Energy Recovery from Organic Wastes Using Microbial Fuel Cells: Traditional and Nonconventional Organic Substrates
Abstract
1. Introduction
2. Factors Affecting MFC Performance
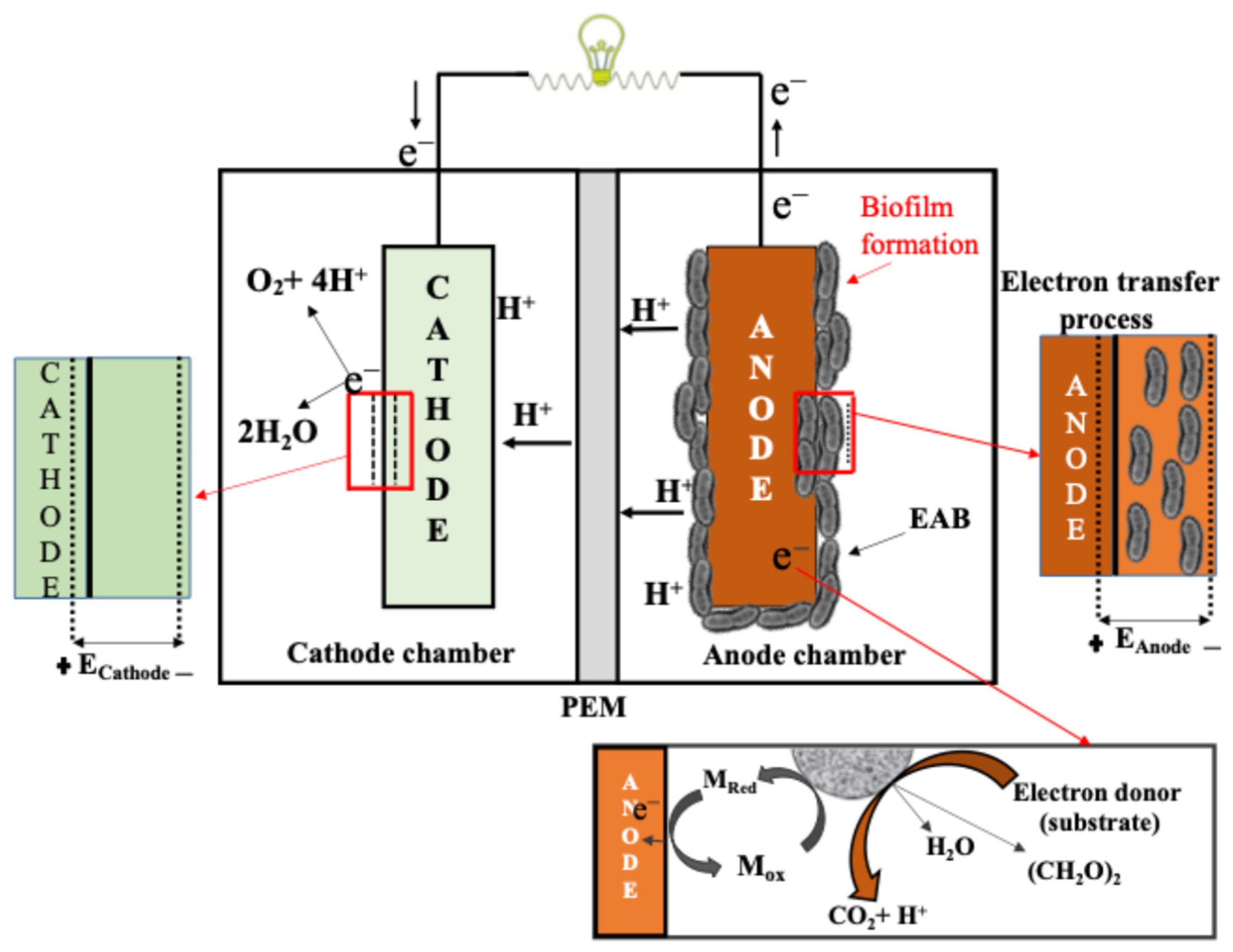
3. Substrates in MFC Technology: Availability and Potential Environmental Benefits
3.1. Acetate, Butyrate, Glucose, Glutamate, Etc., Are Traditionally Employed in MFCs as Feedstock
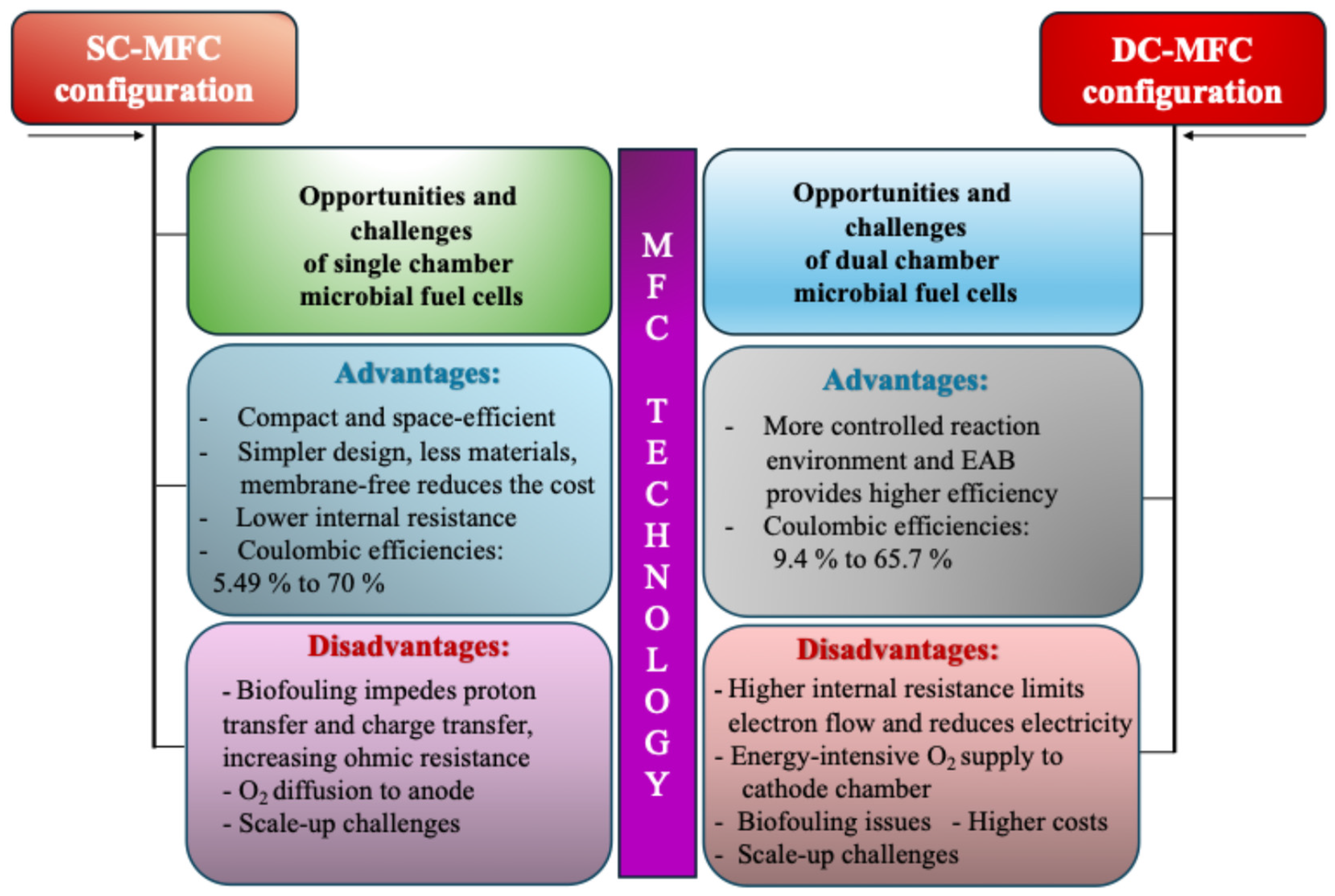
3.2. Conventional and Nonconventional Sources for MFCs: Industrial and Household Wastes
3.2.1. Sugar and Meat Food Industry Wastewater as Promising Substrates for MFC Technology
3.2.2. Synthetic Wastewater and Dye Wastewater as Sources for MFCs
3.2.3. Industrial Waste from Oil and Wood as Sources of Complex and Stable Compounds for Nonconventional MFC Feeding
3.3. Industrial and Domestic Food Wastes: Potato, Mango, Banana, and Orange Wastes as Nonconventional Substrates for MFCs
3.4. Human Urine Domestic Waste as a Successfully Applied Real-World MFC Substrate
3.5. Agricultural Wastes
3.5.1. Livestock Urine Waste as an Essential Nonconventional Renewable Substrate for MFCs
3.5.2. Farm Manures
4. Application of Power Management Systems in MFCs
5. Challenges and Future Perspectives
5.1. Sustainable Substrates and Adaptation to Small-Scale Applications
5.2. Avocado, Petroleum, Urine, and Manure Waste as Valuable Bioresources
5.3. Metabolic-Engineered Microorganisms
5.4. Incorporation of Green Electrodes, Nanomaterials, and Plants into MFC Technology
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ExxonMobil. 2022 Outlook for Energy. 2022. Available online: https://corporate.exxonmobil.com/-/media/global/files/outlook-for-energy/2022/2022-outlook-for-energy-executive-summary.pdf?la=en&hash=240E8F434D13911739A734EF8281F1AA7DEB7271 (accessed on 27 November 2022).
