Potential of Icariin–Glucosamine Combination in the Treatment of Osteoarthritis by Topical Application: Development of Topical Formulation and In Vitro Permeation Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of a Formulation Containing Icariin and Glucosamine
2.2.1. Preparation of Cream
2.2.2. Preparation of Gel
2.2.3. Preparation of Ointment
2.2.4. Acceptance Criteria and Specifications
2.3. Physicochemical Tests
2.3.1. Appearance and Homogeneity
2.3.2. Determination of pH of Formulations
2.3.3. Density
2.3.4. Dynamic Viscosity
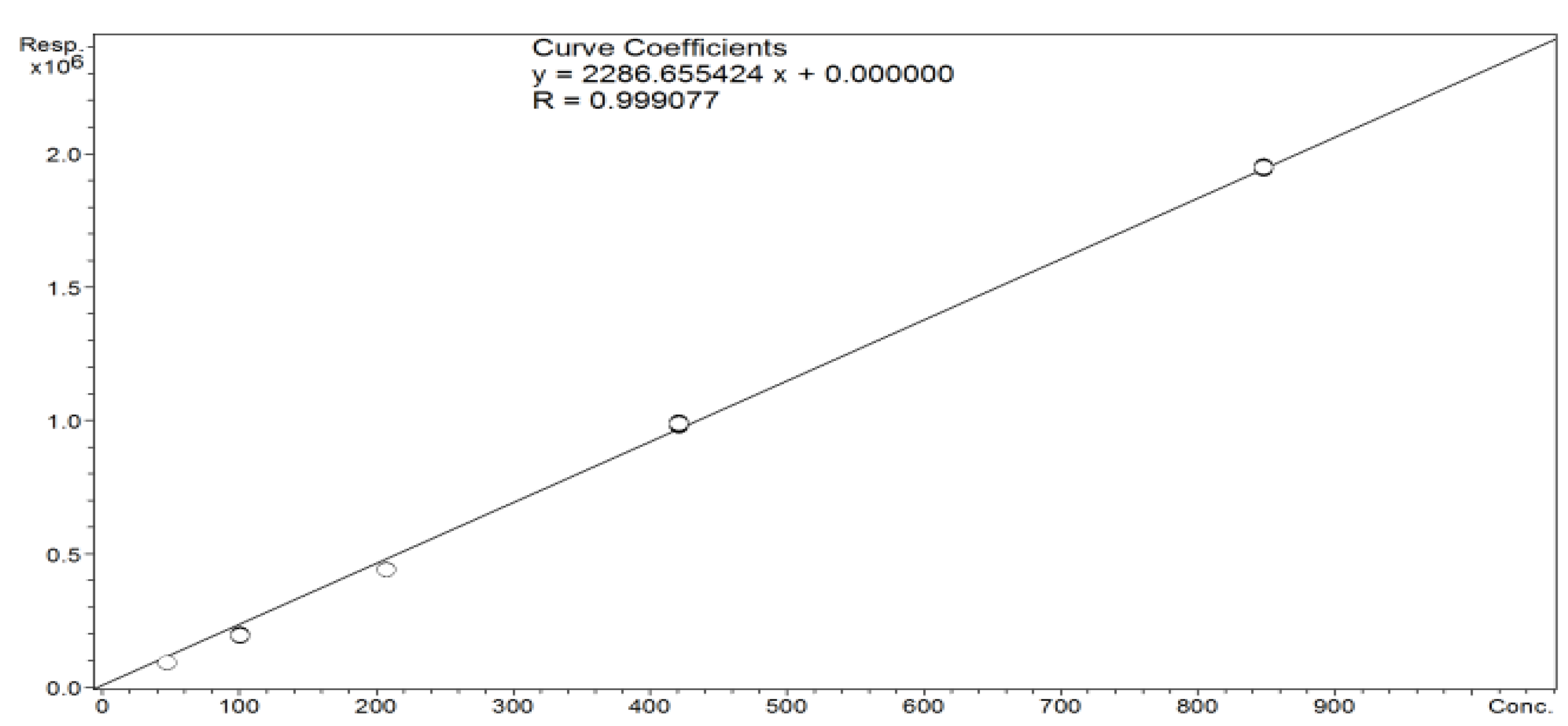
2.3.5. Assay of Icariin Content in Epimedium L. (Species: Epimedium Koreanum) Extract—LC-MS
2.3.6. Release of Icariin
2.3.7. Release of Glucosamine
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence Trends of Site-Specific Osteoarthritis From 1990 to 2019: Findings From the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sport. Health A Multidiscip. Approach 2009, 1, 461–468. [Google Scholar]
- Frenkel, S.R.; Clancy, R.M.; Ricci, J.L.; Di Cesare, P.E.; Rediske, J.J.; Abramson, S.B. Effects of nitric oxide on chondrocyte migration, adhesion, and cytoskeletal assembly. Arthritis Rheum. 1996, 39, 1905–1912. [Google Scholar] [CrossRef]
- Klein, T.J.; Rizzi, S.C.; Reichert, J.C.; Georgi, N.; Malda, J.; Schuurman, W. Strategies for zonal cartilage repair using hydrogels. Macromol. Biosci. 2009, 9, 1049–1058. [Google Scholar] [CrossRef]
- Grunke, M. Successful treatment of inflammatory knee osteoarthritis with tumour necrosis factor blockade. Ann. Rheum. Dis. 2006, 65, 555–556. [Google Scholar] [CrossRef]
- Deshpande, P.; Patil, K.; Guledgud, M.V.; D’souza, R.S. Diagnostic Imaging in TMJ Osteoarthritis: A Case Report and Overview. Int. J. Dent. Sci. Res. 2015, 3, 56–59. [Google Scholar]
- Kean, W.F.; Kean, R.; Buchanan, W.W. Osteoarthritis: Symptoms, signs and source of pain. InflammoPharmacology 2004, 12, 3–31. [Google Scholar] [CrossRef]
- Hunter, D.J.; McDougall, J.J.; Keefe, F.J. The Symptoms of Osteoarthritis and the Genesis of Pain. Rheum. Dis. Clin. North Am. 2008, 34, 623–643. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Pelletier, J.P.; Martel-Pelletier, J.; Rannou, F.; Cooper, C. Efficacy and safety of oral NSAIDs and analgesics in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin. Arthritis Rheum. 2016, 45, S22–S27. [Google Scholar] [CrossRef] [PubMed]
