Abstract
The circular economy and the clean-energy transition are inextricably linked and interdependent. One of the most important areas of the energy transition is the development of hydrogen energy. This study aims to review and systematize the data available in the literature on the environmental and economic parameters of hydrogen storage and transportation technologies (both mature and at high technological readiness levels). The study concluded that salt caverns and pipeline transportation are the most promising methods of hydrogen storage and transportation today in terms of a combination of all parameters. These methods are the most competitive in terms of price, especially when transporting hydrogen over short distances. Thus, the average price of storage will be 0.35 USD/kg, and transportation at a distance of up to 100 km is 0.3 USD/kg. Hydrogen storage underground in a gaseous state and its transportation by pipelines have the least consequences for the environment: emissions and leaks are insignificant, and there is no environmental pollution. The study identifies these methods as particularly viable given their lower environmental impact and potential for seamless integration into existing energy systems, therefore supporting the transition to a more sustainable and circular economy.
1. Introduction
The circular economy and the clean-energy transition are inextricably linked and interdependent. The energy transition and the transition to a circular economy are fundamental to a sustainable future [1]. On the one hand, the transition to a circular economy does not eliminate the need for energy [2]. Rather, it encourages the efficient use of energy, the reduction of primary energy consumption, and the use of waste heat and renewable energy [3]. On the other hand, the energy transition depends on the transition to a circular economy. With the rapid expansion of renewable energy infrastructure, the demand for several critical minerals is projected to increase tremendously [4]. Supply shortages are likely in the coming years. The energy sector cannot afford to use scarce materials just once [5,6]. Some researchers call this relationship the energy–circular economy nexus [7].
The development of hydrogen energy is now seen as an essential step in the energy-transition process [8]. The use of hydrogen can make a significant contribution to decarbonization [9], especially in those sectors of the economy where the introduction of “clean” energy sources is most challenging from a technical point of view or requires too many investment resources (energy-intensive industries or long-distance transportation) [10,11]. According to the International Energy Agency [12], 40 countries have already adopted strategies or roadmaps to develop hydrogen production, storage, transport, and end-use technologies. For example, the Hydrogen and Fuel Cells Program in the United States in 2004, the Hydrogen Industry Development Plan (2021–2035) adopted in 2022 in China, the Package for the Future—Hydrogen Strategy in 2021 in Germany, Hydrogen Strategy 2030 in 2020 in Portugal, etc. Despite differences in plans, all major economies expect hydrogen to play a significant role in their decarbonization.
To date, priority is given to so-called “low-carbon hydrogen”, which does not produce fossil fuels. Electrolysis technologies are well-developed to make “clean” hydrogen using wind, solar, hydro, or nuclear power. However, their practical application still needs to be improved due to high production costs [13]. In addition, a significant share of the final price of hydrogen is associated with its storage and transportation. Although the literature pays much attention to the problems of reducing production costs [14], the economic and environmental efficiency of hydrogen storage and transportation processes have yet to be studied.
Today, issues of transition to hydrogen energy take a significant place in the scientific literature, but most often, they are not considered in the complex of such problems as cost, technological readiness, and environmental safety. For best results, great attention should be paid to the combination of all these aspects of hydrogen storage and transportation. There is a gap in the scientific literature for such a comprehensive study, which we have tried to fill in this paper. This study aims to systematize the data available in the literature on the environmental and economic parameters of hydrogen storage and transportation technologies (both mature and at high technological readiness levels).
The rest of this paper is organized as follows: Section 2 presents the results of the literature review; Section 3 describes the methodology and data used to obtain the research results; Section 4 includes the results of the study, divided into the following parts: options for storing, transporting hydrogen; in Section 5 there is a discussion of the findings based on a combination of possible methods of transporting hydrogen depending on the storage technology, questions related to the environmental consequences of storing and transporting hydrogen occupy a special place; Section 6 concludes the paper with a summary of the key findings, study limitations, and directions for future work.
2. Literature Review
The transition towards a hydrogen-based energy economy highlights pivotal challenges and solutions, mainly focusing on the storage, transportation, and associated safety and environmental impacts of hydrogen. To systematically address these complexities, it is imperative to scrutinize the existing literature through a structured and detailed lens. This review is segmented into four distinct but interrelated sections: Methods of Hydrogen Storage, Hydrogen Transportation Technology, Hydrogen Transportation Safety, and Environmental Safety of Hydrogen Storage and Transportation.
Each of these sections delves into specific facets of hydrogen use in the energy transition, reflecting the breadth of technological intricacies and the depth of safety and environmental considerations. The Methods of Hydrogen Storage section explores various technologies and approaches to storing hydrogen effectively and efficiently, highlighting innovations and their economic implications. Hydrogen Transportation Technology focuses on the mechanisms and infrastructures necessary for moving hydrogen from production sites to points of use, which are critical for widespread adoption. Meanwhile, Hydrogen Transportation Safety and Environmental Safety of Hydrogen Storage and Transportation deal with the risk-management and mitigation strategies essential to ensure public and ecological well-being in the face of increasing hydrogen use. This categorical division not only clarifies existing technologies and issues but also sets the stage for identifying future research directions essential for advancing the role of hydrogen in sustainable energy systems.
2.1. Methods of Hydrogen Storage
One of the primary keys to fully developing the hydrogen economy is safe, compact, lightweight, and cost-effective hydrogen storage [15]. Development of the hydrogen economy requires different hydrogen storage systems: mobile and stationary [16]. Typically, hydrogen can be stored as a compressed gas, a cryogenic liquid, or physically or chemically bound to a suitable solid-state material [17].
The attractiveness of specific methods of storing and transporting hydrogen depends on many factors: price, infrastructure readiness, safety, environmental impact, etc. [18]. The choice of transport method depends on the distance, volume, and urgency of hydrogen delivery, as well as infrastructure and safety considerations [19].
To enable large-scale hydrogen storage in the renewable energy era, UHS (underground hydrogen storage) has attracted significant attention due to its cost-effectiveness and scalability [20]. Despite the tremendous opportunities offered by UHS, the maturity level is considered low [21], and therefore, UHS as a storage method is associated with a number of uncertainties and challenges [22]. Underground storage is likely to be the best solution for significant storage needs [23] as it has numerous advantages in terms of environmental protection, safety, and, above all, capital expenses (CAPEX) for high storage capacity and operating expenses (OPEX).
In addition, UHS offers security benefits and significant storage capacity, often orders of magnitude greater than land-based storage methods [24]. Underground hydrogen storage sites include salt caves, saline aquifers, and depleted hydrocarbon deposits [25].
Depleted hydrocarbon reservoirs are becoming attractive targets for USHS (Underground Seasonal Hydrogen Storage) due to their storage capacity, proven integrity, previous knowledge of reservoir characteristics, and existing infrastructure (e.g., gas pipeline network). However, their maturity is still considered low, with several uncertainties and issues [21]. From a geological perspective, underground formations are suitable for storing hydrogen, which can be used as a chemical energy carrier. They are kept for several months and recovered for re-electrification when needed [26].
Another underground storage that can be used under certain conditions is salt caverns as high-pressure gas storage [27]. Many years of operational experience with hydrogen storage in salt caves provides strong evidence of its feasibility and best practices [28]. Depending on energy storage capacity and release timing, hydrogen storage in salt caves can provide long-term, utility-scale energy storage to meet market demand. Salt caverns may hold great promise due to the self-compacting nature of salt and the ability to tune the size and shape of the caverns [29].
Compared to depleted oil and gas reservoirs, the key advantages of storing hydrogen in salt caverns are: the salt surrounding the caverns is virtually impervious to leakage since the only possibility of gas leakage is through leaky wells [29], the salt does not react with hydrogen [30], and the release of the hydrogen “blow” is very flexible in speed, duration and volume with less buffer gas requirements to avoid rock failure. Caverns are a mature and cost-effective storage technology that has been successfully used to store compressed gases for over 75 years and can potentially expand to USHS. However, the inaccessibility of salt caves in hydrogen production may become a limiting factor.
Among hydrogen storage systems, metal hydride-based solid-state storage systems show more potential [31]. Solid-state hydrogen storage is a possible breakthrough in realizing the unique future of hydrogen as a clean fuel [32]. However, to date, none of the presented metal hydrides meet all the requirements for the hydrogen economy, mainly due to low hydrogen storage capacity, sluggish kinetics, and unreasonable hydrogen absorption (de)sorption temperatures [33,34]. Depleted gas fields, rock caves, and liquid organic H2 carriers may offer economical options but still need to be technologically viable [35].