- Apollon, W.; Luna-Maldonado, A.I.; Kamaraj, S.K.; Vidales-Contreras, J.A.; Rodríguez-Fuentes, H.; Gómez-Leyva, J.F.; Aranda-Ruíz, J. Progress and recent trends in photosynthetic assisted microbial fuel cells: A review. Biomass Bioenergy 2021, 148, 106028. [Google Scholar] [CrossRef]
- Potter, M.C. Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. Lond. Ser. B Contain. Pap. Biol. Character 1911, 84, 260–267. [Google Scholar] [CrossRef]
- Davis, J.B.; Yarbrough, H.F. Preliminary experiments on a microbial fuel cell. Science 1962, 137, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Zhao, Y.; Wu, Y.; Tian, J.; Liu, J.; Wang, R.; Yang, Y.; Liu, Y. Research progress in the application of metal organic frameworks and its complex in microbial fuel cells: Present status, opportunities, future directions and prospects. Int. J. Hydrogen Energy 2023, 48, 23956–23966. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, L.; Hermanowicz, S.W. Influence of water transport characteristics on membrane internal conductive structure in forward osmosis microbial fuel cell. J. Mol. Liq. 2023, 380, 121704. [Google Scholar] [CrossRef]
- Zadeh, P.G.; Rezania, S.; Fattahi, M.; Dang, P.; Vasseghian, Y.; Aminabhavi, T.M. Recent advances in microbial fuel cell technology for energy generation from wastewater sources. Process. Saf. Environ. Prot. 2024, 189, 425–439. [Google Scholar] [CrossRef]
- Ahanchi, M.; Jafary, T.; Yeneneh, A.M.; Rupani, P.F.; Shafizadeh, A.; Shahbeik, H.; Pan, J.; Tabatabaei, M.; Aghbashlo, M. Review on waste biomass valorization and power management systems for microbial fuel cell application. J. Clean. Prod. 2022, 380, 134994. [Google Scholar] [CrossRef]
- Jalili, P.; Ala, A.; Nazari, P.; Jalili, B.; Ganji, D.D. A comprehensive review of microbial fuel cells considering materials, methods, structures, and microorganisms. Heliyon 2024, 10, e25439. [Google Scholar] [CrossRef]
- Mashkour, M.; Rahimnejad, M.; Mashkour, M.; Soavi, F. Increasing bioelectricity generation in microbial fuel cells by a high-performance cellulose-based membrane electrode assembly. Appl. Energy 2021, 282, 116150. [Google Scholar] [CrossRef]
- Zhu, Q.; Hu, J.; Liu, B.; Hu, S.; Liang, S.; Xiao, K.; Yang, J.; Hou, H. Recent Advances on the Development of Functional Materials in Microbial Fuel Cells: From Fundamentals to Challenges and Outlooks. Energ. Environ. Mater. 2021, 5, 401–426. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Lu, J.; Ren, L.L.; Li, C.; Yang, X.; Zhang, X.; Liu, J.; Song, Z. In situ growth of nanoflowers Zn-HHTP@Ni/Co3DHFLM modified air cathode MFC enhances power generation performance. Int. J. Hydrogen Energy 2024, 96, 217–226. [Google Scholar] [CrossRef]
- Bagchi, S.; Behera, M. Pharmaceutical wastewater treatment in ceramic separator MFC: Optimization of operating parameters to improve organic removal and power generation. Clean. Circ. Bioeconomy 2023, 6, 100063. [Google Scholar] [CrossRef]
- Gu, W.; Wang, Y.; Hu, X.; Deng, F. MFC-residual sludge coupled treatment for simulated chromium(VI) wastewater: Electricity production performance and microbial communities. J. Water Process. Eng. 2024, 67, 106097. [Google Scholar] [CrossRef]
- Tong, W.; Zhang, S.; Xiong, G.; Lin, J.; Gao, Q. Study on Optimization of MFC Electric Generation Parameters based on Box–Behnken Design. Procedia Comput. Sci. 2024, 243, 235–244. [Google Scholar] [CrossRef]
- Kumar, G.G.; Kirubaharan, C.J.; Yoo, D.J.; Kim, A.R. Graphene/poly(3,4-ethylenedioxythiophene)/Fe3O4 nanocomposite—An efficient oxygen reduction catalyst for the continuous electricity production from wastewater treatment microbial fuel cells. Int. J. Hydrogen Energy 2016, 41, 13208–13219. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Sathishkumar, Y.; Lee, Y.S.; Kim, A.R.; Yoo, D.J.; Kumar, G.G. The Influence of Chitosan Substrate and Its Nanometric Form Toward the Green Power Generation in Sediment Microbial Fuel Cell. J. Nanosci. Nanotechnol. 2017, 17, 558–563. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Ali, A.E.; Hassouna, M.E.; Elhusseiny, A.F.; Kashyout, A.E.; He, Z.; Moustafa, H. Performance evaluation of microbial fuel cell fabricated using green nanographene oxide as coating anode material. Biomass Convers. Biorefinery 2023, 15, 297–310. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, H.; Liu, W.; Zhang, R.; Guo, J.; Xian, M.; Liu, H. Electricigens in the anode of microbial fuel cells: Pure cultures versus mixed communities. Microb. Cell Factories 2019, 18, 39. [Google Scholar] [CrossRef]
- Naaz, T.; Kumari, S.; Sharma, K.; Singh, V.; Khan, A.A.; Pandit, S.; Priya, K.; Jadhav, D.A. Bioremediation of hydrocarbon by coculturing of biosurfactant-producing bacteria in microbial fuel cell with Fe2O3-modified anode. J. Environ. Manag. 2024, 351, 119768. [Google Scholar] [CrossRef]
- Simoska, O.; Cummings, D.A.; Gaffney, E.M.; Langue, C.; Primo, T.G.; Weber, C.J.; Witt, C.E.; Minteer, S.D. Enhancing the Performance of Microbial Fuel Cells via Metabolic Engineering of Escherichia coli for Phenazine Production. ACS Sustain. Chem. Eng. 2023, 11, 11855–11866. [Google Scholar] [CrossRef]
- Rusyn, I.; Fihurka, O.; Dyachok, V. Effect of Plants Morphological Parameters on Plant-Microbial Fuel Cell Efficiency. Innov. Biosyst. Bioeng. 2022, 6, 161–168. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Fan, Y.; Long, F.; Qiu, Y.; Geier, W.; Liu, H. Novel trickling microbial fuel cells for electricity generation from wastewater. Chemosphere 2020, 248, 126058. [Google Scholar] [CrossRef]
- Yang, J.; Cao, X.; Sun, Y.; Yang, G.; Yi, W. Recovery of microbial fuel cells with high COD molasses wastewater and analysis of the microbial community. Biomass Bioenergy 2022, 161, 106450. [Google Scholar] [CrossRef]
- Sun, F.; Chen, J.; Sun, Z.; Zheng, X.; Tang, M.; Yang, Y. Promoting bioremediation of brewery wastewater, production of bioelectricity and microbial community shift by sludge microbial fuel cells using biochar as anode. Sci. Total Environ. 2024, 929, 172418. [Google Scholar] [CrossRef]
- Sreelekshmy, B.R.; Basheer, R.; Sivaraman, S.; Casudevan, V.; Elias, L.; Shibli, S.M.A. Sustainable electric power generation from live anaerobic digestion of sugar industry effluents using microbial fuel cells. J. Mater. Chem. A 2020, 8, 6041–6056. [Google Scholar] [CrossRef]
- Hamza, H.M.C.; Duraisamy, P.; Periyasamy, S.; Pokkiladathu, H.; Muthuchamy, M. Simultaneous electricity generation and heavy metals reduction from distillery effluent by microbial fuel cell. Indian J. Sci. Technol. 2017, 10, 1–13. [Google Scholar] [CrossRef]
- Kumar, G.; Kim, S.H.; Lay, C.H.; Ponnusamy, V.K. Recent developments on alternative fuels, energy and environment for sustainability. Bioresour. Technol. 2020, 317, 124010. [Google Scholar] [CrossRef]
- Apollon, W.; Rusyn, I.; González-Gamboa, N.; Kuleshova, T.; Luna-Maldonado, A.I.; Vidales-Contreras, J.A.; Kamaraj, S.K. Improvement of zero waste sustainable recovery using microbial energy generation systems: A comprehensive review. Sci. Total Environ. 2022, 817, 153055. [Google Scholar] [CrossRef]
- Sevda, S.; Garlapati, V.K.; Sreekrishnan, T.R. Role of electrode and proton exchange membrane configurations on microbial fuel cell performance toward bioelectricity generation integrated wastewater treatment. J. Environ. Sci. Health Part A 2023, 58, 13–23. [Google Scholar] [CrossRef]
- Torlaema, T.A.M.; Ibrahim, M.N.M.; Ahmad, A.; Guerrero-Barajas, C.; Alshammari, M.B.; Oh, S.E.; Hussain, F. Degradation of Hydroquinone Coupled with Energy Generation through Microbial Fuel Cells Energized by Organic Waste. Processes 2022, 10, 2099. [Google Scholar] [CrossRef]
- Segundo, R.F.; Magaly, D.L.C.N.; Benites, S.M.; Daniel, D.N.; Angelats-Silva, L.; Díaz, F.; Luis, C.-C.; Fernanda, S.P. Increase in Electrical Parameters Using Sucrose in Tomato Waste. Fermentation 2022, 8, 335. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent Advances in Anodes for Microbial Fuel Cells: An Overview. Materials 2020, 13, 2078. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Das, P.P.; Sood, T.; Chakraborty, A.; Purkait, M.K. Reduced graphene oxide incorporated polyvinylidene fluoride/cellulose acetate proton exchange membrane for energy extraction using microbial fuel cells. J. Electroanal. Chem. 2022, 907, 115890. [Google Scholar] [CrossRef]
- Sasaki, D.; Sasaki, K.; Tsuge, Y.; Kondo, A. Less biomass and intracellular glutamate in anodic biofilms lead to efficient electricity generation by microbial fuel cells. Biotechnol. Biofuels 2019, 12, 72. [Google Scholar] [CrossRef]
- Ding, K.; Liu, D.; Chen, X.; Zhang, H.; Shi, S.; Guo, X.; Zhou, L.; Han, L.; Xiao, W. Scalable lignocellulosic biorefineries: Technoeconomic review for efficient fermentable sugars production. Renew. Sustain. Energy Rev. 2024, 202, 114692. [Google Scholar] [CrossRef]