- Permuy, M.; Guede, D.; López-Peña, M.; Muñoz, F.; Caeiro, J.R.; González-Cantalapiedra, A. Comparison of various SYSADOA for the osteoarthritis treatment: An experimental study in rabbits. BMC Musculoskelet Disord. 2015, 16, 120. [Google Scholar] [CrossRef]
- Veronese, N.; Ecarnot, F.; Cheleschi, S.; Fioravanti, A.; Maggi, S. Possible synergic action of non-steroidal anti-inflammatory drugs and glucosamine sulfate for the treatment of knee osteoarthritis: A scoping review. BMC Musculoskelet Disord. 2022, 23, 1084. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Cooper, C.; Bruyère, O.; Al-Daghri, N.M.; Branco, J.; Cavalier, E.; Reginster, J.Y. Multimodal Multidisciplinary Management of Patients with Moderate to Severe Pain in Knee Osteoarthritis: A Need to Meet Patient Expectations. Drugs. 2022, 82, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Wu, C.F.; Lai, W.P.; Yang, X.J.; Cheung, P.Y.; Yao, X.S.; Leung, P.C.; Wong, M.S. The osteoprotective effect of Herba epimedii (HEP) extract in vivo and in vitro. Evid Based Complement Altern. Med. 2005, 2, 353–361. [Google Scholar] [CrossRef]
- Pei, L.K.; Guo, B.L. A review on research of raw material and cut crude drug of Herba epimedii in last ten years. China J. Chin. Mater. Medica. 2007, 32, 466–471. [Google Scholar]
- Liu, R.; Li, A.; Sun, A.; Cui, J.; Kong, L. Preparative isolation and purification of three flavonoids from the Chinese medicinal plant Epimedium koreanum Nakai by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1064, 53–57. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Yang, X.; Wang, K.; Ni, J.; Qu, X. Inhibitory effect of Epimedium extract on S-adenosyl-L-homocysteine hydrolase and biomethylation. Life Sci. 2005, 78, 180–186. [Google Scholar] [CrossRef]
- Wu, H.; Lien, E.J.; Lien, L.L. Chemical and pharmacological investigations of Epimedium species: A survey. Prog. Drug Res. 2003, 60, 1–57. [Google Scholar]
- Ma, H.P.; He, X.R.; Yang, Y.; Li, M.X.; Hao, D.J.; Jia, Z.P. The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011, 134, 519–541. [Google Scholar] [CrossRef]
- Guo, B.; Xiao, P. Determination of flavonoids in different parts of five Epimedium plants. China J. Chin. Mater. Med. 1996, 21, 523–525. [Google Scholar]