2.2. Hydrogen Transportation Technology
Hydrogen energy transport technology can be divided into several types: high-pressure hydrogen gas, liquid hydrogen at low temperatures, hydrogen from solids, organic liquid hydrogen, and mixed with natural gas [36]. The most reasonable solution for a cost-effective supply of hydrogen energy is to transport hydrogen-mixed natural gas using the existing distribution network or transport pure hydrogen after minor modifications to the existing natural gas distribution network [37]. The key to limiting the development of hydrogen energy remains to achieve large-scale, safe, and reliable transportation of hydrogen energy with good economic benefits [38]. From the point of view of large-scale and long-term development of hydrogen energy, methods for transporting gaseous hydrogen at high pressure and liquid hydrogen at low temperatures have high costs and low efficiency. Pipeline hydrogen transport will be an inevitable trend in future development. However, in some countries, the pipeline transportation of pure hydrogen cannot meet the needs of hydrogen energy development due to its high cost. The hydrogen economy requires an extensive gas transportation infrastructure. It has been suggested that existing networks of natural gas pipelines could be used to transport hydrogen [39]. The most sensible solution for the future of cost-effective, large-scale, and sustainable hydrogen energy supply is to transport hydrogen-blended natural gas using the existing natural gas distribution network and infrastructure or to transport pure hydrogen after minor modifications to the existing natural gas distribution network and infrastructure [40]. Pipelines or tanks can also be used for above-ground hydrogen storage. These storage systems are more easily deployed than underground solutions. Still, they are more expensive than underground storage options, ranging from USD 930 to USD 2200/kg H2 storage [41].
The cost of transporting pure hydrogen through a pipeline is 30–50% higher than natural gas [42].
The best-known method of transporting hydrogen is by using hydrogen pipelines. An analysis of studies by various international organizations has shown the following: there is a consensus on the competitiveness of hydrogen pipelines, but the European Commission sees them as attractive for transportation distances of up to 3000 km, IRENA for distances from 3000 to 8000 km, and IEA for up to 8000 km; for distances outside the competitive zone of hydrogen pipelines, IRENA sees the most attractive solutions using ammonia, IEA high-molecular-weight hydrocarbons, and the European Commission cryo-transport; however, it stipulates that at distances over 16,000 km, this method is inferior to ammonia and high-molecular-weight hydrocarbons [43].
2.3. Hydrogen Transportation Safety
Safety in hydrogen transport is a focus of research worldwide. There are several serious issues; for example, when interacting with a hydrogen mixture, the interaction of hydrogen with a pipe leads to the deterioration of mechanical properties such as hardness, ductility, and toughness, which affects the safety of pipelines [44,45]. Mixing hydrogen with natural gas significantly increases the flame speed and temperature, resulting in violent combustion and even explosion [46]. Legally binding vehicle approval and use criteria are needed to achieve safety in hydrogen transport [47].
Automotive transport for liquid hydrogen has advantages and promise but faces serious ventilation problems [48]. The effectiveness of the ventilation system for the safety of hydrogen storage and transportation can be achieved by correctly selecting ventilation systems for enclosed spaces by adequately selecting the ventilation system in enclosed spaces [49]. Transmitted hydrogen losses are possible through the pipeline wall due to diffusion. The main issues that need to be resolved are related to the influence of hydrogen on the mechanical properties of steels, including those used for the manufacture of pipelines, as well as justification for the possibility of using existing gas pipelines for transporting compressed hydrogen [50].
A limitation of the use of hydrogen is its cost during production, storage, and transportation. In addition to the factors determining production costs, the form of hydrogen storage and the chosen method of transporting it to the consumer also influence the final price of hydrogen (if necessary). The priorities for today are clean hydrogen, low cost, efficient, and safe hydrogen delivery and storage. The goals outlined in the US Department of Energy’s Hydrogen Program are to reduce the cost of delivering hydrogen to USD 2 per kg. by 2030 [41].
2.4. Environmental Safety of Hydrogen Storage and Transportation
Issues related to the environmental consequences of hydrogen storage and transportation occupy a special place. Greenhouse gas emissions from hydrogen production, storage, and transportation are dominated by those associated with capital costs. However, most researchers believe they should be improved over fossil energy sources and alternatives [51]. Hydrogen emissions from infrastructure can be divided into intentional (ventilation, blowdown), unintentional (residual hydrogen, boil-off), and leaks from pipelines, equipment, and diffuse penetration [52].
At the same time, any hydrogen transportation and storage method requires special attention to safety requirements. Upon contact with air, it forms an explosive mixture—an explosive gas classified as a substance of a high-hazard class. It raises uncertainty about the impact of hydrogen on emissions into the atmosphere.
On the one hand, using hydrogen in clean fuel cells reduces emissions of local air pollutants. On the other hand, hydrogen emissions from leaks in its storage and transportation systems are expected to change its atmospheric concentration and behavior.
It is worth paying attention to the main problems of transport and storage of hydrogen in comparison with natural gas:
- -
- high “percolation” of liquid hydrogen at temperatures above minus 253 degrees Celsius due to the small size of its molecules;
- -
- embrittlement and destruction of metals under the influence of atomic hydrogen;
- -
- explosion and fire hazard arising from mixing hydrogen with oxygen.
All these problems give rise to the study of new transportation and storage methods and the development and application of new technologies and materials.
The report “Atmospheric Consequences of Increased Hydrogen Use” explains that hydrogen is an indirect greenhouse gas that reacts with other greenhouse gases in the atmosphere, increasing their global warming potential. The study states that the global warming potential of hydrogen ranges from 6 to 16, with the average being 11, while the global warming potential of CO2 is one [53]. All this confirms that it is necessary to minimize leaks from pipes and equipment since any hydrogen leakage will lead to indirect global warming, offsetting the reduction in greenhouse gas emissions achieved by switching from fossil fuels to hydrogen, the study notes.
The report «Fugitive Hydrogen Emissions in the Future Hydrogen Economy» states that with 99% confidence, electrolytic hydrogen production will result in 9.2% of the hydrogen produced being released into the atmosphere through “venting and purging.” At the same time, the maximum hydrogen leakage can occur when transporting it by tankers, with up to 13.2% of its cargo entering the air. Also, large amounts of hydrogen can enter the atmosphere from underground compressed gas storage facilities (6.52%), fuel cells (2.64%), and gas stations [53].
CICERO (Centre for International Climate and Environmental Research) calculated that the greenhouse effect of hydrogen was almost 12 times stronger than carbon dioxide. Experts used five models to analyze atmospheric chemistry to determine the impact of hydrogen interacting with methane, ozone, and water vapor. The authors say the total amount of emissions (leaks) from existing hydrogen systems is unknown. A 1% leak is a “best-case scenario”, but in some cases, it can be as high as 10%.
A leak of 1% would add only about 0.025 °C to global warming by 2050, but a 5% or 10% leak could raise average global temperatures by more than 0.1 °C or 0.4 °C, respectively.
Hydrogen is prone to material damage, which may lead to leakage. High-pressure leaking hydrogen is highly susceptible to spontaneous combustion due to its combustion characteristics, which may cause jet fire or explosion accidents, resulting in severe casualties and property damage [46].
The lack of accurate data to quantify hydrogen leakage across the value chain remains a significant challenge in understanding the magnitude of the impact; a critical first step is to develop technologies that can be used in the field to accurately determine the extent of hydrogen leakage [54].
Thus, the existing literature provides different estimates of the environmental and environmental impacts of hydrogen storage and transport technologies. The optimal technological chain has not yet been determined. In addition, many innovative technologies for storing and transporting hydrogen still need higher technology readiness levels. Therefore, their economic and environmental parameters can only be assessed roughly.
3. Materials and Methods
The main method of this study is a meta-analysis of data on economic and environmental parameters of existing hydrogen storage and transportation technologies. Various literature sources, analytical reports, and company data were used as the basis for the meta-analysis. All data were converted into comparable units to obtain average estimates. In addition, we considered data on the safety and technological maturity of each of the technologies studied. For these parameters, however, a simple systematization and summarization of the available information was performed, as there is not enough data for a full meta-analysis in this case. The data sources are summarized in Table 1.

Table 1.
Data sources for meta-analysis of cost, technological readiness, and emissions from hydrogen storage and transportation.
To identify the prospects for hydrogen energy, we considered not only the current cost of hydrogen storage and transportation but also the expected cost.
To assess technological readiness, we used the technology readiness level (TRL) scale offered by IRENA. It is now widely used by research institutes and technology developers around the world to identify research priorities and develop programs to support innovation.
The technology path begins with defining its basic principles (TRL 1). As the concept and application mature, the technology progresses to TRL 2 and reaches TRL 3 when a proof-of-concept experiment is conducted. The technology then enters a phase where the concept must be validated, from a prototype developed in a laboratory setting (TRL 4) to testing in the environment in which it will be deployed (TRL 6). The technology then moves to the demonstration phase, where it is tested in a real-world environment (TRL 7), eventually reaching a first-of-its-kind commercial demonstration (TRL 8) on its way to the entire retail operation in the relevant environment (9 TRL). More than reaching the stage where technology can be considered commercially available (TRL 9), it must describe its readiness to meet energy policy objectives, for which scale is often critical. The IEA has expanded the TRL scale to include two additional readiness levels: one where the technology is commercial and competitive. However, this requires further innovation efforts to integrate the technology into energy systems and value chains once it is implemented at scale (TRL 10) and once the technology achieves predictable growth (TRL 11) [58].
Next, we conducted a comparative analysis of combinations of possible options for storing and transporting hydrogen and identified the most competitive ones.
For the overall TRL assessment of a combined hydrogen storage and transportation method, we assessed availability as low if at least one component of this method was assessed as low availability.
For ease of perception and meaningful interpretation of the research results, we used the following notations for methods of storing and transporting hydrogen (Table 2).

Table 2.
Accepted designation.