- Fadzli, F.S.; Bhawani, S.A.; Adam Mohammad, R.E. Microbial fuel cell: Recent developments in organic substrate use and bacterial electrode interaction. J. Chem. 2021, 2021, 4570388. [Google Scholar] [CrossRef]
- Apollon, W.; Rusyn, I.; Kuleshova, T.; Luna-Maldonado, A.I.; Pierre, J.F.; Gwenzi, W.; Kumar, V. An overview of agro-industrial wastewater treatment using microbial fuel cells: Recent advancements. J. Water Process. Eng. 2024, 58, 104783. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Franzetti, A.; Prasad, S.; Formicola, F.; Rosatelli, A.; Hassani, A.; Aminabhavi, T.M.; Ritmi, S. Microbial electrochemical bioremediation of petroleum hydrocarbons (PHCs) pollution: Recent advances and outlook. Chem. Eng. J. 2022, 452, 139372. [Google Scholar] [CrossRef]
- Walter, X.A.; You, J.; Gajda, I.; Greenman, J.; Ieropoulos, I.A. Impact of Feedstock Dilution on the Performance of Urine-Fed Ceramic and Membrane-Less Microbial Fuel Cell Cascades Designs. J. Power Sources. 2023, 561, 232708. [Google Scholar] [CrossRef]
- Permana, R.; Ihsan, Y.N.; Riyantini, I.; Agung, M.U. Comparative study between single-chamber and dual-chamber microbial fuel cell on petroleum hydrocarbon degradation in contaminated sediment. Appl. J. Environ. Eng. Sci. 2020, 6, 135–142. [Google Scholar] [CrossRef]
- Meignanalakshmi, S.; Kumar, S.V. Bioelectricity production by using goat (capra hircus) rumen fluid from slaughterhouse waste in mediator-less microbial fuel cells. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 1364–1369. [Google Scholar] [CrossRef]
- Javed, M.M.; Nisar, M.A.; Muneer, B.; Ahmad, M.U. Production of bioelectricity from vegetable waste extract by designing a U-shaped microbial fuel cell. Pak. J. Zool. 2017, 49, 711–716. [Google Scholar] [CrossRef]
- Apollon, W.; Kamaraj, S.K.; Rodríguez-Fuentes, H.; Gómez-Leyva, J.F.; Vidales-Contreras, J.A.; Mardueño-Aguilar, M.V.; Luna-Maldonado, A.I. Bioelectricity production in a single-chamber microbial fuel cell using urine as a substrate. Biofuels 2023, 15, 665–675. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, S.; Logan, B.E. Production of Electricity from Acetate or Butyrate Using a Single-Chamber Microbial Fuel Cell. Environ. Sci. Technol. 2005, 39, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Min, B.; Huang, L.; Angelidaki, I. Electricity generation and microbial community response to substrate changes in microbial fuel cell. Bioresour Technol. 2011, 102, 1166–1173. [Google Scholar] [CrossRef]
- Sun, G.; Thygesen, A.; Meyer, A.S. Acetate is a superior substrate for microbial fuel cell initiation preceding bioethanol effluent utilization. Appl. Microbiol. Biotechnol. 2015, 99, 4905–4915. [Google Scholar] [CrossRef]
- Asensio, Y.; Fernandez-Marchante, C.M.; Lobato, J.; Cañizares, P.; Rodrigo, M.A. Influence of the fuel and dosage on the performance of double-compartment microbial fuel cells. Water Res. 2016, 99, 16–23. [Google Scholar] [CrossRef]
- Yamashita, T.; Ishida, M.; Ogino, A.; Yokoyama, H. Evaluation of organic matter removal and electricity generation by using integrated microbial fuel cells for wastewater treatment. Environ. Technol. 2016, 37, 228–236. [Google Scholar] [CrossRef]
- Khater, D.Z.; El-Khatib, K.M.; Hassan, H.M. Microbial diversity structure in acetate single chamber microbial fuel cell for electricity generation. J. Genet. Eng. Biotechnol. 2017, 15, 127–137. [Google Scholar] [CrossRef]
- Wen, Q.; Wu, Y.; Cao, D.; Zhao, L.; Sun, Q. Electricity generation and modeling of microbial fuel cell from continuous beer brewery wastewater. Bioresour. Technol. 2009, 100, 4171–4175. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.E.-H.; Gomaa, O.M.; Fathey, R.; Kareem, H.A.E.; Zaid, M.A. Optimization of double chamber microbial fuel cell for domestic wastewater treatment and electricity production. J. Fuel Chem. Technol. 2015, 43, 1092–1099. [Google Scholar] [CrossRef]
- Liu, J.; Guo, T.; Wang, D.; Ying, H. Clostridium beijerinckii mutant obtained atmospheric pressure glow discharge generates enhanced electricity in a microbial fuel cell. Biotechnol. Lett. 2015, 37, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Jiang, Y.; Alvarado-Morales, M.; Treu, L.; Angelidaki, I.; Zhang, Y. Electricity generation and microbial communities in microbial fuel cell powered by macroalgal biomass. Bioelectrochemistry 2018, 123, 145–149. [Google Scholar] [CrossRef]
- Gao, C.; Wang, A.; Wu, W.M.; Yin, Y.; Zhao, Y.G. Enrichment of anodic biofilm inoculated with anaerobic or aerobic sludge in single chambered air-cathode microbial fuel cells. Bioresour. Technol. 2014, 167, 124–132. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Zhang, Y.; She, Z.; Shi, Y.; Wang, M.; Gao, M.; Guo, L. Effect of Substrate Conversion on Performance of Microbial Fuel Cells and Anodic Microbial Communities. Environ. Eng. Sci. 2017, 34, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Zang, G.L.; Sheng, G.P.; Tong, Z.H.; Liu, X.W.; Teng, S.X.; Li, W.W.; Yu, H.Q. Direct electricity recovery from Canna indica by an air-cathode microbial fuel cell inoculated with rumen microorganisms. Environ. Sci. Technol. 2010, 44, 2715–2720. [Google Scholar] [CrossRef]
- Catal, T.; Li, K.; Bermek, H.; Liu, H. Electricity production from twelve monosaccharides using microbial fuel cells. J. Power Sources 2008, 175, 196–200. [Google Scholar] [CrossRef]
- Rismani-Yazdi, H.; Christy, A.D.; Dehority, B.A.; Morrison, M.; Yu, Z.; Tuovinen, O.H. Electricity generation from cellulose by rumen microorganisms in microbial fuel cells. Biotechnol. Bioeng. 2007, 97, 1398–1407. [Google Scholar] [CrossRef]
- Chen, C.K.; Pai, T.Y.; Lin, K.L.; Ganesan, S.; Ponnusamy, V.K.; Lo, F.C.; Chiu, H.-Y.; Banks, C.J.; Lo, H.M. Electricity production from municipal solid waste using microbial fuel cells with municipal solid waste incinerator bottom ash as electrode plate. Bioresour. Technol. Rep. 2022, 19, 101210. [Google Scholar] [CrossRef]
- Ishii, S.; Suzuki, S.; Norden-Krichmar, T.M.; Wu, A.; Yamanaka, Y.; Nealson, K.H.; Bretschger, O. Identifying the microbial communities and operational conditions for optimized wastewater treatment in microbial fuel cells. Water Res. 2013, 47, 7120–7130. [Google Scholar] [CrossRef]
- Tarasov, S.; Plekhanova, Y.; Kashin, V.; Gotovtsev, P.; Signore, M.A.; Francioso, L.; Kolesov, V.; Reshetilov, A. Gluconobacter Oxydans-Based MFC with PEDOT:PSS/Graphene/Nafion Bioanode for Wastewater Treatment. Biosensors 2022, 12, 699. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.K.; Chang, I.S.; Moon, H.; Kang, K.H.; Kim, B.H. Nitrilotriacetic acid degradation under microbial fuel cell environment. Biotechnol. Bioeng. 2006, 95, 772–774. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.J.; Dwivedi, K.A.; Kumar, S.; Wang, C.T.; Yadav, A.K. Binder-free NiO/MnO2 coated carbon based anodes for simultaneous norfloxacin removal, wastewater treatment and power generation in dual-chamber microbial fuel cell. Environ. Pollut. 2023, 317, 120578. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.R.; Navidjouy, N.; Rahimnejad, M.; Alizadeh, S.; Samarghandi, M.R.; Nematollahi, D. Effect of different concentrations of substrate in microbial fuel cells toward bioenergy recovery and simultaneous wastewater treatment. Environ. Technol. 2022, 43, 1–9. [Google Scholar] [CrossRef]
- Suransh, J.; Mungray, A.K. Reduction in particle size of vermiculite and production of the low-cost earthen membrane to achieve enhancement in the microbial fuel cell performance. J. Environ. Chem. Eng. 2022, 10, 108787. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Guerrero–Barajas, C.; Ibrahim, M.N.M.; Umar, K.; Yaakop, A.S. Local fruit wastes driven benthic microbial fuel cell: A sustainable approach to toxic metal removal and bioelectricity generation. Environ. Sci. Pollut. Res. 2022, 29, 32913–32928. [Google Scholar] [CrossRef]
- Huang, L.; Logan, B.E. Electricity production from xylose in fed-batch and continuous-flow microbial fuel cells. Appl. Microbiol. Biotechnol. 2008, 80, 655–664. [Google Scholar] [CrossRef]
- Firdous, S.; Jin, W.; Shahid, N.; Bhatti, Z.A.; Iqbal, A.; Abbasi, U.; Mahmood, Q.; Ali, A. The performance of microbial fuel cells treating vegetable oil industrial wastewater. Environ. Technol. Innov. 2018, 10, 143–151. [Google Scholar] [CrossRef]
- Vargas, I.T.; Tapia, N.; Regan, J.M. Rumen Inoculum Enhances Cathode Performance in Single-Chamber Air-Cathode Microbial Fuel Cells. Materials 2022, 15, 379. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; De La Cruz-Noriega, M.; Nazario-Naveda, R.; Benites, S.M.; Delfín-Narciso, D.; Rojas-Villacorta, W.; Romero, C.V. Bioelectricity through microbial fuel cells using avocado waste. Energy Rep. 2022, 8, 376–382. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; De La Cruz-Noriega, M.; Nazario-Naveda, R.; Benites, S.M.; Delfín-Narciso, D.; Angelats-Silva, L.; Murga-Torres, E. Use of Banana Waste as a Source for Bioelectricity Generation. Processes 2022, 10, 942. [Google Scholar] [CrossRef]
