Evaluation of the Activity and Tolerability of a Cosmetic Treatment for the Periocular Area on the Aging Face: Controlled Clinical and Instrumental Evaluation vs. Placebo
Abstract
:1. Introduction
2. Experimental Section
2.1. Formulation
2.2. Trial Design
2.3. Clinical and Instrumental Evaluation
- Wrinkles grade (visual score) of the area around the eyes (deep and fine wrinkles) using a reference photographic scale: 0, no wrinkles; 1, very mild; 2, mild; 3, moderately evident; 4, evident; 5, very evident; 6, marked; 7, very marked;
- Circles under the eye (visual score): 0, absent, 1, quite visible; 2, visible; 3, marked; 4, very marked;
- Skin dryness (clinical score): 0, extremely hydrated; 1, hydrated; 2, normal; 3, quite dry; 4, dry; 5, very dry;
- Skin smoothness (clinical score): 0, very good; 1, good; 2, moderate; 3, low; 4, very low;
- Visualization of the skin aging grade of the area around the eyes by the Spiderming® graph (DermIng S.r.l. Clinical Research Institute, Monza, Italy), that allowed us to evaluate the results and visualize the effect of the cosmetic products. Taking into account that a smaller area of the graph coincides with a younger skin, the activity of the products in terms of anti-age activity could be quantified;
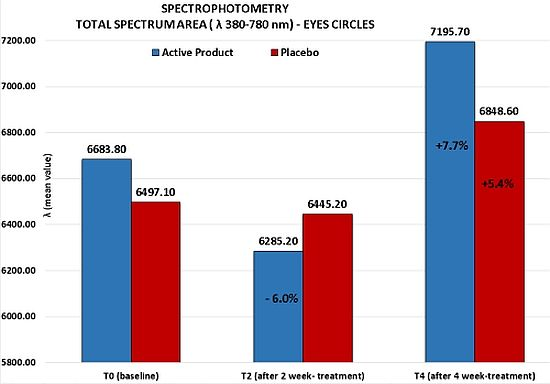
- Skin electrical capacitance measurement for stratum corneum hydration (corneometry) [16,17]. Skin hydration was measured by the instrument Corneometer CM820 (Courage-Khazaka, Köln, Germany). To reduce the variability of measurements, three repeated measures on the same skin area (periocular area) were performed and their adjusted mean was considered as the real measure value; spectrophotometry [18]. The efficacy of the test product was evaluated with a spectrophotometric measurement (Ocean Optics, Dunedin, FL, USA) of the skin color on the circles under the eyes. Spectrophotometric evaluations employed a spectrophotometer for the spectra of visible, ultraviolet and infrared (λ from 188 to 1100 nm) using a tungsten halogen lamp and a deuterium lamp compliant to CIE (Commission Internationale de l’Eclairage). The light source was turned on 30 min prior to the use of the equipment in order to stabilize the lamp emissions. The inclination of the probe was 90° on the surface to be examined, on an area of ca. 2 mm2. The wavelength range was 380–780 nm, corresponding to the visible spectrum;
- Photographic documentation and profilometry [19,20,21,22,23,24,25] of the wrinkles around the eyes (“crow’s feet”). The photographic documention was performed with Primos® compact portable system (GFMesstechnik, Berlin, Germany), a phase-shift rapid in vivo measurement which provides high-resolution profilometry of skin surfaces by using phase-shifted light stripes projected. Defining an area within the image and tracing a segment of known length in a defined position across the wrinkle and perpendicular to it, it is possible to calculate the profilometric paramet as follows:
- Ra, roughness average parameter which is the arithmetic mean of all ordinates from mean line of profile;
- Rt, wrinkles depth mean value;
- Rz, wrinkles height mean value;
- Rmax, maximum wrinkles height;
- Rv, maximum wrinkles depth.
- Eyelid signs: erythema, oedema, desquamation;
- Ocular signs: conjunctival injection (redness).
3. Statistical Analysis
- Evaluation of each study product vs. basal conditions (T2 and T4 vs. T0);
- Clinical assessment: Friedmann test followed, in case of statistically significant result, by Dunnett test for multiple comparisons;
- Instrumental measurements: analysis of variance (ANOVA) test followed, in case of statistically significant result, by Dunnett test for multiple comparisons;
- Comparison study product vs. placebo time by time;
- Clinical assessment: Wilcoxon test (Rank Sum);
- Instrumental measurements: Student’s t test (two-sample t).