Through comprehensive comparative analysis, this study systematically evaluated a range of hydrogen storage and transportation methods, drawing on a rich ensemble of data sources and applying a robust meta-analytical framework. Each method was scrutinized for its potential benefits and constraints, focusing particularly on costs, technological readiness, and safety measures that are critical for scalable, sustainable deployment.
4. Results
4.1. Hydrogen Storage Options
According to the US Department of Energy classification, hydrogen fuel storage methods can be divided into two groups.
The first group includes physical methods that use physical processes (mainly compression or liquefaction) to convert hydrogen gas into a compact state. Storing hydrogen in compressed gas can be in massive stationary systems, including gas holders, underground tanks, gas cylinders, pipelines, and glass microspheres. Liquid hydrogen is stored in stationery and transportable cryogenic containers.
The second group includes chemical methods in which hydrogen storage is ensured by physical or chemical processes of its interaction with certain materials. These methods are characterized by the strong interaction of molecular or atomic hydrogen with the support material. We can distinguish the following options: reserves of adsorbed hydrogen in zeolites, related compounds, activated carbon, and hydrocarbon nanomaterials. In practice, the following types of storage are used: metal hydrides, ammonia, sponge iron, and others.
4.1.1. Underground Storage
Underground storage may be an option for large-scale hydrogen storage, especially in locations with suitable geoecology. This storage method has been around for quite some time. Natural gas fields stored hydrocarbons hermetically for millions of years before commercial exploitation of the deposits began. In this regard, the same geological formations can hold hydrogen for seasonal storage and cyclic operation. However, because hydrogen has a greater diffusivity than methane, it is necessary to assess the scale of its losses [59].
At the same time, underground storage offers significant economies of scale, high efficiency (the amount of hydrogen injected divided by the amount that can be recovered), low operating costs, and low land costs. These characteristics mean they are likely to be the cheapest hydrogen storage option, even though hydrogen has a low energy density compared to natural gas.
In turn, porous and fractured media (depleted gas, gas condensate, and oil fields; aquifers), underground reservoirs in rock salt (salt caverns), and exhausted mine workings are used as underground storage facilities. Hydrogen can be stored in underground caves or geological structures in one of four ways [60].
Salt Cavern Storage
The easiest way to store hydrogen is in salt cavern storage. These are artificial cavities in underground salt formations created by injecting fresh or low-salinity water down a well down to the salt geologic layer to extract the brine. There are more than 1900 salt caves worldwide. Creating a 500,000 m3 salt cave takes from six months to two years. Salt caves are not lined, as the salt acts as a sealant. This type of storage is suitable for storing hydrogen at extremely high pressures when the salt layer is deep enough. Salt cave storage is flexible and allows for several cycles of gas injection and withdrawal per year. The disadvantage of this storage method is that the geographical accessibility of the salt caves is limited.
Depleted Gas Field Storage
The second underground storage method for large amounts of hydrogen is injecting pure hydrogen into the porous rock in a depleted gas field storage. Depleted natural gas reservoirs are underground geological structures that naturally contain hydrocarbons and, once finished, can be used for gas storage. Hydrogen content can vary from a few percent to 100 percent. Depleted reservoirs consist of porous, permeable sedimentary rocks located under an impermeable seal and closed on all sides by impermeable rocks. The share of buffer gas in pore storage is usually 50–60% of their total gas capacity, which is higher than in salt caverns.
However, this storage method still needs further study, given the lower viscosity of hydrogen, as it is more difficult to contain than natural gas. Additional verification and testing are required in the laboratory and actual underground conditions. The advantages of depleted gas fields as hydrogen storage are that they are more volumetrically significant than salt caves, and their geology is already well understood through natural gas exploitation. Compared to developing new salt caves, they already have an infrastructure of natural gas wells, some of which could be upgraded or repurposed to produce hydrogen.
Aquifer Storage
Aquifers are similar to natural gas reservoirs in that they are porous sedimentary rock structures but contain water instead of natural gas. Porous aquifer rocks determine storage capacity. The main requirements for storage are the presence of a dome-shaped reservoir or a structural fault that ensures gas capture at the top of the structure, as well as the presence of a tire over the reservoir consisting of an impermeable layer. All problems identified for depleted gas fields apply to aquifers, but aquifers present additional challenges. The aquifers must be better developed and have production wells or surface structures. Because aquifers contain liquid, water movement can result in significant hydrogen uptake during hydrogen injection, resulting in hydrogen loss. Compared to salt caves and depleted gas reservoirs, aquifers have the advantage of being more widely accessible.
Lined Hard-Rock Cavern Storage
Finally, hydrogen can be stored underground by pumping directly into a Lined hard-rock cavern storage (LRC). Lined hard-rock caverns are artificial structures created in metamorphic or igneous rocks in geographic areas where salt or depleted deposits cannot be developed. These are artificial tunnels and caves built in low-permeability rock formations. Reliable containment of gases is usually achieved by lining the cave walls with gas-impermeable material. It may be a form of compressed storage (hydrogen gas) or cryogenic storage (liquid hydrogen). Storing hydrogen in a lined rock cave involves several technical difficulties that have yet to be resolved.
Because hard-rock caverns are carefully lined, they are not at risk of contamination and can be operated at higher pressures than other structures. However, steel embrittlement due to hydrogen must be avoided. Hard-rock caverns can undergo multiple injection and withdrawal cycles yearly, making them well-suited to peak loads. They require relatively small amounts of buffer gas but are expensive to develop. Compared to salt caverns or depleted deposits, rock caverns are mined at shallower depths (up to several hundred meters) and require shallow foundation rock, which is only sometimes available.
These four underground hydrogen storage methods differ in technology readiness level (TRL) and cost.
The only underground storage technology that can be considered commercially available is storage in salt caverns (Table 3).

Table 3.
Technological readiness levels of underground hydrogen storage facilities.
According to various estimates, the cheapest underground storage is salt caverns (Table 4).

Table 4.
Costs of hydrogen storage (underground storage), USD/kg.
Barring new design standards, capital costs for salt caverns and porous hydrogen reservoirs should remain the same as for underground natural gas storage. Capital costs for salt caves can range from USD 35 to USD 38/kg H2 stored, depending on storage pressure. Other underground storage solutions, such as lined rock caves, require higher costs, ranging from USD 56 to USD 116/kg H2 [62]. Mine cavern solutions will be more expensive than salt cavern solutions because the drill and blast method cannot compete with the leaching method of salt mining. Inserts will also require additional costs, both for purchase and installation. Thus, the cost of a pressurized hydrogen or ammonia LRC is expected to be 50–100% higher than the cost of an unlined rock cave, while the cost of a cryogenic hydrogen LRC is expected to be 100% higher than the cost of an LRC with cryogenic hydrogen. Underground storage in salt caves would be the preferred solution as it offers significant cost savings and flexibility.
However, hydrogen storage in salt caverns poses several problems that require further research. In salt caves, hydrogen leaks are possible due to the permeability of the base and leaks associated with the process plant at the surface—venting and purging due to maintenance or malfunction.
Leakage due to salt permeability or pipe/wellhead leakage is predicted to be negligible and is estimated at 1.76 × 10−11 kg/s per cavern [63]. Most of the releases will come from the surface treatment facility and include intentional releases from the planned annual shutdown of the entire plant, annual releases from component maintenance, etc. According to conversations with industry experts, hydrogen leakage from a typical onshore installation serving 15–20 caverns would be about 25 tons per year. The above-ground power plant, serving 17 caverns, will have a total storage capacity of approximately 2.6 TWh or 65,000 tons annually. Thus, the hydrogen loss is predicted to be about 0.04% [64].
4.1.2. Pressure Vessel Storage (Containers)
Pressurized gas containers are the most common hydrogen storage technology and involve the physical storage of compressed hydrogen gas in high-pressure tanks for stationary or mobile (such as tube trailers) applications. There are different types of containers, depending on the amount of hydrogen they can hold and the pressure. This storage method has been used for quite some time and mainly consists of all-steel tanks.
According to the IEA, this method of hydrogen storage is at TRL 11. This technology has matured; it is widely used, and only minor innovations are expected.
The cost of hydrogen storage is relatively low, where the method is already widely used. Also, as in the case of salt caverns, the cost of storing H2 in this case, namely in pressure containers, is <1/kg (Table 5).

Table 5.
Costs of hydrogen storage (Pressure vessel storage (containers)), USD/kg.
This method of hydrogen storage has relatively high storage losses of 1–3% per day. At the same time, no CO2 emissions [43].
4.1.3. Ammonia Storage
This storage method has been used for approximately 100 years. Hydrogen was initially stored in pressurized systems, typically with a capacity of about 2000 tons. Today, atmospheric hydrogen storage tanks store up to 50,000 tons. Low-pressure hydrogen storage has become widespread because it requires much less capital per unit volume.
According to the IEA, this method of hydrogen storage is at TRL 11. This technology has matured; it is widely used, and only minor innovations are expected.
However, the cost of storing ammonia is higher than in underground storage and gas containers (Table 6).

Table 6.
Costs of hydrogen storage (Ammonia storage), USD/kg.
Ammonia is much more expensive and has more stringent safety regulations and concerns. Storage loss <0.5% per day. At the same time, no CO2 emissions [43].