- Zhao, G.; Ma, F.; Wei, L.; Chua, H.; Chang, C.-C.; Zhang, X.-J. Electricity generation from cattle dung using microbial fuel cell technology during anaerobic acidogenesis and the development of microbial populations. Waste Manag. 2012, 32, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Brar, Y.S.; Kaur, J.; Gupta, A.; Sharma, K.K.; Chohan, J.S.; Kumar, R.; Sharma, S.; Chattopadhyaya, S.; Dwivedi, S.P.; et al. Management of cattle dung and novel bioelectricity generation using microbial fuel cells: An Ingenious experimental approach. Int. J. Chem. Eng. 2021, 2021, 5536221. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zaveri, P.; Patel, R.; Shah, M.T.; Munshi, N.S. Optimization of microbial fuel cell process using a novel consortium for aromatic hydrocarbon bioremediation and bioelectricity generation. J. Environ. Manag. 2021, 298, 113546. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gao, J.; Meng, L.; Liu, J.; Zhao, D. The effect of crude oil on hydrolyzed polyacrylamide-containing wastewater treatment using microbial fuel cell biosystem. Process Saf. Environ. Prot. 2023, 179, 89–98. [Google Scholar] [CrossRef]
- Walter, X.A.; Merino-Jiménez, I.; Greenman, J.; Ieropoulos, I. PEE POWER® urinal II—Urinal scale-up with microbial fuel cell scale-down for improved lighting. J. Power Sources 2018, 15, 150–158. [Google Scholar] [CrossRef]
- Sharma, R.; Kumari, R.; Pant, D.; Malaviya, P. Bioelectricity generation from human urine and simultaneous nutrient recovery: Role of Microbial Fuel Cells. Chemosphere 2021, 292, 133437. [Google Scholar] [CrossRef]
- Walter, X.A.; Madrid, E.; Gajda, I.; Greenman, J.; Ieropoulos, I. Microbial fuel cell scale-up options: Performance evaluation of membrane (c-MFC) and membrane-less (s-MFC) systems under different feeding regimes. J. Power Sources 2022, 520, 230875. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Jain, S.C.; Ghangrekar, M.M. Cow’s urine as a yellow gold for bioelectricity generation in low cost clayware microbial fuel cell. Energy 2016, 113, 76–84. [Google Scholar] [CrossRef]
- Apollon, W.; Luna-Maldonado, A.I.; Kamaraj, S.K.; Vidales-Contreras, J.A.; Rodríguez-Fuentes, H.; Gómez-Leyva, J.F.; Maldonado-Ruelas, V.A.; Ortiz-Medina, R.A. Self-sustainable nutrient recovery associated to power generation from livestock’s urine using plant-based biobatteries. Fuel 2023, 332, 126252. [Google Scholar] [CrossRef]
- Cui, R.; Wang, J.; Liu, L.; Yu, T.; Li, Y.; Gao, C. Removal of radioactive ions in low-concentration nuclear industry wastewater with carbon-felt based iron/magnesium/zirconium polycrystalline catalytic cathode in a dual-chamber microbial fuel cell. J. Power Sources 2022, 528, 231208. [Google Scholar] [CrossRef]
- Kirmizakis, P.; Doherty, R.; Mendonca, C.A.; Costeira, R.; Allen, C.C.R.; Ofterdinger, U.S.; Kulakov, L. Enhancement of gasworks groundwater remediation by coupling a bioelectrochemical and activated carbon system. Environ. Sci. Pollut. Res. 2019, 26, 9981–9991. [Google Scholar] [CrossRef]
- Jabbar, N.; Alardhi, S.; Al-Jadir, T.M.; Abed Dhahad, H. Contaminants Removal from Real Refinery Wastewater Associated with Energy Generation in Microbial Fuel Cell. J. Ecol. Eng. 2023, 24, 107–114. [Google Scholar] [CrossRef]
- Sato, C.; Paucar, N.E.; Chiu, S.; Mahmud, M.Z.; Dudgeon, J. Single-Chamber Microbial Fuel Cell with Multiple Plates of Bamboo Charcoal Anode: Performance Evaluation. Processes 2021, 9, 2194. [Google Scholar] [CrossRef]
- Toczylowska-Maminska, R.; Szymona, K.; Kloch, M. Bioelectricity production from wood hydrothermal-treatment wastewater:Enhanced power generation in mfc-fed mixed wastewaters. Sci. Total Environ. 2018, 634, 586–594. [Google Scholar] [CrossRef]
- Toczyłowska-Mamińska, R.; Pielech-Przybylska, K.; Sekrecka-Belniak, A.; Dziekońska-Kubczak, U. Stimulation of electricity production in microbial fuel cells via regulation of syntrophic consortium development. Appl. Energy 2020, 271, 115184. [Google Scholar] [CrossRef]
- Jenol, M.A.; Ibrahim, M.F.; Kamal Bahrin, E.; Kim, S.W.; Abd-Aziz, S. Direct Bioelectricity Generation from Sago Hampas by Clostridium beijerinckii SR1 Using Microbial Fuel Cell. Molecules 2019, 24, 2397. [Google Scholar] [CrossRef]
- Christwardana, M.; Prabowo, A.K.; Tiarasukma, A.P.; Ariyanti, D. Microbial Fuel Cells for Simultaneous Electricity Generation and Organic Degradation from Slaughterhouse Wastewater. Int. J. Renew. Energy Dev. 2016, 5, 107–112. [Google Scholar] [CrossRef]
- Cabello-Torres, R.J.; Torres, E.V.; Pino, L.F.; Castañeda-Olivera, C.A.; Valdiviezo-Gonzales, L. Modeling of Renewable Energy Production from the Treatment of Slaughterhouse Wastewater with Ruminal Liquor in Microbial Fuel Cells. Chem. Eng. Trans. 2022, 91, 643–648. [Google Scholar] [CrossRef]
- Arulmani SR, B.; Ganamuthu, H.L.; Ashokkumar, V.; Govindarajan, G.; Kandasamy, S.; Zhang, H. Biofilm formation and electrochemical metabolic activity of Ochrobactrum Sp. JSRB-1 and Cupriavidus Sp JSRB-2 for energy production. Environ. Technol. Innov. 2020, 20, 101145. [Google Scholar] [CrossRef]
- Franks, A.E.; Malvankar, N.; Nevin, K.P. Bacterial biofilms: The powerhouse of a microbial fuel cell. Biofuels 2010, 1, 589–604. [Google Scholar] [CrossRef]
- Mahmoud, R.H.; Gomaa, O.M.; Hassan, R.Y. Bioelectrochemical frameworks governing microbial fuel cell performance: Technical bottlenecks and proposed solutions. RSC Adv. 2022, 12, 5749–5764. [Google Scholar] [CrossRef] [PubMed]
- Hemdan, B.A.; El-Taweel, G.E.; Naha, S.; Goswami, P. Bacterial community structure of electrogenic biofilm developed on modified graphite anode in microbial fuel cell. Sci. Rep. 2023, 13, 1255. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Chen, G.; Yu, R.; Li, H.; Wang, C. Enhanced simultaneous nitrification/denitrification in the biocathode of a microbial fuel cell fed with cyanobacteria solution. Process Biochem. 2016, 51, 80–88. [Google Scholar] [CrossRef]
- Ndayisenga, F.; Yu, Z.; Yan, G.; Phulpoto, I.A.; Li, Q.; Kumar, H.; Fu, L.; Zhou, D. Using easy-to-biodegrade cosubstrate to eliminate microcystin toxic on electrochemically active bacteria and enhance bioelectricity generation from cyanobacteria biomass. Sci. Total Environ. 2021, 751, 142292. [Google Scholar] [CrossRef]
- Imanthi, K.P.A.; Madusanka, D.A.T.; Pathmalal, M.M.; Idroos, F.S. Emerging trends of cyanobacteria-based microbial fuel cells as an alternative energy source. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2023; pp. 99–119. [Google Scholar] [CrossRef]
- Baniasadi, B.; Vahabzadeh, F. The performance of a cyanobacterial biomass-based microbial fuel cell (MFC) inoculated with Shewanella oneidensis MR-1. J. Environ. Chem. Eng. 2021, 9, 106338. [Google Scholar] [CrossRef]
- Gatti, M.N.; Milocco, R.H. A biofilm model of microbial fuel cells for engineering applications. Int. J. Energy Environ. Eng. 2017, 8, 303–315. [Google Scholar] [CrossRef]
- Yang, D.; Li, H.; Jia, X.; Yu, F.; Wang, G.; Zhang, Y.; Wang, W.; Zang, L.; Shi, F. Carbon Footprint of Monosodium Glutamate Production in China. Chem. Eng. Trans. 2023, 103, 739–744. [Google Scholar] [CrossRef]
- Tsiropoulos, I.; Cok, B.; Patel, M.K. Energy and greenhouse gas assessment of European glucose production from corn—A multiple allocation approach for a key ingredient of the biobased economy. J. Clean. Prod. 2013, 43, 182–190. [Google Scholar] [CrossRef]
- de Figueiredo, E.B.; Panosso, A.R.; Romão, R.; La Scala, N.J. Greenhouse gas emission associated with sugar production in southern Brazil. Carbon Balance Manag. 2010, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Ieropoulos, I.; Melhuish, C.; Greenman, J. Artificial Metabolism: Toward True Energetic Autonomy in Artificial Life. In Proceedings of the Advances in Artificial Life, 7th European Conference in Artificial Life (ECAL), Dortmund, Germany, 14–17 September 2003; Springer: Berlin/Heidelberg, Germany, 2003; pp. 792–799. [Google Scholar] [CrossRef]
- Zafar, H.; Peleato, N.M.; Roberts, D.J. Bioaugmentation with Bacillus subtilis and Cellulomonas fimi to Enhance the Biodegradation of Complex Carbohydrates in Mfc-Fed Fruit Waste. Biomass Bioenergy 2023, 174, 106843. [Google Scholar] [CrossRef]
- EEA (European Environment Agency). The European Environment—State and Outlook 2020: Knowledge for Transition to a Sustainable Europe. 2020. Available online: https://www.eea.europa.eu/soer/2020 (accessed on 1 December 2024).
- EPA (Environmental Protection Agency). 2020. Available online: https://www.epa.gov/air-emissions-inventories/2020-national-emissions-inventory-nei-data (accessed on 1 December 2024).
