- Zheng, H.Q.; Hu, F.L.; Dietemann, V. Changes in composition of royal jelly harvested at different times: Consequences for quality standards. Apidologie 2011, 42, 39–47. [Google Scholar] [CrossRef]
- Balkanska, R.; Zhelyazkova, I.; Ignatova, M.; Kashamov, B. Effect of supplementary honey and artificial sugar feeding of bees on the composition of royal jelly. Agric. Sci. Technol. 2013, 5, 335–338. [Google Scholar]
- Liolios, V.; Tananaki, C.; Dimou, M.; Kanelis, D.; Rodopoulou, M.A.; Thrasyvoulou, A. Exploring the sugar profile of unifloral bee pollen using high performance liquid chromatography. J. Food Nutr. Res. 2018, 57, 341–350. [Google Scholar]
- Bacandritsos, N.; Saitanis, C.; Papanastasiou, I. Morphology and life cycle of Marchalina hellenica (Gennadius) (Hemiptera: Margarodidae) on pine (Parnis Mt.) and fir (Helmos Mt.) forests of Greece. Ann. Soc. Entomol. 2004, 40, 169–176. [Google Scholar] [CrossRef]
- Thrasyvoulou, A.; Tananaki, C.; Goras, G.; Karazafiris, E.; Dimou, M.; Liolios, V.; Kanelis, D.; Gounari, S. Legislation of honey criteria and standards. J. Apic. Res. 2018, 57, 88–96. [Google Scholar] [CrossRef]
- Tsavea, E.; Vardaka, F.-P.; Savvidaki, E.; Kellil, A.; Kanelis, D.; Bucekova, M.; Grigorakis, S.; Godocikova, J.; Gotsiou, P.; Dimou, M.; et al. Physicochemical characterization and biological properties of pine honey produced across Greece. Foods 2022, 11, 943. [Google Scholar] [CrossRef]
- Genç, M.; Aslan, A. Determination of trans-10-hydroxy-2-decenoic acid content in pure royal jelly and royal jelly products by column liquid chromatography. J. Chromatogr. A 1999, 839, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Ferioli, F.; Marcazzan, G.L.; Caboni, M.F. Determination of (E)-10-hydroxy-2-decenoic acid content in pure royal jelly: A comparison between a new CZE method and HPLC. J. Sep. Sci. 2007, 30, 1061–1069. [Google Scholar] [CrossRef]
- Mărghitaş, L.A.; Morar, O.; Bobiş, O.; Bonta, V.; Dezmirean, D.S. Comparative evaluation of chemical composition for three categories of royal jelly. J. Agroalim. Proc. Technol. 2010, 16, 399–401. [Google Scholar]
- Kolayli, S.; Sahin, H.; Can, Z.; Yildiz, O.; Malkoc, M.; Asadov, A. A Member of Complementary Medicinal Food: Anatolian Royal Jellies, Their Chemical Compositions, and Antioxidant Properties. J. Evid. Based Complement. Altern. Med. 2016, 21, NP43–NP48. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods. 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Wytrychowski, M.; Chenavas, S.; Daniele, G.I.; Casabianca, H.; Batteau, M.; Guibert, S.; Brion, B. Physicochemical characterisation of French royal jelly: Comparison with commercial royal jellies and royal jellies produced through artificial bee-feeding. J. Food Comp. Anal. 2013, 29, 126–133. [Google Scholar] [CrossRef]