4.1.4. Liquid Organic Hydrogen Carriers (LOHC)
Liquid organic hydrogen carriers (LOHC) are organic molecules that can store hydrogen through a catalytic exothermic hydrogenation reaction at a certain pressure and moderate temperature to produce a hydrogen-rich molecule, releasing heat. LOHCs must provide sufficiently high hydrogen storage capacity (>5.5 wt.%), be safe to handle (non-toxic, non-flammable, non-explosive), be available in large quantities, and be inexpensive. However, currently, the hydrogenation and dehydrogenation processes require energy corresponding to approximately 35–40% of the energy content of stored hydrogen because, among other things, dehydrogenation temperatures are high.
According to the IEA, this hydrogen storage method is at TRL 6–7, i.e., this technology is at the testing and demonstration stage. The research aims to improve the overall efficiency of LOHC use by finding improved catalysts that enable dehydrogenation at lower temperatures (<150 °C) with limited use of precious metals, efficient heat management, and higher hydrogen recovery rates after purification.
Hydrogen storage losses <0.5% per day. This method involves emissions of CO and CO4 [43]. Today, this is the most expensive method of storing hydrogen (Table 7).

Table 7.
Costs of hydrogen storage (LOHC), USD/kg.
4.1.5. Liquid Hydrogen Storage
Large-scale liquid hydrogen storage technology today is similar to that of the 1960s; however, innovation in design is required to increase tank size further.
The density of pure hydrogen increases due to its liquefaction due to the low boiling point of hydrogen (−253 °C) compared to natural gas (−162 °C); cryogenic storage tanks are designed to minimize flash gas and prevent heat loss. However, liquefaction is an energy-intensive process compared to compression.
According to the IEA, this method of hydrogen storage is at TRL 8–9. The technology is heading from commercial demonstration (TRL 8) to entire commercial operation in the appropriate environment (TRL9). Liquid hydrogen storage has one of the highest costs (Table 8).

Table 8.
Costs of hydrogen storage (Liquid hydrogen storage), USD/kg.
Storage losses are 0.06–3, an additional 15% when refrigerated per day. At the same time, no CO2 emissions [43].
4.1.6. Adsorbents Storage
A relatively new chemical method for storing hydrogen is hydrogen adsorption. The hydrogen capacity of sorbent-based systems is intermediate between compressed gas and intermetallic compounds (metal hydrides), suggesting the transfer of hydrogen molecules to the surface of the pores of solid materials due to physical interaction and the subsequent release of hydrogen, when necessary, by thermal stimulation or other methods.
According to the IEA, this hydrogen storage method is at TRL 2–3. This technology is experimental and has a long way to go before commercialization.
Adsorption hydrogen can be transported in special containers or tanks using adsorbents to ensure its retention. Special precautions are required to avoid leaks and ensure safe transport. To date, it is impossible to assess its cost and environmental impact.
4.1.7. Metal Hydrides Storage
It is the chemical storage of hydrogen by absorption/desorption, which involves the chemical bonding of atomic hydrogen within the structure of a solid material. Hydrogen from metal hydrides can be released in two ways: mainly by heating (thermolysis) or by reaction with water (hydrolysis).
According to the IEA, this method of hydrogen storage is at TRL 4–5. The technology is entering a phase where the concept itself must be proven, from a prototype developed in a laboratory setting to testing in the conditions in which it will be deployed. Primary research aims to improve hydrogen absorption/desorption kinetics at moderate temperatures and high storage capacity by adding catalysts, doping with other elements, and nanostructuring. Due to low technological readiness, this storage method has yet to have a cost estimate.
Storing hydrogen in metal hydrides does not pose any safety concerns. It is stored at low pressure, relatively low temperatures are used, and hydrogen is released from the material only when heated. Storage loss <0.5% per day. At the same time, no CO2 emissions [43]. Thus, today, the most outstanding technological readiness among hydrogen storage methods is ammonia storage, pressure containers, salt cavern storage, and liquid hydrogen storage. Other storage methods still have several stages before they are commercially viable.
The selection of the most appropriate method will depend on specific conditions and requirements such as required capacity, availability, cost, security, and other factors. A comparative analysis of hydrogen storage methods is presented in Table 9.

Table 9.
Main advantages and disadvantages of storage methods.
Thus, in terms of cost, the cheapest methods are those already using salt cavern storage and pressure containers. These methods are already well known. From a safety point of view, the safest methods are underground vision and the most dangerous—ammonia storage.
4.2. Hydrogen Transportation Options
To transport H2, traditional delivery methods for gas raw materials can be used: in gaseous form using pipelines, as well as in compressed or liquefied form using land (road, rail) and sea transport using container shipping, as well as in cryogenic tanks or carriers such as ammonia or metal hydrides.
4.2.1. Transportation through Pipelines
Pipelines remain the primary method of transporting compressed hydrogen. Several options exist for pipeline transportation of hydrogen gas: through special hydrogen pipelines (new hydrogen pipelines) and existing natural gas pipelines, for which they are being repurposed (repurposed natural gas pipelines).
Repurposing involves converting an existing gas pipeline into a dedicated hydrogen pipeline. Due to differences in chemical properties, hydrogen can accelerate the deterioration of pipes through a process known as hydrogen embrittlement, in which hydrogen causes cracks in steel. There are still problems with repurposing offshore gas pipelines since monitoring the pipeline using current technologies is complex, and sometimes there is a lack of detailed documentation of the pipeline’s operation over recent years—repurposed natural gas pipelines—TRL 8.
Although an established technology, the construction of hydrogen pipelines has characteristics that differ from existing hydrogen pipelines compared to those required for new pipelines. Currently, the largest hydrogen pipelines are 18 inches in diameter, while new hydrogen pipelines may be up to 36–48 inches in diameter. Low grades of steel are used (usually below X52), whereas, in new pipelines, higher grades of steel may be preferred to reduce the amount of steel required without compromising integrity; existing pipelines operate under static load conditions, while future pipelines must be able to withstand pressure changes due to cyclic loading and linear packing. In addition, there is no standard for constructing offshore hydrogen pipelines. Research is underway to determine criteria that will ensure the highest level of safety while reducing costs—new hydrogen pipelines—TRL 9. By 2040, experts predict a 23,000 km hydrogen network, 75% of which will consist of converted gas pipelines.
The use of pipelines has several advantages:
- Highest cost-effectiveness for large volumes of hydrogen;
- There are no thermodynamic limitations to reducing transportation costs;
- Low power consumption;
- Transportation safety;
- Environmentally friendly;
- Use of existing pipeline systems for natural gas and oil.
At the same time, this method of transportation also has several disadvantages, for example:
- Significant capital investments in the construction of special pipelines;
- Very high transportation costs for small volumes;
- Complex and expensive procedure for obtaining permits for land acquisition, construction, etc.;
- Geographical accessibility.
According to IRENA estimates, capital costs for constructing 20-inch gas pipelines range from 600,000 to 1,600,000 USD/km, and for completing 40-inch ones, they range from 1,500,000 to 4,400,000 USD/km.
The cost of transporting hydrogen through 20-inch hydrogen pipelines will be 0.2 USD/kg over a distance of 100 km; 0.2–4.2 USD/kg for a distance from 100 to 2000 km; 4.2–16 USD/kg for a distance from 2000 to 8000 km. The cost of transporting hydrogen through 48-inch hydrogen pipelines will be 0.4 USD/kg over a distance of 100 km; 0.4–1.2 USD/kg for a distance from 100 to 2000 km; and 1.2–3.4 USD/kg for a distance from 2000 to 8000 km [43]. Thus, for short distances, this method of transportation has no price competitors.
Many authors propose to solve the problem of preventing leaks and emissions of hydrogen into the environment by installing highly sensitive automation and many shut-off valves and fittings capable of shutting off the hydrogen supply in short sections of the pipe. Hydrogen pipelines pose a particular danger as critical infrastructure facilities that can be damaged as a result of terrorist attacks or during military operations.
4.2.2. Transportation of Hydrogen Fuel for Freight Transport
The most traditional way to transport hydrogen gas is by truck (Truck transport). It includes road transport and railway transport.
Hydrogen is transported in special tubes or containers. Trucks that transport hydrogen gas in steel tubes compress it to a pressure of about 180–250 bar, carrying about 380 kg on board and limited by the weight of the tubes. However, recently, lightweight composite storage vessels with a capacity of 560–900 kg of hydrogen per trailer (350–500 bar) have been increasingly used, significantly increasing transport efficiency per trip. Large volumes of hydrogen can also be transported in cryogenic vessel trailers, which can carry around 1500–3000 kg of hydrogen per trip. Liquid hydrogen trailers are thermally insulated to minimize the hydrogen boil-off rate.
Rail transport for the transportation of liquid hydrogen is used quite limitedly due to the small branching of transport railway lines. In cryogenic railway tanks, hydrogen losses are approximately the same as in road tanks. With a single cooling in tank trucks, up to 15% of hydrogen is lost, and losses associated with imperfect thermal insulation amount to 0.5% per day of the volume of transported hydrogen.
According to IRENA, the readiness level of this transportation technology is the highest, 11.