- Sun, M.; Sheng, G.P.; Mu, Z.X.; Liu, X.W.; Chen, Y.Z.; Wang, H.L.; Yu, H.Q. Manipulating the hydrogen production from acetate in a microbial electrolysis cell-microbial fuel cell-coupled system. J. Power Sources 2009, 191, 338–343. [Google Scholar] [CrossRef]
- Stager, J.L.; Zhang, X.; Logan, B.E. Addition of acetate improves stability of power generation using microbial fuel cells treating domestic wastewater. Bioelectrochemistry 2017, 118, 154–160. [Google Scholar] [CrossRef]
- Obileke, K.C.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial fuel cells, a renewable energy technology for bioelectricity generation: A mini-review. Electrochem. Commun. 2021, 125, 107003. [Google Scholar] [CrossRef]
- Ullah, Z.; Zeshan, S. Effect of substrate type and concentration on the performance of a double chamber microbial fuel cell. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2020, 81, 1336–1344. [Google Scholar] [CrossRef]
- Biffinger, J.C.; Byrd, J.N.; Dudley, B.L.; Ringeisen, B.R. Oxygen exposure promotes fuel diversity for Shewanella oneidensis microbial fuel cells. Biosens. Bioelectron. 2008, 23, 820–826. [Google Scholar] [CrossRef]
- Amjad, M.; Hussain, S.; Mubashir, A. A Perspective Review on Sugar Industry Wastes, Uses and Treatment Techniques. Open Access J. Waste Manag. Xenobiotics 2023, 6, 1–10. [Google Scholar] [CrossRef]
- Zhong, C.; Zhang, B.; Kong, L.; Xue, A.; Ni, J. Electricity generation from molasses wastewater by an anaerobic baffled stacking microbial fuel cell. J. Chem. Technol. Biotechnol. 2011, 86, 406–413. [Google Scholar] [CrossRef]
- Kushwaha, J.P. A review on sugar industry wastewater: Sources, treatment technologies, and reuse. Desalin. Water Treat. 2015, 53, 309–318. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, H.; Zhou, S.; Shi, C.; Wang, C.; Ni, J. A novel uasb–mfc–baf integrated system for high strength molasses wastewater treatment and bioelectricity generation. Bioresour. Technol. 2009, 100, 5687–5693. [Google Scholar] [CrossRef] [PubMed]
- Savvidou, M.G.; Pandis, P.K.; Mamma, D.; Sourkouni, G.; Argirusis, C. Organic Waste Substrates for Bioenergy Production via Microbial Fuel Cells: A Key Point Review. Energies 2022, 15, 5616. [Google Scholar] [CrossRef]
- Wu, C.H.; Liu, S.H.; Chu, H.L.; Li, Y.C.; Lin, C.W. Feasibility study of electricity generation and organics removal for a molasses wastewater by a waterfall-type microbial fuel cell. J. Taiwan Inst. Chem. Eng. 2017, 78, 150–156. [Google Scholar] [CrossRef]
- Bhatti, Z.A.; Syed, M.; Maqbool, F.; Zhao, Y.G.; Ying, X.; Siddiqui, M.F.; Mahmood, Q. Potential of molasses substrate for bioelectricity production in microbial fuel cell with the help of active microbial community. Int. J. Energy Res. 2022, 46, 11185–11199. [Google Scholar] [CrossRef]
- Fan, L.; Shi, J.; Xi, Y. PVDF-modified nafion membrane for improved performance of MFC. Membranes 2020, 10, 185. [Google Scholar] [CrossRef]
- Sakr, E.A.; Khater, D.Z.; Kheiralla, Z.M.; El-Khatib, K. Statistical optimization of waste molasses-based exopolysaccharides and self-sustainable bioelectricity production for dual chamber microbial fuel cell by Bacillus piscis. Microb. Cell Factories 2023, 22, 202. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, J.; Cheng, W.; Li, X.; Zhao, Y.; Wang, F.; Geng, Z.; Wang, Q.; Dong, Y. Synergistic interactions of microbial fuel cell and microbially induced carbonate precipitation technology with molasses as the substrate. Environ. Res. 2023, 228, 115849. [Google Scholar] [CrossRef]
- Miran, W.; Nawaz, M.; Kadam, A.; Shin, S.; Heo, J.; Jang, J.; Lee, D.S. Microbial community structure in a dual chamber microbial fuel cell fed with brewery waste for azo dye degradation and electricity generation. Environ. Sci. Pollut. Res. 2015, 22, 13477–13485. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ahmad, D.; Lesa, R. Electrolytic treatment of beer brewery wastewater. Ind. Eng. Chem. Res. 2006, 45, 6854–6859. [Google Scholar] [CrossRef]
- Khalid, S.; Alvi, F.; Fatima, M.; Aslam, M.; Riaz, S.; Farooq, R.; Zhang, Y. Dye degradation and electricity generation using microbial fuel cell with graphene oxide modified anode. Mater. Lett. 2018, 220, 272–276. [Google Scholar] [CrossRef]
- Ismail, Z.Z.; Mohammed, A.J. Biotreatment of Slaughterhouse Wastewater Accompanied with Electrcity Generation and Nutrients Recovery in Microbial Fuel Cell. J. Eng. 2017, 23, 94–105. [Google Scholar] [CrossRef]
- Mohammed, A.J.; Ismail, Z.Z. Slaughterhouse wastewater biotreatment associated with bioelectricity generation and nitrogen recovery in hybrid system of microbial fuel cell with aerobic and anoxic bioreactors. Ecol. Eng. 2018, 125, 119–130. [Google Scholar] [CrossRef]
- Mkilima, T.; Saspugayeva, G.; Dakieva, K.Z.; Tussupova, Z.; Zhaken, A.; Kumarbekuly, S.; Daribay, A.; Khussainov, M. Enhancing slaughterhouse wastewater treatment through the integration of microbial fuel cell and Electro-Fenton systems: A comprehensive comparative analysis. J. Water Process Eng. 2024, 57, 104743. [Google Scholar] [CrossRef]
- El Moussaoui, T.; Kessraoui, A.; Ouazzani, N.; Seffen, M.; Mandi, L. Synthetic urban wastewater treatment by an activated sludge reactor: Evolution of bacterial biomass and purifying efficiency. J. Mater. Environ. Sci. 2018, 9, 817–827. [Google Scholar] [CrossRef]
- Oveisi, F.; Fallah, N.; Nasernejad, B. Biodegradation of synthetic wastewater containing styrene in microbial fuel cell: Effect of adaptation of microbial community. Fuel 2021, 305, 121382. [Google Scholar] [CrossRef]
- Aldrovandi, A.; Marsili, E.; Stante, L.; Paganin, P.; Tabacchioni, S.; Giordano, A. Sustainable power production in a membrane-less and mediator-less synthetic wastewater microbial fuel cell. Bioresour. Technol. 2009, 100, 3252–3260. [Google Scholar] [CrossRef]
- Flimban, S.G.A.; Ismail, I.M.I.; Kim, T.; Oh, S.-E. Overview of Recent Advancements in the Microbial Fuel Cell from Fundamentals to Applications: Design, Major Elements, and Scalability. Energies 2019, 12, 3390. [Google Scholar] [CrossRef]
- Rajaguru, P.; Kalaiselvi, K.; Palanivel, M.; Subburam, V. Biodegradation of azo dyes in a sequential anaerobic-aerobic system. Appl. Microbiol. Biotechnol. 2000, 54, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xie, W.; Wang, R.; Wu, X.; Yu, L.; Qiao, Y. Fast Start-Up Microfluidic Microbial Fuel Cells with Serpentine Microchannel. Front. Microbiol. 2018, 9, 2816. [Google Scholar] [CrossRef]
- Apollon, W.; Flores-Breceda, H.; Méndez-Zamora, G.; Gómez-Leyva, J.F.; Luna-Maldonado, A.I.; Kamaraj, S.-K. Importance of Genetically Engineered Microbes (GEMs) in Bioremediation of Environmental Pollutants: Recent Advances and Challenges. In Omics for Environmental Engineering and Microbiology Systems; Kumar, V., Garg, V.K., Kumar, S., Biswas, J.K., Eds.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Sonu, K.; Sogani, M.; Syed, Z.; Rajvanshi, J.; Sengupta, N. Effectiveness of rice husk in the removal of methyl orange dye in Constructed Wetland-Microbial Fuel Cell. Bioresour. Technol. Rep. 2022, 20, 101223. [Google Scholar] [CrossRef]
- Ahmadpour, T.; Aber, S.; Ghasem Hosseini, M. Visible-light enhanced azo dye degradation and power generation in a microbial photoelectrochemical cell using AgBr/ZnO composite photocathode. Bioelectrochemistry 2022, 146, 108139. [Google Scholar] [CrossRef] [PubMed]
- Rusyn, I.B.; Moroz, O.M.; Karabyn, V.V.; Kulachkovs’kiĭ, O.R.; Hudz, S.P. Biodegradation of oil hydrocarbons by Candida yeast. Mikrobiolohichnyi Zhurnal 2003, 65, 36–42. [Google Scholar]
- Ghosh, S.; Rusyn, I.; Dmytruk, O.V.; Dmytruk, K.V.; Onyeaka, H.; Gryzenhout, M.; Gafforov, Y. Filamentous fungi for sustainable remediation of pharmaceutical compounds, heavy metal and oil hydrocarbons. Front. Bioeng. Biotechnol. 2023, 11, 1106973. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Tang, S.; Xie, S.X.; Wang, P.; Huang, C.; Geng, X.; Jia, X.; Huo, H.; Li, X.; Zhang, J.; et al. Oil removal and the characteristics of changes in the composition of bacteria based on the oily sludge bioelectrochemical system. Sci. Rep. 2020, 10, 15474. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, Q.; Mo, F.; Li, T.; Liu, J. Bioelectrochemical degradation of petroleum hydrocarbons: A critical review and future perspectives. Environ. Pollut. 2022, 306, 119344. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Govindarajan, D.; Mohanakrishna, G.; Thatikayala, D.; Abu-Reesh, I.M.; Min, B.; Nambi, I.M.; Al-Raoush, R.I.; Aminabhavi, T.M. Sustainable bioelectrochemical systems for bioenergy generation via waste treatment from petroleum industries. Fuel 2023, 331, 125632. [Google Scholar] [CrossRef]