4. Results
4.1. Clinical Evaluation
- Deep wrinkles on lateral corner of the eyes: reduction of the visual score of at least one grade in 52.5% of treated cases;
- Fine wrinkles on lateral corner of the eyes: reduction of the visual score of at least one grade in 67% of subjects;
- Circles under the eyes: reduction of the clinical score of at least one grade in 76% of volunteers; this reduction was statistically significant in respect to the one obtained for the placebo (Wilcoxon test p < 0.06 T4active vs. T4placebo);
- Skin smoothness: decrease of the clinical score of at least one grade in 53% of subjects;
- Skin dryness: reduction of the clinical score of at least one grade in 67% of volunteers, although the statistical significance showed at T2 was not confirmed at T4.


4.2. Instrumental Evaluation

- 11% at T2 and −8% at T4 for Ra parameter, that represents the average roughness;
- 13% at T2 for Rt and Rz parameters that represent respectively wrinkles average depth and average height;
- 16.3% at T2 (Dunnett test p < 0.05) and −10% at T4 for Rmax parameter that represents the wrinkles maximum height;
- 6% at T2 and −4% at T4 for Rv parameter that represents the wrinkles maximum depth.
- 13% at T2 for Ra;
- 19% at T2 and −14% at T4 for Rt;
- 12% at T2 and −9% at T4 for Rz;
- 16% at T2 and −9% at T4 for Rmax;
- 10% at T2 and −4% at T4 for Rv.

4.3. Tolerability


4.4. Self Assessment Evaluation
5. Discussion
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Love, L.P.; Farrior, E.H. Periocular anatomy and aging. Facial Plast. Surg. Clin. North Am. 2010, 18, 411–417. [Google Scholar] [CrossRef]
- Galatoire, O.; Morax, S. Periocular aging: Physiopathogenesis, clinical aspect, and treatment. Ann. Dermatol. Venereol. 2009, 136, 137–141. [Google Scholar] [CrossRef]
- Bombardelli, E.; Spelta, M. Phospholipid-polyphenol complexes—A new concept in skin care ingredients. Cosmet. Toilet. 1991, 106, 69. [Google Scholar]
- Eliton, C.; Davindsonl, B.C. A comparison of the lipid and fatty acid profiles from the kernels of the fruit (nuts) of Ximenia caffra and Ricinodendron rautanenii from Zimbabwe. Ind. Crops Prod. 2008, 27, 29–32. [Google Scholar] [CrossRef]
- Croft, K.D.; Beilin, L.J.; Ford, G.L. Differential inhibition of thromboxane B2 and leukotriene B4 biosynthesis by two naturally occurring acetylenic fatty acids. Biochim. Biophys. Acta 1987, 921, 621–624. [Google Scholar] [CrossRef]
- Desai, V.B.; Shankaranarayana, K.H. On the utilization aspects of sandal seed oil. Res. Ind. 1990, 35, 232–233. [Google Scholar]
- Liu, Y.; Longmore, R.B. Dietary sandalwood seed oil modifies fatty acid composition of mouse adipose tissue, brain, and liver. Lipids 1997, 32, 965–969. [Google Scholar] [CrossRef]
- Nugteren, D.H.; Christ-Hazelhof, E. Naturally occuring conjugated octadecatrienoic acids are strong inhibitors of prostaglandin biosynthesis. Prostaglandins 1987, 33, 403–417. [Google Scholar] [CrossRef]
- Cristoni, A.; Guglielmini, G.; Stucchi, P.; Bouet, A. An unsaturated fatty acid from traditional African cosmesis. In Proceedings of Cosmetics USA, New York, NY, USA, 16–18 November 1999.
- Comini, M.; Lenzini, M.; Guglielmini, G. Nanoemulsions Comprising Lipoaminoacids and Monoglycerides, Diglycerides and Polyglycerides of Fatty Acids. Ital. Patent MI2005A000218, 24 August 2006. [Google Scholar]
- ICH Harmonised Tripartite Guideline. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6_R1/Step4/E6_R1__Guideline.pdf (accessed on 14 May 2014).
- The World Medical Association. Available online: http://www.wma.net/en/30publications/10policies/b3/ (accessed on 14 May 2014).