The US Department of Energy estimates the cost of transporting 1 kg of hydrogen in a gaseous state by road over a distance of 100 km at 3–5 USD; by rail, it is 2.1–2.4 USD. A hydrogen decompression installation additionally increases the delivery cost by 0.4–0.8 USD/kg.
Delivery by road transport is more expensive than delivery by pipeline. Also, due to the low density of hydrogen, a large volume of containers or tanks is required for transportation, and these are additional costs because a small volume of product is transported in one cycle. The cost of transporting cylinders with compressed hydrogen will be 0.8–4 USD/kg over a distance of 100 km; 10–50 USD/kg for a distance from 100 to 2000 km; 50–150 USD/kg for a distance from 2000 to 8000 km [43].
The US Department of Energy estimates the cost of hydrogen liquefaction at 2.75 USD/kg and the regasification of liquid hydrogen at 0.39 USD/kg. Over short distances, liquid hydrogen is transported, especially in equipped tank trucks or by rail. The costs of such transportation are comparable to balloons.
Transportation of hydrogen cylinders is carried out with the obligatory presence of spacers and unique strapping inside the container, which is necessary for safety reasons; the containers are specially marked.
There are no emissions or leaks of hydrogen from the cylinders themselves, but they are from freight transport.
Hydrogen-pressurized trailers are effective in meeting the needs of small consumers, and the lack of waste can offset the high delivery costs. It is currently the easiest method, especially in areas without pipelines.
There is evidence that hydrogen losses during transportation in cryogenic tanks due to imperfect thermal insulation amount to approximately 0.5% per day of the volume of transported hydrogen. In addition, up to 15% is lost during a single cooling of a cryogenic tank.
Hydrogen can also be delivered by truck in metal hydrides. However, since the technologies for storing hydrogen in metal hydrides are in the early stages of readiness, there are still very few practical assessments of their transportation.
The cost of creating hydride compounds ranges around 1 USD/kg; transportation by road or rail is estimated to range from 1.5 to 2 USD/kg, 10–30% cheaper than transporting hydrogen in cylinders.
The method of transporting and storing hydrogen in metal hydrides is relatively safe since, under normal conditions, hydrogen is not released from compounds and, therefore, is not explosive. There are no leaks or emissions of hydrogen.
4.2.3. Liquefied Hydrogen Tanker
A liquefied hydrogen tanker is a vessel designed to transport liquefied hydrogen (LH2). Ships that transport liquid hydrogen are typically purpose-built vessels with specialized cryogenic tanks and other safety features such as safety valves and leak detection systems. LH2 ships aim to use stripped hydrogen on busy routes, providing low-emission marine fuel while preventing emissions.
According to IRENA TRL for Liquefied hydrogen tanker, today it is 7. This technology is tested in actual conditions.
Cryo-tankers transport liquid hydrogen over long distances by sea, the first of which, Suiso Frontie, is already operating flights from Australia to Japan. The project participants do not disclose the cost of freighting an existing hydrogen tanker. The range of estimates of the cost of transportation in the future is extensive: according to the European Commission, the cost of transportation for every 100 km will be from 0.012 to 0.07 USD/kg of hydrogen, IEA—0.04 USD/kg hydrogen, IRENA—0.06 USD/kg hydrogen.
The cost of transporting liquid hydrogen will be 4.2 USD/kg over a distance of 100 km; 4.2–5 USD/kg for a distance from 100 to 2000 km; and 5–7.8 USD/kg for a distance from 2000 to 8000 km [43].
Transporting hydrogen in liquid form reduces the risks of explosion and fire hazards during transportation. However, given the extremely low temperature at which hydrogen is transported in cryotanks, there are threats of cold injury.
Just like during transportation by road, there are losses in cryogenic tanks during transportation. There are no emissions from tanks, but there are emissions from sea tankers.
4.2.4. Ammonia Tanker
Transport of ammonia as a carrier of hydrogen, which is transported in fully refrigerated, non-pressurized containers, is often designed to transport liquefied petroleum gas (LPG) as it has a lower boiling point than ammonia. Conveyors for liquefied petroleum gas can be used provided that there are no parts containing copper, zinc, or alloys in contact with the cargo.
It is a mature technology, rated IRENA TRL 11.
The cost of “immersing” hydrogen in ammonia is between 0.2 and 2.1EUR/kg [56]. The indicator is highly sensitive to the initial cost of nitrogen, the specific technology used, the installation’s tonnage, and the cooling costs. The subsequent release of hydrogen is from 1.2 to 2 EUR/kg. The cost of freighting a tanker is about 40,000 USD/day.
The cost of transporting ammonia will be 1.4–4.1 USD/kg over a distance of 100 km; 1.43–4.13 USD/kg for a distance from 100 to 2000 km; and 1.6–4.3 USD/kg for a distance from 2000 to 8000 km [43].
It is important to remember that liquid ammonia itself is also a dangerous substance: it causes severe burns if it comes into contact with the skin, is easily flammable in the presence of an open source of fire, can explode when the container is heated, and is toxic if inhaled.
There are no emissions from ammonia transport, but there are emissions from sea tankers.
4.2.5. Liquid Organic Hydrogen Carrier Tanker
Liquid organic hydrogen (LOHC) will be transported using existing ships and port infrastructure. LOHC can be transported in chemical tankers whose tanks are specially coated with, for example, phenolic epoxy paint, stainless steel, or zinc paint, and they may have special piping for transporting various cargoes. The type of coating may determine which chemical can be transported. Product tankers, a kind of oil tanker, carry refined oil, are often designed to carry chemical cargo, and can also take LOHC. Depending on the chemicals used as LOHC, the type of tanker that can be used may vary, and there may also be some size restrictions at ports for safety reasons. This technology is well known; it is TRL–11.
The cost of producing “organic containers,” depending on the complexity of hydrogenation and the price of the initial hydrogenated substance, varies from 0.3 USD/kg (for methanol) to 44 USD/kg for N-ethyl carbazole. The cost of “immersing” hydrogen in such containers is low—less than 0.5 USD/kg, but the cost of “removing” it ranges from 0.6 to 4 USD/kg [57]. Considering that conventional oil tankers can carry out transportation, it is inexpensive compared to all other methods.
The cost of transporting LOHC by sea will be 1.1–4.5 (depending on the price of immersion in LOHC) USD/kg over a distance of 100 km; 1.1–4.7 USD/kg for a distance from 100 to 2000 km; 1.1–4.7 USD/kg for a distance from 2000 to 8000 km [43]. Thus, the price practically does not change depending on the distance.
On the consignee’s side, high-tech equipment is required to release hydrogen and generate harmful emissions. This process requires significant amounts of heat and produces associated emissions. In terms of safety, this method of transportation is comparable to the usual transportation of gasoline or diesel fuel.
The main advantages and disadvantages of hydrogen transportation methods are presented in Table 10.

Table 10.
Main advantages and disadvantages of hydrogen transportation methods.
Thus, the easiest and cheapest method of transportation is to use existing pipelines, although there are significant geographical limitations.
4.3. Hydrogen Emissions during Storage and Transportation
According to Frazer-Nash Consultancy, hydrogen emissions during storage and transportation can range from 0.04% to 13.2%, depending on the chosen method (Table 11).

Table 11.
Hydrogen emissions during storage and transportation.
It should be kept in mind that most storage systems are not entirely sealed, so there are values for permissible leakage volumes. Strict standards apply to how hydrogen is stored and transported. According to US Department of Energy standards, Technical Targets for Hydrogen Delivery Components should be at most 0.5% [41].
In underground hydrogen storage, leaks occur due to sealing, depressurization, leakage, and accidents [65]. There are also risks of natural leakage due to base permeability (predicted negligible), ventilation, and surface blowing due to maintenance and faults/When compressed hydrogen gas is stored in containers. Cylinders may leak as the rate depends on pressure, cylinder and valve material, and cylinder size [64].
Liquid organic hydrogen carriers (LOHC) require significant heat and generate associated emissions. The most extensive ranges in emission estimates are associated with liquefaction (0.15–10%), transport and handling of liquid hydrogen (2–20%), and liquid hydrogen refilling (2–15%). Moreover, current and future estimates of value chain emissions vary widely (0.2–20%) [52]. Liquid hydrogen storage tanks, such as those used by NASA, lose 1–5% of their H2 daily due to boil-off [66].
Most authors think that the impact of the storage of adsorbents on the environment has yet to be thoroughly studied and requires additional research. When storing hydrogen in metal hydrides and ammonia, the environmental impact is generally low as they do not emit pollutants [19]. At the same time, it is necessary to note the highest safety requirements for ammonia storage.
Dutch researcher Van Ruijven synthesized leakage estimates for different hydrogen transport methods and obtained interesting results. Estimates include long-haul vessels (0–2%), long-haul pipelines (0.1–5%), short-haul trucks (2–5.5%), short-haul pipelines (0.1–5%), airborne storage (0.3–1%), and fuel cells and on-board systems (0.1–1%) [67].
The primary potential H2 emissions associated with hydrogen transport through pipelines are leakage from pipelines and equipment, as well as operational purging during maintenance, removal of impurities, and in the event of failure [52].
According to some estimates, hydrogen leaks during pipeline transportation and storage will be from 1% to 2% by 2050, and when transported by freight transport, they will be from 2.5% to 5% [68].