- Kamyab, S.; Gharachorloo, M.; Honarvar, M.; Ghavami, M. Quantitative analysis of bioactive compounds present in Iranian royal jelly. J. Apic. Res. 2020, 59, 42–52. [Google Scholar] [CrossRef]
- Sesta, G. Determination of sugars in royal jelly by HPLC. Apidologie 2006, 37, 84–90. [Google Scholar] [CrossRef]
- Wang Wang, X.L.; Lin, H.; Ren, S.Y.; Ding, T.; Wang, Y.; Liu, Y.; Zhang, P.X.; Deng, X.; Yi, X.H.; Han, F. Determination of the 6 kinds of sugars content in royal jelly by high performance liquid chromatograph. J. Food Saf. Qual. 2021, 12, 4474–4479. [Google Scholar]
- Karlidag, S.; Koseman, A.; Akyol, A.; Saatcioglu, G.; Seker, I.; Uyumlu, A.B.; Yilmaztekin, M.; Erdogan, S. The effect of different industrial sugars on royal jelly production. J. Hell. Vet. Med. Soc. 2022, 73, 4989–4996. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J. Quantitative analysis of trans-10-hydroxy-2-decenoic acid in rojal jelly products purshased in USA by high performance liqued chromatography. J. Apic. Sci. 2010, 54, 77–85. [Google Scholar]
- Yang, X.; Li, Y.; Wang, L.; Liqunm, L.; Guo, L.; Yang, M.; Huang, F.; Zhao, H. Determination of 10-HDA in royal jelly by ATR-FTMIR and NIR spectral combining with data fusion strategy. Optik 2020, 203, 164052. [Google Scholar] [CrossRef]
- Keskin, M.; Ozkok, A.; Karahalil, F.Y.; Kolayli, S. What should be the amount of 10-Hydroxi-2-Decanoic Acid (10-HDA) in royal jelly? Mediterr. Agric. Sci. 2020, 33, 347–350. [Google Scholar] [CrossRef]







| Fresh Royal Jelly | ||||||
|---|---|---|---|---|---|---|
| Collection Date | Moisture (%) | Protein Content (%) | Fructose (%) | Glucose (%) | Sucrose (%) | 10-HDA (%) |
| 1-May | 68.1 ± 0.7 | 11.9 ± 0.4 | 3.5 ± 1.2 | 3.2 ± 0.9 | 0.4 ± 1.3 | 2.8 ± 1.7 |
| 17-May | 68.0 ± 0.6 | 12.7 ± 3.0 | 3.7 ± 1.1 | 2.9 ± 0.9 | 0.4 ± 1.7 | 3.5 ± 1.2 |
| 1-June | 67.3 ± 1.0 | 12.7 ± 1.2 | 3.6 ± 1.6 | 3.3 ± 1.6 | 0.4 ± 0.2 | 3.3 ± 1.4 |
| 16-June | 67.7 ± 0.6 | 12.4 ± 2.2 | 3.5 ± 0.7 | 3.0 ± 0.9 | 0.2 ± 0.8 | 3.5 ± 1.8 |
| 1-July | 65.9 ± 0.8 | 13.3 ± 1.3 | 3.2 ± 1.7 | 3.1 ± 1.2 | 0.4 ± 0.8 | 3.5 ± 0.6 |
| 15-July | 66.3 ± 1.1 | 13.4 ± 1.0 | 3.0 ± 1.9 | 3.1 ± 0.4 | 0.3 ± 0.5 | 3.8 ± 0.4 |
| 1-August | 66.7 ± 0.8 | 13.5 ± 1.6 | 3.2 ± 2.5 | 2.9 ± 1.6 | 0.2 ± 0.3 | 3.8 ± 0.2 |
| 16-August | 67.7 ± 0.8 | 13.1 ± 1.7 | 3.3 ± 1.3 | 3.6 ± 1.2 | 0.1 ± 0.4 | 3.6 ± 0.1 |