- Abdel-Hamid, A.M.; Solbiati, J.O.; Cann, I.K.O. Insights into lignin degradation and its potential industrial applications. Adv. Appl. Microbiol. 2013, 82, 1–28. [Google Scholar] [CrossRef]
- Börcsök, Z.; Pásztory, Z. The role of lignin in wood working processes using elevated temperatures: An abbreviated literature survey. Eur. J. Wood Wood Prod. 2020, 79, 511–526. [Google Scholar] [CrossRef]
- Rezaei, F.; Richard, T.L.; Logan, B.E. Analysis of chitin particle size on maximum power generation, power longevity, and Coulombic efficiency in solid-substrate microbial fuel cells. J. Power Sources 2009, 192, 304–309. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Khawdas, W.; Aso, Y.; Ohara, H. Microbial fuel cells using Cellulomonas spp. with cellulose as fuel. J. Biosci. Bioeng. 2017, 123, 358–363. [Google Scholar] [CrossRef]
- Justin Cook, C. Potatoes, Milk, and the Old World Population Boom. J. Dev. Econ. 2014, 110, 123–138. [Google Scholar] [CrossRef]
- Du, H.; Li, F. Enhancement of solid potato waste treatment by microbial fuel cell with mixed feeding of waste activated sludge. J. Clean. Prod. 2017, 143, 336–344. [Google Scholar] [CrossRef]
- Sato, C.; Apollon, W.; Luna-Maldonado, A.I.; Paucar, N.E.; Hibbert, M.; Dudgeon, J. Integrating Microbial Fuel Cell and Hydroponic Technologies Using a Ceramic Membrane Separator to Develop an Energy–Water–Food Supply System. Membranes 2023, 13, 803. [Google Scholar] [CrossRef] [PubMed]
- Din, M.I.; Iqbal, M.; Hussain, Z.; Khalid, R. Bioelectricity generation from waste potatoes using single chambered microbial fuel cell. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 46, 12596–12606. [Google Scholar] [CrossRef]
- Radeef, A.Y.; Ismail, Z.Z. Resource recovery in potato chips processing industry: Green bioelectricity production using continuous mediatorless microbial fuel cell. Int. J. Green Energy 2021, 18, 910–919. [Google Scholar] [CrossRef]
- Siddique, M.N.I.; Zaied, B.K.; Ibrahim, M.Z.B. Feasibility and synergistic effect of glucoamylase hydrolysis and microbial fuel cell on electricity generation from potato-waste. Ecol. Environ. Conserv. 2022, 28, 615–620. [Google Scholar] [CrossRef]
- Santoro, C.; García, M.J.; Walter, X.A.; You, J.; Theodosiou, P.; Gajda, I.; Obata, O.; Winfield, J.; Greenman, J.; Ieropoulos, I.A. Urine in Bioelectrochemical Systems: An Overall Review. Chemelectrochem 2020, 7, 1312–1331. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Greenman, J.; Melhuish, C. Urine utilization by microbial fuel cells; Energy fuel for the future. Phys. Chem. Chem. Phys. 2012, 14, 94–98. [Google Scholar] [CrossRef]
- Kuntke, P.; Śmiech, K.; Bruning, H.; Zeeman, G.; Saakes, M.; Sleutels, T.; Hamelers, H.V.; Buisman, C.J. Ammonium recovery and energy production from urine by a microbial fuel cell. Water Res. 2012, 46, 2627–2636. [Google Scholar] [CrossRef]
- Papaharalabos, G.; Greenman, J.; Melhuish, C.; Santoro, C.; Cristiani, P.; Li, B.; Ieropoulos, I.A. Increased power output from micro porous layer (MPL) cathode microbial fuel cells (MFC). Int. J. Hydrogen Energy 2013, 38, 11552–11558. [Google Scholar] [CrossRef]
- Ieropoulos, I.A.; Ledezma, P.; Stinchcombe, A.; Papaharalabos, G.; Melhuish, C.; Greenman, J. Waste to real energy: The first MFC powered mobile phone. Phys. Chem. Chem. Phys. PCCP 2013, 15, 15312–15316. [Google Scholar] [CrossRef]
- Ieropoulos, I.A.; Stinchcombe, A.; Gajda, I.; Forbes, S.; Merino-Jimenez, I.; Pasternak, G.; Sanchez-Herranz, D.; Greenman, J. Pee power urinal—Microbial fuel cell technology field trials in the context of sanitation. Environ. Sci. Water Res. Technol. 2016, 2, 336–343. [Google Scholar] [CrossRef]
- Walter, X.A.; Santoro, C.; Greenman, J.; Ieropoulos, I.A. Scalability and stacking of self-stratifying microbial fuel cells treating urine. Bioelectrochemistry 2020, 133, 107491. [Google Scholar] [CrossRef] [PubMed]
- Mali, B.M.; Gavimath, C.C.; Hooli, V.R.; Patil, A.B.; Gaddi, D.P.; Ternikar, C.R.; Ravishankera, B.E. Generation of bioelectricity using waste water. Int. J. Adv. Biotechnol. Res. 2012, 3, 537–540. [Google Scholar]
- Vignesh, H.; Rani, H.K. Generation of bioelectricity from waste water and cow’s urine. Indian J. Appl. Res. 2012, 1, 16–19. [Google Scholar]
- Javalkar, P.D.; Alam, J.M. Comparative Study on Sustainable Bioelectricity Generation from Microbial Fuel Cell Using Biowaste as Fuel. Int. J. Sci. Res. Publ. 2013, 3, 1–6. [Google Scholar]
- Gireeshan, M.G.; Vasuki, R.; Krishnakumar, T. High power production from elephant’s urine. Int. J. Pharm. Technol. 2014, 6, 6714–6718. [Google Scholar]
- Cheng, P.; Shan, R.; Yuan, H.; Shen, W.; Chen, Y. Bioelectricity generation from the salinomycin-simulated livestock sewage in a Rhodococcus pyridinivorans inoculated microbial fuel cell. Process Saf. Environ. Prot. 2020, 138, 76–79. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, T.; Chen, J.; Guo, J.; Luo, H.; Chen, W.; Mo, Y.; Wei, Z.; Huang, X. The reduction and fate of antibiotic resistance genes (ARGs) and mobile genetic elements (MGEs) in microbial fuel cell (MFC) during treatment of livestock wastewater. J. Contam. Hydrol. 2022, 247, 103981. [Google Scholar] [CrossRef]
- Cheng, P.; Yang, C.; Zhou, S.; Huang, J.; Liu, R.; Yan, B. Degradation efficiency of antibiotics by the sewage-fed microbial fuel cells depends on gram-staining property of exoelectrogens. Process. Saf. Environ. Prot. 2023, 176, 421–429. [Google Scholar] [CrossRef]
- Saxena, D.; Sandhwar, V.K. Cow farm wastes: A bioresource for sustainable development. In Advanced Zero Waste Tools, Bio-Based Materials and Waste for Energy Generation and Resource Management; Hussain, C.M., Kushwaha, A., Bharagava, R.N., Goswami, L.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 5, pp. 411–429. [Google Scholar] [CrossRef]
- Apollon, W.; Vidales-Contreras, J.A.; Rodríguez-Fuentes, H.; Gómez-Leyva, J.F.; Olivares-Sáenz, E.; Maldonado-Ruelas, V.A.; Ortiz-Medina, R.A.; Kamaraj, S.-K.; Luna-Maldonado, A.I. Livestock’s Urine-Based Plant Microbial Fuel Cells Improve Plant Growth and Power Generation. Energies 2022, 15, 6985. [Google Scholar] [CrossRef]
- Apollon, W.; Valera-Montero, L.L.; Perales-Segovia, C.; Maldonado-Ruelas, V.A.; Ortiz-Medina, R.A.; Gómez-Leyva, J.F.; Vázquez-Gutiérrez, M.A.; Flores-Benítez, S.; Kamaraj, S.K. Effect of ammonium nitrate on novel cactus pear genotypes aided by biobattery in a semiarid ecosystem. Sustain. Energy Technol. Assess. 2022, 49, 101730. [Google Scholar] [CrossRef]
- Rusyn, I. Role of microbial community and plant species in performance of plant microbial fuel cells. Renew. Sustain. Energy Rev. 2021, 152, 111697. [Google Scholar] [CrossRef]
- Kuleshova, T.E.; Galushko, A.S.; Panova, G.G.; Volkova, E.N.; Apollon, W.; Shuang, C.; Sevda, S. Bioelectrochemical systems based on the electroactivity of plants and microorganisms in the root environment (review). Sel’skokhozyaistvennaya Biol. [Agric. Biol.] 2022, 57, 425–440. [Google Scholar] [CrossRef]
- Sophia, A.C.; Sreeja, S. Green energy generation from plant microbial fuel cells(PMFC) using compost and a novel clay separator. Sustain. Energy Technol. 2017, 21, 59–66. [Google Scholar] [CrossRef]
- Oodally, A.; Gulamhussein, M.; Randall, D.G. Investigating the performance ofconstructed wetland microbial fuel cells using three indigenous South Africanwetland plants. J. Water Process Eng. 2019, 32, 100930. [Google Scholar] [CrossRef]
- Rusyn, I.; Hamkalo, K.R. Use of Carex hirta in electrobiotechnological systems on green roofs. Regul. Mech. Biosyst. 2019, 10, 39–44. [Google Scholar] [CrossRef]
- Manitoba. Properties of Manure. 2015. Available online: https://www.gov.mb.ca/agriculture/environment/nutrient-management/pubs/properties-of-manure.pdf (accessed on 11 October 2022).