Pipelines are the most critical hydrogen delivery systems, including dedicated hydrogen pipelines and natural gas blending systems. These systems themselves demonstrate a low risk of leakage. Weller Z. D., Hamburg S. P., and Von Fischer J. C. [69] and Hormaza M. and Brouwer J. [70] found that the leakage rate of hydrogen simply passing through the pipeline was approximately 0.4%. Pipelines have a low carbon footprint and do not emit pollutants.
5. Discussion
The study results showed the presence of various options for combinations of hydrogen storage and transportation, taking into account the type of hydrogen forms and the consumer’s location. In Figure 1. possible methods of transporting hydrogen are shown depending on storage technology.
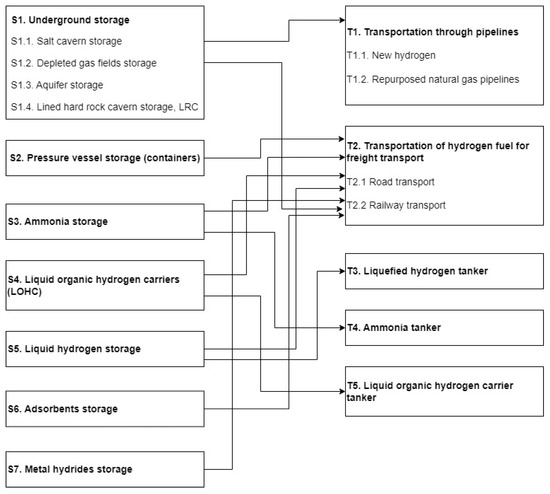
Figure 1.
Hydrogen transportation options depending on storage technology.
The prospects for using storage and transportation technologies depend on technological readiness, price, safety issues, etc. The use of the IRENA’s Technological Readiness Level (TRL) scale proved instrumental in assessing the readiness of each technology, allowing for a nuanced understanding of their developmental stages. This approach facilitated the identification of technologies that, despite their nascent stage, exhibit promising potential due to their innovative characteristics and adaptability to evolving market demands and energy policies. From the technological readiness level, the most promising storage and transportation options will be storing compressed hydrogen gas in containers, transporting it by truck, transporting ammonia by truck, and transporting it in an ammonia tanker (Table 12). There are also good prospects for transporting hydrogen from salt caves through pipelines and trucks. Technologies for transporting liquid hydrogen by freight transport have already been sufficiently mastered. These technologies are on track to, or have already transitioned to, full commercialization in their respective environments and have achieved predictable growth.

Table 12.
Comparative analysis of technological readiness of possible options for storing and transporting hydrogen.
We want to notice that geographical and geological characteristics limit the use of salt caverns. The availability of roads or railways often limits transportation by freight transport. It leads us to believe that it is necessary to expand research and new technological developments in the field of storing hydrogen in chemical compounds that will remove dependence on the geological structure of the area, as well as research that has already begun in the field of transporting hydrogen by other modes of transport, such as air.
The technologies are still at a low technological readiness level and have a long way to go before full commercial use. Moreover, this path requires significant capital investments at each stage [71], and it is also necessary to prepare end users to accept the technology [72].
Storage and transportation costs depend on the method and distance the hydrogen is transported in most cases; the further the hydrogen needs to be transported, the higher the price. Table 13 presents average data for hydrogen storage and transportation.

Table 13.
Comparative analysis of the competitiveness of possible options for storing and transporting hydrogen at average prices.
The results show that the most cost-competitive technologies are those for transporting hydrogen through pipelines from underground storage facilities. At the same time, transportation through 48 mm pipelines is cheaper, as are technologies for transporting ammonia by special tankers and liquid organic hydrogen carrier tankers. Moreover, the cost of transporting hydrogen in the form of ammonia and LOCH is practically independent of the distance.
From a safety and environmental perspective, hydrogen is a flammable gas, and its release may create a fire or explosion risk, mainly if the release occurs near a spark or open flame. Hydrogen is burned at shallow threshold explosive concentrations. A hydrogen leak can create hazardous situations for people near the leak. In this regard, increased requirements for warehouse and transport vehicles are necessary, and exceptional safety measures and strict standards must be observed.
Hydrogen released into the natural environment can affect living organisms and ecosystems. It may depend on the hydrogen concentration and duration of exposure. Hydrogen leaks during storage and transportation can lead to its accumulation, increasing the concentration of gases in the greenhouse effect. It could increase global warming. Transportation by truck has higher emissions of greenhouse gases and air pollutants than pipelines. At the same time, the use of renewable energy sources in transport can significantly reduce them.
The study results showed that storing hydrogen in underground storage facilities in a gaseous state and transporting it through pipelines have the most negligible environmental impact (Table 14).

Table 14.
Comparative analysis of possible options for combining hydrogen storage and transportation in terms of environmental impact.
From this point of view, adsorbent storage and metal hydride storage have good prospects. However, these technologies are still at a low stage of readiness, and they still face numerous tests before full commercialization and significant investments. At the same time, reductions in emissions and leakages can be expected due to the latest developments and innovations.
6. Conclusions
The review and meta-analysis of the data available in the literature on the environmental and economic parameters of hydrogen storage and transportation technologies showed that salt caverns and pipelines are the most promising methods for storing and transporting hydrogen today. This method has been well studied, is at a high stage of readiness, is competitive in price, and does not cause significant environmental damage. Our data showed that the average storage price in this aggregate method was 0.35 USD/kg. At the same time, the price of transportation ranges from 0.3 to 7.2 USD/kg, depending on the distance. This method is attractive because it is already well known and does not have a negative impact on the environment.
Our findings coincide with the studies of other scientists. Considering the operating footprint of storage and transportation, gaseous hydrogen transported via a pipeline is a better alternative from an environmental point of view, and it has a lower energy footprint (38–85%) than the other options. Storage and transport (without construction) could have accounted for around 35.5% of the total GHG footprint of a hydrogen value chain (production, storage, transportation, and losses) if liquidated and transported via road transport instead of a pipeline [73].
Pipelines are the most economical way to transport hydrogen, but their construction requires significant investments. Material-based storage technologies are preferred in application scenarios involving medium to long-term hydrogen storage due to their higher bulk density, chemical stability, and safety. However, pipelines have significant limitations. These methods are only competitive where pipelines exist; constructing new pipelines will involve substantial costs and many procedures related to obtaining construction permits. Adsorbent and metal hydride storage technologies have good prospects but require further research and development. At the same time, reductions in emissions and leakages can be expected due to the latest developments and innovations.
As the main contribution of this study to the existing literature, we can highlight the obtained estimates of economic, environmental, and safety parameters not of separate technologies but of the related stages of the hydrogen supply chain: storage and transportation. Combining the obtained results with detailed studies of hydrogen production technologies, it is possible to obtain estimates of the complete hydrogen supply chain.
At the same time, our study has some limitations. To obtain the results, we relied on secondary data obtained from various organizations. Data accounting methods are constantly updated, including, over time, indicators are updated and supplemented with retrospective information. The use of such estimates is often controversial and limits research. It is worth noting that there is a limited amount of data on the impact of hydrogen energy on the environment, which has recently become increasingly important. Further research requires a more thorough study of this particular aspect.
In addition, this study only considered carbon emissions as an environmental parameter, whereas in practice, other categories of environmental impacts need to be considered. Meta-analysis of this type of data will be the next step in the development of this study in the future.
Author Contributions
The main activities of the team of authors can be described as follows: conceptualization, S.R., I.L. and B.G.; methodology, S.R., I.L. and B.G.; software, S.R., T.S. and Y.H.; validation, S.R., I.L. and B.G.; formal analysis, I.L.; investigation, I.L., T.S. and Y.H.; resources, S.R. and B.G.; data curation, S.R., I.L. and T.S.; writing—original draft preparation, S.R., I.L., B.G., T.S. and Y.H.; writing—review and editing, S.R., I.L., B.G., T.S. and Y.H.; visualization, S.R.; supervision, S.R.; project administration, I.L.; funding acquisition, S.R. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Russian Science Foundation grant No. 22-78-10089, https://rscf.ru/project/22-78-10089/.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xie, J.; Xia, Z.; Tian, X.; Liu, Y. Nexus and synergy between the low-carbon economy and circular economy: A systematic and critical review. Environ. Impact Assess. Rev. 2023, 100, 107077. [Google Scholar] [CrossRef]
- Naidoo, D.; Nhamo, L.; Lottering, S.; Mpandeli, S.; Liphadzi, S.; Modi, A.T.; Mabhaudhi, T. Transitional Pathways towards Achieving a Circular Economy in the Water, Energy, and Food Sectors. Sustainability 2021, 13, 9978. [Google Scholar] [CrossRef]
- Ratner, S.; Gomonov, K.; Lazanyuk, I.; Revinova, S. Barriers and drivers for Circular Economy 2.0 on the firm level: Russian case. Sustainability 2021, 13, 11080. [Google Scholar] [CrossRef]
- Nizhegorodtsev, R.; Ratner, S. Trends in the development of industrially assimilated renewable energy: The problem of resource restrictions. Therm. Eng. 2016, 63, 197–207. [Google Scholar] [CrossRef]
- Schmidt, M. The Resource-Energy Nexus as a key factor for circular economy. Chem. Ing. Tech. 2021, 93, 1707–1716. [Google Scholar] [CrossRef]
- Ratner, S.; Lychev, A. Evaluating environmental impacts of photovoltaic technologies using Data Envelopment Analysis. Adv. Syst. Sci. Appl. 2019, 19, 12–30. [Google Scholar] [CrossRef]
- Chen, W.; Kim, H. Circular economy and energy transition: A nexus focusing on the non-energy use of fuels. Energy Environ. 2019, 30, 586–600. [Google Scholar] [CrossRef]
- Zhang, Y.; Davis, D.; Brear, M.J. The role of hydrogen in decarbonizing a coupled energy system. J. Clean. Prod. 2022, 346, 131082. [Google Scholar] [CrossRef]
- Griffiths, S.; Sovacool, B.K.; Kim, J.; Bazilian, M.; Uratani, J.M. Industrial decarbonization via hydrogen: A critical and systematic review of developments, socio-technical systems and policy options. Energy Res. Soc. Sci. 2021, 80, 102208. [Google Scholar] [CrossRef]
- Ratner, S.; Zaretskaya, M. Forecasting the Ecology Effects of Electric Cars Deployment in Krasnodar Region (Russia): Learning Curves Approach. J. Environ. Manag. Tour. 2018, 9, 82–94. [Google Scholar] [CrossRef]
- Ratner, S.; Chepurko, Y.; Hiển, N.H. Prospects of Transition of Air Transportation to Clean Fuels: Economic and Environmental Management Aspects. Int. Energy J. 2019, 19, 125–138. Available online: http://www.rericjournal.ait.ac.th/index.php/reric/article/view/2084/718 (accessed on 25 June 2024).