- Rieger, M.M.; Battista, G.W. Some experiences in the safety testing of cosmetics. J. Soc. Cosmet. Chem. 1964, 15, 161–172. [Google Scholar]
- Curry, A.S.; Gettings, S.D.; McEwen, G.N. CTFA Safety Testing Guidelines; The Cosmetic, Toiletry and Fragrance Association: Washington, DC, USA, 1991. [Google Scholar]
- Camarosa, J.G.; Anthoine, P.; Tribo Boixareu, M.J.; Serra Baldrich, E.; Aubert, L. Demonstration of the anti-wrinkle efficacy of a cosmetic product. Correlation between clinical observations and instrumental methods. J. Appl. Cosmetol. 1997, 15, 13–20. [Google Scholar]
- Elsner, P.; Berardesca, E.; Maibach, H. Bioengineering of the Skin: Water and the Stratum Corneum; CRC Press: Boca Raton, FL, USA, 1994; pp. 171–175. [Google Scholar]
- Tagami, H. Evaluation of skin surface hydration in vivo by electrical measurements. J. Invest. Dermatol. 1980, 75, 500–507. [Google Scholar] [CrossRef]
- Zomios, G.; Bykowski, J.; Kollias, N. Skin melanin, hemoglobin and light scattering properties can be quantitatively assessed in vivo using diffuse reflectance spectroscopy. J. Invest. Dermatol. 2001, 117, 1452–1457. [Google Scholar] [CrossRef]
- Cook, T.H. Profilometry of the skin: A useful tool for the substantiation of cosmetic efficacy. J. Soc. Cosmet. Chem. 1980, 31, 339–359. [Google Scholar]
- Hatzis, J. Skin surface profile technique and its applications. Int. J. Cosmet. Sci. 1991, 13, 281–291. [Google Scholar] [CrossRef]
- Jaspers, S.; Hopermann, H. Rapid in vivo measurement of the topography of human skin by active image triangulation using a digital micromirror device. Skin Res. Technol. 2006, 5, 195–207. [Google Scholar]
- Leveque, J.L. EEMCO guidance for the assessment of skin topography. J. Eur. Acad. Derm. Ven. 1999, 12, 103–114. [Google Scholar]
- Wilhelm, K.P.; Elsner, P.; Berardesca, E.; Maibach, H.I. Bioengineering of the Skin: Skin Surface Imaging and Analysis; CRC Press: Boca Raton, FL, USA, 1997; pp. 129–143. [Google Scholar]
- Frankowsky, G.; Hainich, R. DLP-Based 3D metrology by structured light or projected fringe technology for life sciences and industrial metrology. Proc. SPIE 2009, 2009. [Google Scholar] [CrossRef]
- Corcuff, P.; Chatenay, F.; Leveque, J.L. The impact of aging on the microrelief of peri-orbital and leg skin. J. Soc. Cosmet. Chem. 1987, 82, 145–152. [Google Scholar]
- Sachs, L. Applied Statistics: A Handbook of Techniques; Springer: Heidelberg, Germany, 1981; pp. 536–539. [Google Scholar]
- Wulc, A.E.; Sharma, P.; Czyz, C.N. The Anatomic Basis of Midfacial Aging. In Midfacial Rejuvenation; Wulc, A.E., Sharma, P., Czyzet, C.N., Eds.; Springer Science Business Media: New York, NY, USA, 2012; pp. 15–28. [Google Scholar]
- Freitag, F.M.; Cestari, T.F. What causes dark circles under the eyes? J. Cosmet. Dermatol. 2007, 6, 211–215. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sparavigna, A.; Tenconi, B.; De Ponti, I.; Guglielmini, G. Evaluation of the Activity and Tolerability of a Cosmetic Treatment for the Periocular Area on the Aging Face: Controlled Clinical and Instrumental Evaluation vs. Placebo. Cosmetics 2014, 1, 105-116. https://doi.org/10.3390/cosmetics1020105
Sparavigna A, Tenconi B, De Ponti I, Guglielmini G. Evaluation of the Activity and Tolerability of a Cosmetic Treatment for the Periocular Area on the Aging Face: Controlled Clinical and Instrumental Evaluation vs. Placebo. Cosmetics. 2014; 1(2):105-116. https://doi.org/10.3390/cosmetics1020105
Chicago/Turabian StyleSparavigna, Adele, Beatrice Tenconi, Ileana De Ponti, and Giancarlo Guglielmini. 2014. "Evaluation of the Activity and Tolerability of a Cosmetic Treatment for the Periocular Area on the Aging Face: Controlled Clinical and Instrumental Evaluation vs. Placebo" Cosmetics 1, no. 2: 105-116. https://doi.org/10.3390/cosmetics1020105
APA StyleSparavigna, A., Tenconi, B., De Ponti, I., & Guglielmini, G. (2014). Evaluation of the Activity and Tolerability of a Cosmetic Treatment for the Periocular Area on the Aging Face: Controlled Clinical and Instrumental Evaluation vs. Placebo. Cosmetics, 1(2), 105-116. https://doi.org/10.3390/cosmetics1020105