- Syed, Z.; Sonu, K.; Sogani, M. Cattle manure management using microbial fuel cells for green energy generation. Biofuels Bioprod. Biorefining 2022, 16, 460–470. [Google Scholar] [CrossRef]
- Magotra, V.K.; Kang, T.W.; Kim, D.; Inamdar, A.I.; Walke, P.D.; Lee, S.; Chavan, H.S.; Kadam, A.A.; Im, H.; Jeon, H.C. Urea fuel cell using cow dung compost soil as a novel biocatalyst for power generation applications. Energy 2022, 239, 122357. [Google Scholar] [CrossRef]
- Mahmoud, M.; El-Kalliny, A.S.; Squadrito, G. Stacked titanium dioxide nanotubes photoanode facilitates unbiased hydrogen production in a solar-driven photoelectrochemical cell powered with a microbial fuel cell treating animal manure wastewater. Energy Convers. Manag. 2022, 254, 115225. [Google Scholar] [CrossRef]
- Adegunloye, D.V.; Olusegun-Awosika, D.B.; Odjegba, P.I. Production potential of cow dung for the generation of electric current using microbial fuel cell. World J. Adv. Res. Rev. 2023, 17, 533–548. [Google Scholar] [CrossRef]
- Vishwanathan, A.S. Microbial fuel cells: A comprehensive review for beginners. 3 Biotech 2021, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, D.A.; Das, I.; Ghangrekar, M.M.; Pant, D. Moving toward practical applications of microbial fuel cells for sanitation and resource recovery. J. Water Process Eng. 2020, 38, 101566. [Google Scholar] [CrossRef]
- Apollon, W. An Overview of Microbial Fuel Cell Technology for Sustainable Electricity Production. Membranes 2023, 13, 884. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Fu, Q.; Liao, Q.; Zhu, X.; Kobayashi, H.; Ye, D. Voltage reversal causes bioanode corrosion in microbial fuel cell stacks. Int. J. Hydrogen Energy 2017, 42, 27649–27656. [Google Scholar] [CrossRef]
- Prasad, J.; Tripathi, R.K. Review on improving microbial fuel cell power management systems for consumer applications. Energy Rep. 2022, 8, 10418–10433. [Google Scholar] [CrossRef]
- Osorio-de-la-Rosa, E.; Vazquez-Castillo, J.; Castillo-Atoche, A.; Heredia-Lozano, J.; Castillo-Atoche, A.; Becerra-Nunez, G.; Barbosa, R. Arrays of plant microbial fuel cells for implementing self-sustainable wireless sensor networks. IEEE Sens. J. 2020, 21, 1965–1974. [Google Scholar] [CrossRef]
- Naik, S.; Jujjavarapu, S.E. Self-powered and reusable microbial fuel cell biosensor for toxicity detection in heavy metal polluted water. J. Environ. Chem. Eng. 2021, 9, 105318. [Google Scholar] [CrossRef]
- Apollon, W.; Kamaraj, S.K.; Silos-Espino, H.; Perales-Segovia, C.; Valera-Montero, L.L.; Maldonado-Ruelas, V.A.; Vázquez-Gutiérrez, M.A.; Ortiz-Medina, R.A.; Flores-Benítez, S.; Gómez-Leyva, J.F. Impact of Opuntia species plant biobattery in a semiarid environment: Demonstration of their applications. Appl. Energy 2020, 279, 115788. [Google Scholar] [CrossRef]
- Rusyn, I.; Medvediev, O. Stacking and design optimization of novel plant microbial fuel cell based on dwarf indoor decorative and culinary plants as a compact biobattery for a low energy consumption devices. Bioresour. Technol. Rep. 2024, 26, 101860. [Google Scholar] [CrossRef]
- Mukherjee, A.; Patel, V.; Shah, M.T.; Jadhav, D.A.; Munshi, N.S.; Chendake, A.D.; Pant, D. Effective power management system in stacked microbial fuel cells for onsite applications. J. Power Sources 2022, 517, 230684. [Google Scholar] [CrossRef]
- Lefebvre, O.; Uzabiaga, A.; Chang, I.S.; Kim, B.H.; Ng, H.Y. Microbial fuel cells for energy self-sufficient domestic wastewater treatment—A review and discussion from energetic consideration. Appl. Microbiol. Biotechnol. 2011, 89, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Wang, S.; Wang, K.; Wang, M.; Chen, B.; Wang, Z.; Tan, T. Improved energy efficiency in microbial fuel cells by bioethanol and electricity co-generation. Biotechnol. Biofuels Bioprod. 2022, 15, 84. [Google Scholar] [CrossRef]
- Pocaznoi, D.; Calmet, A.; Etcheverry, L.; Erable, B.; Bergel, A. Stainless steel is a promising electrode material for anodes of microbial fuel cells. Energy Environ. Sci. 2012, 5, 9645–9652. [Google Scholar] [CrossRef]
- Mahanta, V.; Raja, M.; Kothandaraman, R. Activated carbon from sugarcane bagasse as a potential positive electrode catalyst for vanadium redox flow battery. Mater. Lett. 2019, 247, 63–66. [Google Scholar] [CrossRef]
- Dhand, V.; Yadav, M.; Kim, S.H.; Rhee, K.Y. A comprehensive review on the prospects of multifunctional carbon nano onions as an effective, high-performance energy storage material. Carbon 2021, 175, 534–575. [Google Scholar] [CrossRef]
- Taer, E.; Taslim, R.; Putri, A.; Apriwandi, A.; Agustino, A. Activated carbon electrode made from coconut husk waste for supercapacitor application. Int. J. Electrochem. Sci. 2018, 13, 12072–12084. [Google Scholar] [CrossRef]
- Gómez-Urbano, J.L.; Moreno-Fernández, G.; Arnaiz, M.; Ajuria, J.; Rojo, T.; Carriazo, D. Graphene-coffee waste derived carbon composites as electrodes for optimized lithium ion capacitors. Carbon 2020, 162, 273–282. [Google Scholar] [CrossRef]
- Abd-Elrahman, N.K.; Al-Harbi, N.; Basfer, N.M.; Al-Hadeethi, Y.; Umar, A.; Akbar, S. Applications of nanomaterials in microbial fuel cells: A review. Molecules 2022, 27, 7483. [Google Scholar] [CrossRef]
- Rusyn, I.B.; Hamkalo, K.R. Electrobiosystems with mosses on the green roofs. Environ. Res. Eng. Manag. 2020, 76, 20–30. [Google Scholar] [CrossRef]
- Rusyn, I.; Gómora-Hernández, J.C. Constructed wetland microbial fuel cell as enhancing pollutants treatment technology to produce green energy. Biotechnol. Adv. 2024, 77, 108648. [Google Scholar] [CrossRef] [PubMed]


| Type of MFC | MFC Prototype/Working Volume, Electrodes | Type of Substrate | Substrate Concentration | Inoculum Source | Maximum Performance | References |
|---|---|---|---|---|---|---|
| Traditionally Used Organic Substrates and Wastes in MFC | ||||||
| SC-MFC | 28 mL MFC with Toray carbon paper as the anode and carbon paper containing 0.35 mg cm−2 Pt as the cathode | Acetate | 141 mg L−1 | DWW | 506 mW m−2 | [46] |
| DC-MFC | 250/300 mL MFC with Toray carbon paper as the anode and cathode (42 cm2) | Acetate, glucose, and butyrate | 1000 mg L−1 (COD) | DWW | 52–72 mW m−2 | [47] |
| DC-MFC | 300 mL MFC with carbon paper sheets as the anode and cathode (7.1 cm2) | Acetate | 1.28 g L−1 | DWW | 362 mW m−2 | [48] |
| DC-MFC | 4 cm3 two reactors with carbon felts as electrodes | Acetate | 5000 mg L−1 (COD) | Activated sludge from a wastewater treatment plant | 500 mW m−2 | [49] |
| SC-MFC | 2 L Fa-MFC with brush anodes floating in a beaker | Acetate | 465–1029 mg L−1 d−1 | Artificial wastewater | 152 mW m−2 cathode area | [50] |
| SC-MFC | 2 L Fa-MFC with brush anodes floating in a beaker | Acetate | 45–119 mg L−1 d−1 | Livestock wastewater | 95 mW m−2 cathode area | [50] |
| SC-MFC | 50 mL MFC with Teflon-treated carbon paper as the cathode and carbon paper as the anode (0.3 mg cm−2 Pt/C loaded); electrode area of 25 cm2) | Acetate | 0.3 mg cm2 | Mixed culture (aerobic activated sludge) | 86.1 mW m−2 | [51] |
| SC-MFC | One-chamber air cathode with carbon fibers as an anode | Beer brewery wastewater | 600 mg L−1 | Anaerobic mixed consortia | 264 mW m−2 | [52] |
| SC-MFC | 28 mL MFC with Toray carbon paper as the anode electrode and carbon paper containing 0.35 mg/cm2 Pt as the cathode electrode | Butyrate | 93 mg L−1 | DWW | 305 mW m−2 | [46] |
| DC-MFC | 100 mL MFC with mild steel coated with Fe2TiO5 and stainless-steel electrodes | Crude sugarcane effluent | 4538 mg L−1 | Anaerobic sludge | 8314 mW m−2 | [27] |
| DC-MFC | 300 mL MFC with a silver anode and cathode (54.57 cm2) | Domestic wastewater | 100 mmol L−1 | Bacterial consortia | 117–209 mW m−2 | [53] |
| DC-MFC | 250 mL MFC with a graphite felt anode and cathode electrodes (effective area of 25 cm2) | Glucose | 1 g L−1 | Clostridium beijerinckii M13 | 79.2 mW m−2 | [54] |
| DC-MFC | 500 mL bottle-MFC with a carbon brush (5.0 cm in diameter, 5.0 cm in length) as the anode and cathode | Glucose and mannitol | 43.65 g L−1 (glucose) and 14 g L−1 (mannitol) | L. digitata 300 mL of DWW | 0.5 V | [55] |
| SC-MFC | 28 mL MFC with a carbon brush as an anode and carbon cloth as an air cathode (7 cm2) | Lactate | 500 mg L−1 | Aerobic sludge | 5.79 W m−3 | [56] |
| SC-MFC | 28 mL MFC with a carbon brush as an anode and carbon cloth as an air cathode (7 cm2) | Lactate | 500 mg L−1 | Anaerobic sludge | 3.66 W m−3 | [56] |
| SC-MFC | 28 mL MFC with a carbon brush anode and carbon cloth cathode | Lactate | 500 mg L−1 | Pretreated sludge | 1.65 W m−3 | [57] |
| SC-MFC | 400 mL MFC with Pt-coated carbon paper (2 × 2 cm2, 2 mg cm−2) as the cathode and plain carbon paper (3 × 3 cm2) as the anode | Lignocellulosic biomass | 4 g L−1 | Rumen microorganisms | 0.405 W m−3 | [58] |
| SC-MFC | 12 mL reactor coated with a carbon, polytetrafluoroethylene, and platinum cathode (7.0 cm2) and anode (2.0 cm2) | Lignocellulosic biomass | 480 mg L−1 (6.7 mM glucuronic acid) | Mixed bacterial culture | 1410–2760 mW m−2 | [59] |
| DC-MFC | 400 mL MFC with graphite plates as electrodes (84 cm2) | Microcrystalline cellulose | 7.5 g L−1 | Rumen microorganisms | 55 mW m−2 | [60] |
| DC-MFC | 1.8 L MFC with an anode and cathode chamber (10 cm × 10 cm × 18 cm) | Municipal solid waste | 0.2 mg L−1 | Anaerobic sludge | 37.808 mW m−2 | [61] |
| SC-MFC | 350 mL MFC with carbon cloth electrodes (84 cm2) | Municipal wastewater | 250 mg L−1 | Microorganisms | 0.2–0.3 mA | [62] |