- IEA Global Hydrogen Review. October 2021. Available online: https://iea.blob.core.windows.net/assets/5bd46d7b-906a-4429-abdae9c507a62341/GlobalHydrogenReview2021.pdf (accessed on 15 January 2024).
- Gomonov, K.; Reshetnikova, M.S.; Ratner, S. Economic analysis of recently announced green hydrogen projects in Russia: A multiple case study. Energies 2023, 16, 4023. [Google Scholar] [CrossRef]
- Revinova, S.; Lazanyuk, I.; Ratner, S.; Gomonov, K. Forecasting development of green hydrogen production technologies using Component-Based learning curves. Energies 2023, 16, 4338. [Google Scholar] [CrossRef]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen storage: Materials, methods and perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- Rusman, N.; Dahari, M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, Y. Recent advances in improving performances of the lightweight complex hydrides Li-Mg-NH system. Prog. Nat. Sci. Mater. Int. 2017, 27, 21–33. [Google Scholar] [CrossRef]
- Ratner, S.; Nizhegorodtsev, R. Analysis of the World Experience of Smart Grid Deployment: Economic Effectiveness Issues. Therm. Eng. 2018, 65, 387–399. [Google Scholar] [CrossRef]
- Ivanova, E.E.; Shumbor, V.V.; Zhirov, D.A.; Gagloev, D.V. Analysis of Optimal Methods of Transport in the UK; ICSP “NEW SCIENCE” eBooks: Petrozavodsk, Russia, 2023. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Heinemann, N.; Alcalde, J.; Miocic, J.; Hangx, S.; Kallmeyer, J.; Ostertag-Henning, C.; Hassanpouryouzband, A.; Thaysen, E.M.; Strobel, G.; Schmidt-Hattenberger, C.; et al. Enabling large-scale hydrogen storage in porous media—The scientific challenges. Energy Environ. Sci. 2021, 14, 853–864. [Google Scholar] [CrossRef]
- Zeng, L.; Sarmadivaleh, M.; Saeedi, A.; Chen, Y.; Zhong, Z.; Xie, Q. Storage integrity during underground hydrogen storage in depleted gas reservoirs. Earth-Sci. Rev. 2023, 247, 104625. [Google Scholar] [CrossRef]
- Jens, J.; Wang, A.; van der Leun, K.; Peters, D.; Buseman, M. Extending the Hydrogen European Backbone: A European Hydrogen Infrastructure Vision Covering 21 Countries. 2021. Available online: https://reglobal.org/wp-content/uploads/2021/05/European-Hydrogen-Backbone_April-2021.pdf (accessed on 17 January 2024).
- Sambo, C.; Dudun, A.; Samuel, S.A.; Esenenjor, P.; Muhammed, N.S.; Haq, B. A review on worldwide underground hydrogen storage operating and potential fields. Int. J. Hydrogen Energy 2022, 47, 22840–22880. [Google Scholar] [CrossRef]
- Tarkowski, R.; Lankof, L.; Luboń, K.; Michalski, J. Hydrogen storage capacity of salt caverns and deep aquifers versus demand for hydrogen storage: A case study of Poland. Appl. Energy 2024, 355, 122268. [Google Scholar] [CrossRef]
- Bauer, S.; Dahmke, A.; Kolditz, O. Subsurface energy storage: Geological storage of renewable energy—Capacities, induced effects and implications. Environ. Earth Sci. 2017, 76, 1–4. [Google Scholar] [CrossRef]
- Gabrielli, P.; Poluzzi, A.; Kramer, G.J.; Spiers, C.J.; Mazzotti, M.; Gazzani, M. Seasonal energy storage for zero-emissions multi-energy systems via underground hydrogen storage. Renew. Sustain. Energy Rev. 2020, 121, 109629. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Joonaki, E.; Edlmann, K.; Haszeldine, R.S. Offshore geological storage of hydrogen: Is this our best option to achieve net-zero? ACS Energy Lett. 2021, 6, 2181–2186. [Google Scholar] [CrossRef]
- Lord, A.S.; Kobos, P.H.; Borns, D.J. Geologic storage of hydrogen: Scaling up to meet city transportation demands. Int. J. Hydrogen Energy 2014, 39, 15570–15582. [Google Scholar] [CrossRef]
- Bünger, U.; Michalski, J.; Crotogino, F.; Kruck, O. Large-Scale Underground Storage of Hydrogen for the Grid Integration of Renewable Energy and Other Applications; Elsevier eBooks: Amsterdam, The Netherlands, 2016; pp. 133–163. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, C.; Shen, S.; Zou, J.; Mao, S.S.; Yao, X. Combination of nanosizing and interfacial effect: Future perspective for designing Mg-based nanomaterials for hydrogen storage. Renew. Sustain. Energy Rev. 2015, 44, 289–303. [Google Scholar] [CrossRef]
- Eftekhari, A.; Fang, B. Electrochemical hydrogen storage: Opportunities for fuel storage, batteries, fuel cells, and supercapacitors. Int. J. Hydrogen Energy 2017, 42, 25143–25165. [Google Scholar] [CrossRef]
- Edalati, K.; Uehiro, R.; Ikeda, Y.; Li, H.; Emami, H.; Filinchuk, Y.; Arita, M.; Sauvage, X.; Tanaka, I.; Akiba, E.; et al. Design and synthesis of a magnesium alloy for room temperature hydrogen storage. Acta Mater. 2018, 149, 88–96. [Google Scholar] [CrossRef]
- BNEF Hydrogen Economy Outlook Key Messages 30 March 2020. 2020. Available online: http://data.bloomberglp.com/professional/sites/24/BNEF-Hydrogen-Economy-Outlook-Key-Messages-30-Mar-2020.pdf (accessed on 10 December 2023).
- EMEA Hydrogen. A Revolution in Need of Realism; Separating Theopportunity from the Optimism. Available online: https://buyhydrogen.com.au/wp-content/uploads/2021/04/J.P.Morgan-CAZENOVE-EMEA-Hydrogen.pdf (accessed on 15 January 2024).
- El-Eskandarany, M.S. Solid-State Hydrogen Storage Nanomaterials for Fuel Cell Applications; Elsevier eBooks: Amsterdam, The Netherlands, 2020; pp. 229–261. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Su, Y.; Wang, P.; Yu, B. Effects of hydrogen blending on hydraulic and thermal characteristics of natural gas pipeline and pipe network. Oil Gas Sci. Technol. 2021, 76, 70. [Google Scholar] [CrossRef]
- Tian, X.F.; Pei, J. Study progress on the pipeline transportation safety of hydrogen-blended natural gas. Heliyon 2023, 9, e21454. [Google Scholar] [CrossRef]
- Melaina, M.; Antonia, O.; Penev, M. Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues; National Renewable Energy Laboratory: Golden, CO, USA, 2013. [CrossRef]
- Qianlu, L.; Liuyi, T. Research progress on application of hydrogen-mixed natural gas in terminal pipe network. Oil Gas Storage Transp. 2022, 41, 381–390. [Google Scholar] [CrossRef]
- Energy.gov. DOE Technical Targets for Hydrogen Delivery. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-hydrogen-delivery (accessed on 15 January 2024).