| 1-September | 67.9 ± 0.9 | 12.6 ± 1.5 | 3.2 ± 1.2 | 3.3 ± 1.1 | 0.3 ± 0.5 | 3.2 ± 1.3 |
| 15-September | 68.2 ± 0.7 | 12.2 ± 2.6 | 3.7 ± 1.0 | 3.2 ± 1.0 | 0.3 ± 0.5 | 3.5 ± 1.6 |
| 1-October | 68.6 ± 0.6 | 12.9 ± 1.6 | 3.2 ± 0.8 | 2.7 ± 0.7 | 0.0 ± 0.2 | 3.3 ± 0.6 |
| 15-October | 67.8 ± 2.2 | 11.6 ± 1.1 | 3.4 ± 2.2 | 3.4 ± 0.7 | 0.1 ± 0.3 | 3.2 ± 2.6 |
| Dry Matter of Royal Jelly | ||||||
| Collection Date | Moisture (%) | Protein Content (%) | Fructose (%) | Glucose (%) | Sucrose (%) | 10-HDA (%) |
| 1-May | - | 37.5 ± 0.2 | 10.9 ± 0.4 | 10.0 ± 0.3 | 1.3 ± 0.4 | 8.9 ± 0.5 |
| 17-May | - | 39.6 ± 0.8 | 11.6 ± 0.4 | 9.0 ± 0.3 | 1.2 ± 0.5 | 11.1 ± 0.4 |
| 1-June | - | 38.7 ± 0.6 | 10.9 ± 0.5 | 10.0 ± 0.5 | 1.4 ± 0.4 | 10.1 ± 0.5 |
| 16-June | - | 37.2 ± 0.3 | 10.5 ± 0.1 | 8.8 ± 0.3 | 0.6 ± 0.3 | 10.6 ± 0.5 |
| 1-July | - | 38.7 ± 0.2 | 9.2 ± 0.6 | 8.9 ± 0.5 | 1.0 ± 0.3 | 10.2 ± 0.3 |
| 15-July | - | 39.1 ± 0.3 | 8.7 ± 0.7 | 9.1 ± 0.1 | 0.8 ± 0.2 | 11.0 ± 0.2 |
| 1-August | - | 39.0 ± 0.6 | 9.1 ± 0.9 | 8.3 ± 0.5 | 0.5 ± 0.2 | 11.0 ± 0.1 |
| 16-August | - | 40.3 ± 0.6 | 10.1 ± 0.3 | 11.0 ± 0.4 | 0.3 ± 0.1 | 11.1 ± 0.1 |
| 1-September | - | 38.7 ± 0.2 | 9.8 ± 0.5 | 10.2 ± 0.3 | 0.9 ± 0.3 | 9.8 ± 0.3 |
| 15-September | - | 38.5 ± 0.6 | 11.7 ± 0.3 | 10.0 ± 0.3 | 1.0 ± 0.3 | 10.9 ± 0.5 |
| 1-October | - | 41.0 ± 0.7 | 10.1 ± 0.3 | 8.6 ± 0.3 | 0.1 ± 0.0 | 10. 5 ± 0.2 |
| 15-October | - | 36.2 ± 0.5 | 10.4 ± 0.8 | 10.5 ± 0.2 | 0.2 ± 0.1 | 9.8 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanelis, D.; Liolios, V.; Rodopoulou, M.-A.; Papadopoulou, F.; Tananaki, C. Production and Quality Characteristics of Royal Jelly in Relation to Available Natural Food Resources. Resources 2024, 13, 55. https://doi.org/10.3390/resources13040055
Kanelis D, Liolios V, Rodopoulou M-A, Papadopoulou F, Tananaki C. Production and Quality Characteristics of Royal Jelly in Relation to Available Natural Food Resources. Resources. 2024; 13(4):55. https://doi.org/10.3390/resources13040055
Chicago/Turabian StyleKanelis, Dimitrios, Vasilios Liolios, Maria-Anna Rodopoulou, Fotini Papadopoulou, and Chrysoula Tananaki. 2024. "Production and Quality Characteristics of Royal Jelly in Relation to Available Natural Food Resources" Resources 13, no. 4: 55. https://doi.org/10.3390/resources13040055
APA StyleKanelis, D., Liolios, V., Rodopoulou, M.-A., Papadopoulou, F., & Tananaki, C. (2024). Production and Quality Characteristics of Royal Jelly in Relation to Available Natural Food Resources. Resources, 13(4), 55. https://doi.org/10.3390/resources13040055