| DC-MFC | 50 mL MFC with graphite rods as the anode and cathode (20 cm2) | Municipal wastewater | 279 mg O2 L−1 | Gluconobacter oxydans | 65 mW m−2 | [63] |
| DC-MFC | 25 mL MFC with graphite felt as electrodes (24 cm2) | Nitrilotriacetic acid | 48.5 mg L−1 | Oligotrophic consortium enriched with river water | 0.0005 mA cm−2 | [64] |
| DC-MFC | Two 250 mL MFC reactors with graphite felt and activated carbon cloth electrodes, coated with NiO/MnO2 | Wastewater treatment plant | 0 mg L−1 (norfloxacin) | n/a | 1696.56 mW m−2 | [65] |
| DC-MFC | Two 250 mL MFC reactors with graphite felt and activated carbon cloth electrodes, coated with NiO/MnO2 | Wastewater treatment plant | 20 mg L−1 (norfloxacin) | n/a | 1295.91 mW m−2 | [63] |
| SC-MFC | 2600 mL MFC with graphite plates electrodes (166.81 cm2) | Raw distillery effluent | 1, 53, 846 mg L−1 (COD) | Microbial community of distillery effluent | 25,194.8 mW m−2 | [28] |
| DC-MFC | 240 mL MFC with carbon felt as the anode and cathode (30 cm2) | Synthetic wastewater with glucose | 100 mg L−1 (COD) 0.5–10 g L−1 (glucose) | Anaerobic sludge | 50.7 mW m−2 | [66] |
| SC-MFC | 150 mL reactor with carbon felt as the anode (16 cm2) and cathode (31 cm2) | Synthetic wastewater | 989.5 mg L−1 (COD) | 1% anaerobic sludge | 995.73 mW m−3 | [67] |
| DC-MFC | 500 mL MFC with graphite rods as the anode and cathode | Synthetic wastewater | 50 mg L−1 | Fruit wastes | 87.71 mA m−2 | [68] |
| SC-MFC | 800 mL MFC with a carbon cloth cathode (144 cm2 = 12 × 12 cm2), containing 0.35 mg cm−2 Pt, and graphite fiber brushes (6 cm in outer diameter and 7 cm long) as the anode electrode, total surface area of 4.2 m2 | Xylose | 20 mM | Mixed bacterial culture | 673 mW m−2 | [69] |
| DC-MFC | 500 mL with two chambers with a titanium rod anode and a carbon cloth cathode | Vegetable oil industrial wastewater | n/a | 100 mL of sewage sludge | 6119 mW m−2 | [70] |
| SC-MFC | 28 mL reactor with a graphite fiber anode and a platinum catalyst–carbon cloth cathode | Wastewater | 1 g L−1 (glucose) | 14 mL of rumen inoculum | 824.5 mW m−2 | [71] |
| Type of MFC | MFC Prototype/Working Volume, Electrodes | Type of Substrate | Substrate Concentration | Inoculum Source | Maximum Performance | References |
|---|---|---|---|---|---|---|
| Nonconventional Organic Sources for MFCs | ||||||
| SC-MFC | 150 mL MFC with a zinc anode and copper cathode | Avocado waste | 100% decomposing avocado | N/A | 5736.112 mW cm−2 | [72] |
| SC-MFC | 150 mL MFC with a zinc anode and copper cathode (both with an area of 80 cm2) | Banana waste | 100% decomposing banana | N/A | 566.80 mW cm−2 | [73] |
| DC-MFC | 15 L MFC with carbon brushes as electrodes | Cattle dung/ acetate and butyrate | 2059.7 and 369.1 mg L−1 | Biogas slurry | 0.220 W m−3 | [74] |
| SC-MFC | 700 mL MFC with zinc–carbon electrodes | Cattle dung | 100% cattle dung slurry | Biogas slurry | 1465 mW m−2 1858 mA m−2 | [75] |
| DC-MFC | 1.2 L MFC with graphite felt as the electrode material with a surface area of 0.0108 m2 | Effluent wastewater with sodium benzoate C7H5NaO2 | 1% sucrose 5 mM C7H5NaO2 | Novel consortium | 18.15 mW m−2 | [76] |
| DC-MFC | 60 cm × 30 cm × 30 cm MFC with 12 L cathode and 36 L anode chambers with PEM and carbon fiber felt electrodes | HPAM-containing oilfield wastewater | 300 mg⋅L−1 crude oil, 508 mg⋅L−1 HPAM | Activated anoxic sludge | 2420 mW m−2 | [77] |
| SC-MFC | 19.2 L cascade of 4 modules. Individual SSM-MFC module (400 mm × 300 mm × 170 mm) with an AC-PTFE cathode and carbon fiber veil anode | Human urine | 100% urine | Activated sludge | 9.9 W m−3 | [78] |
| DC-MFC | 10 L scale MFC stack with individual 0.5 L module (17.5 × 14.5 × 2 cm3), CEM and stainless-steel mesh electrodes | Human urine | 100% urine | Anaerobic digestate | 14.5 mW m−2 | [79] |
| SC-MFC | 2211 mL stack. Each 435 mL c-MFC module consists of 8 individual earthenware ceramic cylinders (50 mm × 21 mm × 28 mm) enclosed in a cylindrical (140 mm) PVC vessel with a parallel-connected carbon veil cathode and anode, coated AC/PTFE, and stapled stainless-steel mesh | Human urine | 100% urine | Activated sludge | 32.2 mW m−3, 19.36 mW per single module | [80] |
| SC-MFC | Each 525 mL s-MFC module consist of 28 parallel-connected carbon veil cathode–anode pairs coated AC/PTFE and assembled on stainless-steel mesh and enclosed in cylindrical PVC vessel | Human urine | 100% urine | Activated sludge | 69.7 mW m−3, 23.43 mW per single module | [80] |
| SC-MFC | Cascade of 3 modules. Each 435 mL c-MFC module consist of 8 individual earthenware ceramic cylinders (50 mm × 21 mm × 28 mm) enclosed in a cylindrical (140 mm) PVC vessel with a parallel-connected carbon veil cathode and anode, coated AC/PTFE, and stapled stainless-steel mesh | Human urine | 100% urine | Activated sludge | 6.45 mW per single module, 17.99 mW per cascade of 3 modules | [41] |
| SC-MFC | Cascade of 3 modules. Each 525 mL s-MFC module consist of 28 parallel connected carbon veil cathode–anode pairs coated AC/PTFE and assembled on stainless-steel mesh and enclosed in a cylindrical PVC vessel | Human urine | 100% urine | Activated sludge | 7.80 mW per single module, 26.45 mW per cascade of 3 modules | [41] |
| DC-MFC | 2.5 L cathodic plastic bucket chamber and 0.4 L inner clayware pot anodic chamber | Livestock urine | 3 kg COD m−3-diluted cow’s urine | Anaerobic sludge | 5230 mW m−3 | [81] |
| SC-MFC | 643 mL P-MFC with a graphite felt anode and a stainless-steel mesh cathode | Livestock urine | 100% cow urine | N/A | 42.79 mW m−2 | [82] |
| SC-MFC | 643 mL P-MFC with a graphite felt anode and a stainless-steel mesh cathode | Livestock urine | 100% goat urine | N/A | 46.97 mW m−2 | [82] |
| SC-MFC | 643 mL P-MFC with a graphite felt anode and a stainless-steel mesh cathode | Livestock urine | 100% sheep urine | N/A | 19.28 mW m−2 | [82] |
| DC-MFC | Two 7 mL reactors with a carbon-felt based iron/magnesium/zirconium polycrystalline catalytic cathode and bioanode | Nuclear industry wastewater | 60Co, 90Sr, 137Cs, 138La, and 144Ce 1000 mg L−1 | Mixed bacteria containing Shewanella oneidensis MR1 | 1400 mW m−2 | [83] |
| DC-MFC | 2.5 L MFC (15 cm × 8 cm × 22 cm) with copper and zinc electrodes | Paddy straw | 10 g | Rumen fluid | 8490 mW m−2 | [43] |
| SC-MFC | 80.5 mL MFC with a graphite and GAC anode and a porous graphite plate cathode | PAHs polluted groundwater | 1546 mg⋅L−1 light PAHs | Bacterial community | 7.8 mA m−2 | [84] |
| DC-MFC | 1 L MFC with a graphite plate anode and cathode | Petroleum refinery wastewater | 350 mg⋅L−1 TPH | Mixed anaerobic bacteria (Bacillus sp.-dominant) | 552 mW m−3 | [85] |
| SC-MFC | 5 L MFC with graphite carbon electrodes | TPH contaminated sediment | 26,000 mg kg−1 TPH | N/A | 50,570 mW m−2 | [42] |
| DC-MFC | 1.5 L with two chambers with graphite carbon electrodes | TPH contaminated sediment | 26,000 mg kg−1 TPH | N/A | 5760 mW m−2 | [42] |
| SC-MFC | 500/530 mL MFC with a Pt-coated carbon cloth cathode and bamboo charcoal as the anode | Potato waste | 1000 mg L−1 | Anaerobic mixed bacterial community | 576 mW m−2 | [86] |
| SC-MFC | 28 mL MFC with a carbon fiber brush anode and a carbon paper with PTFE and Pt cathode | Raw WHTW and MWW | 3343 mg⋅L−1 COD | Microbial community of WHTW and MWW | 360 mW m−2 | [87] |
| SC-MFC | 28 mL MFC with a carbon fiber brush anode and a carbon paper with PTFE and Pt cathode | Raw WHTW | 280 mg⋅L−1 cellulose, 250 mg⋅L−1 lignin, and other | Pretreatedmicrobial consortium of WHTW at 45 °C | 334 mW m−2 | [88] |
| DC-MFC | 200 mL MFC with carbon cloth as the anode and cathode (1.5 × 1.5 cm) | Sago hampas | 20 g L−1 | Clostridium beijerinckii SR1 | 73.8 mW cm−2 | [89] |
| DC-MFC | 200 mL MFC with carbon cloth as the anode and cathode (1.5 × 1.5 cm) | Sago hampas | 5.04 g L−1 | Clostridium beijerinckii SR1 | 61.5 mW m−2 | [89] |
| DC-MFC | 1 L MFC with graphite and copper electrodes (31.4 cm2) | Slaughterhouse wastewater | 1:10 waste–rumen microbes | Rumen microbes | 700 mW m−2 | [90] |
| DC-MFC | 120 mL MFC with copper–graphite electrodes (17.6 cm2) | Slaughterhouse wastewater | 10:2.4 waste–ruminal liquor | Ruminal liquor | 568 mW m−3 | [91] |
| DC-MFC | U-shaped MFC with graphite rods as the anode and cathode electrodes (0.0015 m2) | Vegetable waste | N/A | Sewage wastewater | 88,990 mW m−2 | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apollon, W.; Rusyn, I.; Paucar, N.E.; Hibbert, M.; Kamaraj, S.-K.; Sato, C. Energy Recovery from Organic Wastes Using Microbial Fuel Cells: Traditional and Nonconventional Organic Substrates. Resources 2025, 14, 47. https://doi.org/10.3390/resources14030047
Apollon W, Rusyn I, Paucar NE, Hibbert M, Kamaraj S-K, Sato C. Energy Recovery from Organic Wastes Using Microbial Fuel Cells: Traditional and Nonconventional Organic Substrates. Resources. 2025; 14(3):47. https://doi.org/10.3390/resources14030047
Chicago/Turabian StyleApollon, Wilgince, Iryna Rusyn, Noris Evelin Paucar, Monte Hibbert, Sathish-Kumar Kamaraj, and Chikashi Sato. 2025. "Energy Recovery from Organic Wastes Using Microbial Fuel Cells: Traditional and Nonconventional Organic Substrates" Resources 14, no. 3: 47. https://doi.org/10.3390/resources14030047
APA StyleApollon, W., Rusyn, I., Paucar, N. E., Hibbert, M., Kamaraj, S.-K., & Sato, C. (2025). Energy Recovery from Organic Wastes Using Microbial Fuel Cells: Traditional and Nonconventional Organic Substrates. Resources, 14(3), 47. https://doi.org/10.3390/resources14030047