- Witkowski, A.; Rusin, A.; Majkut, M.; Stolecka, K. Analysis of compression and transport of the methane/hydrogen mixture in existing natural gas pipelines. Int. J. Press. Vessel. Pip. 2018, 166, 24–34. [Google Scholar] [CrossRef]
- Ekologiya Energetika Energosberezhenie: Byulleten’/Pod Redakciej Akademika, R.A.N.A.V. Klimenko.—Moskva: PAO «Mosenergo», 2023. Vyp. 2. Vodorodnaya Energetika: Za i Protiv/(V.A. Kulagin, D.A. Grushevenko).—2023.—36 p. Available online: https://mosenergo.gazprom.ru/d/textpage/45/837/06-vodorod.pdf (accessed on 15 January 2024).
- Zhang, J.; Wang, T.; Ng, W.W.; Pedrycz, W. Ensembling perturbation-based oversamplers for imbalanced datasets. Neurocomputing 2022, 479, 1–11. [Google Scholar] [CrossRef]
- Cristello, J.B.; Yang, J.M.; Hugo, R.; Lee, Y.; Park, S.S. Feasibility analysis of blending hydrogen into natural gas networks. Int. J. Hydrogen Energy 2023, 48, 17605–17629. [Google Scholar] [CrossRef]
- Li, H.; Cao, X.; Liu, Y.; Shao, Y.; Nan, Z.; Teng, L.; Peng, W.; Bian, J. Safety of hydrogen storage and transportation: An overview on mechanisms, techniques, and challenges. Energy Rep. 2022, 8, 6258–6269. [Google Scholar] [CrossRef]
- Mair, G.W.; Thomas, S.; Schalau, B.; Wang, B. Safety criteria for the transport of hydrogen in permanently mounted composite pressure vessels. Int. J. Hydrogen Energy 2021, 46, 12577–12593. [Google Scholar] [CrossRef]
- Ahluwalia, R.; Roh, H.-S.; Peng, J.-K.; Papadias, D.; Baird, A.; Hecht, E.; Ehrhart, B.; Muna, A.; Ronevich, J.; Houchins, C.; et al. Liquid hydrogen storage system for heavy duty trucks: Configuration, performance, cost, and safety. Int. J. Hydrogen Energy 2023, 48, 13308–13323. [Google Scholar] [CrossRef]
- Gojić, M.; Tanasić, N.; Arandjelović, I.; Milivojević, A. Influence of ventilation system effectiveness on the safety of hydrogen storage and transportation. Procedia Struct. Integr. 2023, 48, 334–341. [Google Scholar] [CrossRef]
- Bolobov, V.I.; Latipov, I.U.; Popov, G.G.; Buslaev, G.V.; Martynenko, Y.V. Estimation of the Influence of Compressed Hydrogen on the Mechanical Properties of Pipeline Steels. Energies 2021, 14, 6085. [Google Scholar] [CrossRef]
- Hydrogen Decarbonization Pathways. A Life-Cycle Assessment, January 2021. Available online: https://hydrogencouncil.com/wp-content/uploads/2021/01/Hydrogen-Council-Report_Decarbonization-Pathways_Executive-Summary.pdf (accessed on 25 June 2024).
- Esquivel-Elizondo, S.; Mejia, A.H.; Sun, T.; Shrestha, E.; Hamburg, S.P.; Ocko, I.B. Wide range in estimates of hydrogen emissions from infrastructure. Front. Energy Res. 2023, 11, 1207208. [Google Scholar] [CrossRef]
- Department for Energy Security and Net Zero. (2022, April 8). Fugitive Hydrogen Emissions in a Future Hydrogen Economy. GOV.UK. Available online: https://www.gov.uk/government/publications/fugitive-hydrogen-emissions-in-a-future-hydrogen-economy (accessed on 25 January 2024).
- Ocko, I.B.; Hamburg, S.P. Climate consequences of hydrogen leakage. Atmos. Chem. Phys. 2022, 22, 9349–9368. [Google Scholar] [CrossRef]
- IEA. Future of Hydrogen, Transitions Commission 2021. 2019. Available online: https://iea.blob.core.windows.net/assets/9e3a3493-b9a6-4b7d-b499-7ca48e357561/The_Future_of_Hydrogen.pdf (accessed on 25 January 2024).
- Collis, J.; Schomäcker, R. Determining the production and transport cost for H2 on a global scale. Front. Energy Res. 2022, 10, 909298. [Google Scholar] [CrossRef]
- Assessment of Hydrogen Delivery Options (Electronic resource)//European Union, 2021—JRC124206. Available online: https://joint-research-centre.ec.europa.eu/system/files/2021-06/jrc124206_assessment_of_hydrogen_delivery_options.pdf (accessed on 25 June 2024).
- Clean Energy Innovation–Energy Technology Perspectives 2020–Analysis-IEA. (n.d.). IEA. Available online: https://www.iea.org/reports/energy-technology-perspectives-2020 (accessed on 25 June 2024).
- Butov, K.A. Podzemnoe hranenie vodoroda. Osnovnye problemy perekhoda na «zelenuyu energetiku». Process. V Geosredah 2020, 1, 553–560. [Google Scholar]
- Olivier. Four Ways to Store Large Quantities of Hydrogen. Geostock. Available online: https://www.geostockgroup.com/en/four-ways-to-store-large-quantities-of-hydrogen/ (accessed on 28 September 2023).
- IEA. ETP Clean Energy Technology Guide. Available online: https://www.iea.org/data-and-statistics/data-tools/etp-clean-energy-technology-guide (accessed on 25 June 2024).
- Ahluwalia, R.; Peng, J.; Hua, T.; Kumar, R.; Satyapal, S. System Level Analysis of Hydrogen Storage Options. Hydrogen and Fuel Cells Program 2019 Annual Merit Review and Peer Evaluation Meeting. 2019. Available online: https://www.hydrogen.energy.gov/docs/hydrogenprogramlibraries/pdfs/review19/st001_ahluwalia_2019_o.pdf (accessed on 25 June 2024).
- Science, Engineering and Evidence-HSE. 2008. Available online: https://www.hse.gov.uk/researcH/ (accessed on 25 June 2024).
- Consultancy, F.N. Fugitive Hydrogen Emissions in a Future Hydrogen Economy. UK Government. FNC 012865-53172R Issue. 2022. Available online: https://assets.publishing.service.gov.uk/media/624ec79cd3bf7f600d4055d1/fugitive-hydrogen-emissions-future-hydrogen-economy.pdf (accessed on 25 June 2024).
- Fan, Z.; Sheerazi, H.; Bhardwaj, A.; Corbeau, A.S.; Longobardi, K.; Castañeda, A.; Merz, A.K.; Woodall, D.C.M. Hydrogen Leakage: A Potential Risk for the Hydrogen Economy; Columbia Center on Global Energy Policy: New York, NY, USA, 2022; Available online: https://triton-hydrogen.com/wp-content/uploads/2023/09/HydrogenLeakageRegulations_CGEP_Commentary_063022.pdf (accessed on 25 June 2024).
- Hydrogeninsight. Hydrogen Leakage Makes H2 Potentially More Dangerous to Climate than Burning Natural Gas’: Rio Tinto. 2020. Available online: https://www.hydrogeninsight.com/industrial/hydrogen-leakage-makes-h2-potentially-more-dangerous-to-climate-than-burning-natural-gas-rio-tinto/2-1-1365074 (accessed on 25 June 2024).
- Van Ruijven, B.; Lamarque, J.; Van Vuuren, D.; Kram, T.; Eerens, H. Emission scenarios for a global hydrogen economy and the consequences for global air pollution. Glob. Environ. Chang. 2011, 21, 983–994. [Google Scholar] [CrossRef]
- Center on Global Energy Policy at Columbia University (CGEP). 2022. Available online: https://www.energypolicy.columbia.edu/wp-content/uploads/2022/07/HydrogenLeakageRegulations_CGEP_Commentary_063022.pdf (accessed on 25 June 2024).
- Weller, Z.D.; Hamburg, S.P.; Von Fischer, J.C. A National Estimate of Methane Leakage from Pipeline Mains in Natural Gas Local Distribution Systems. Environ. Sci. Technol. 2020, 54, 8958–8967. [Google Scholar] [CrossRef]
- Mejia, H.; Brouwer, J. Gaseous Fuel Leakage From Natural Gas Infrastructure. In Proceedings of the ASME 2018 International Mechanical Engineering Congress and Exposition, Pittsburgh, PA, USA, 9–15 November 2018; Volume 6A: Energy, p. V06AT08A069. [Google Scholar] [CrossRef]
- Ratner, S.; Chepurko, Y.; Drobyshecskaya, L.; Petrovskaya, A. Management of Energy Enterprises: Energy-efficiency Approach in Solar Collectors Industry: The Case of Russia. Int. J. Energy Econ. Policy 2018, 8, 237–243. Available online: https://www.econjournals.com/index.php/ijeep/article/view/6740 (accessed on 25 June 2024).
- Ratner, S.; Salnikov, A.A.; Berezin, A.; Sergi, B.S.; Sohag, K. Customer engagement in innovative smart grid deployment projects: Evidence from Russia. Environ. Sci. Pollut. Res. 2021, 29, 5902–5911. [Google Scholar] [CrossRef]
- Hren, R.; Vujanović, A.; Van Fan, Y.; Klemeš, J.J.; Krajnc, D.; Čuček, L. Hydrogen production, storage and transport for renewable energy and chemicals: An environmental footprint assessment. Renew. Sustain. Energy Rev. 2023, 173, 113113. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).