- Wang, Y.A.; Guo, Z.M.; Jin, Y.; Zhang, X.L.; Wang, L.; Xue, X.Y.; Liang, X.M. Identification of prenyl flavonoid glycosides and phenolic acids in Epimedium koreanum Nakai by Q-TOF-MS combined with selective enrichment on click oligo (ethylene glycol) column. J. Pharm. Biomed. Anal. 2010, 51, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Zhang, X.; Yang, X.H.; Qiu, N.X.; Wang, Y.; Wang, Z.Z. Microwave assisted extraction of flavonoids from cultivated Epimedium sagittatum: Extraction yield and mechanism, antioxidant activity and chemical composition. Ind. Crop. Prod. 2013, 50, 857–865. [Google Scholar] [CrossRef]
- Sze, S.C.; Tong, Y.; Ng, T.B.; Cheng, C.L.; Cheung, H.P. Herba Epimedii: A antioxidative properties and its medical implications. Molecules 2010, 15, 7861–7870. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Song, J.; Jia, X.B. Phytochemistry and ethnopharmacology of Epimedium L. species. Chin. Herb. Med. 2015, 7, 204–222. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Han, X.F.; Liu, T.G.; Cheng, M.C.; Xiao, H.B. A cell-based model of bone remodeling for identifying activity of icarrin in the treatment of osteoporosis. Biotechnol. Lett. 2015, 37, 219–226. [Google Scholar] [CrossRef]
- Pan, Y.; Kong, L.D.; Xia, X.; Zhang, W.Y.; Xia, Z.H.; Jiang, F.X. Antidepressant-like effect of icariin and its possible mechanism in rats. Pharmacol. Biochem. Behav. 2005, 82, 686–694. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Lou, Y.J. Proliferation-stimulating effects of icariin and desmethylicaritin in MCF-7 cells. Eur. J. Pharmacol. 2004, 504, 147–153. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Huang, Y.; Wismeijer, D.; Liu, Y. Icariin: Does It Have An Osteoinductive Potential for Bone Tissue Engineering? Phytother. Res. 2013, 28, 498–509. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Y.; Huang, Y. Icariin Reduces Cartilage Degeneration in a Mouse Model of Osteoarthritis and is Associated with the Changes in Expression of Indian Hedgehog and Parathyroid Hormone-Related Protein. Med Sci Monit. 2018, 24, 6695–6706. [Google Scholar] [CrossRef]
- Zhao, J.; Ohba, S.; Komiyama, Y.; Shinkai, M.; Chung, U.; Nagamune, T. Icariin: A Potential Osteoinductive Compound for Bone Tissue Engineering. Tissue Eng. Part A 2010, 16, 233–243. [Google Scholar] [CrossRef]
- Hamerman, D. The biology of osteoarthritis. N. Engl. J. Med. 1989, 320, 1322–1330. [Google Scholar] [PubMed]
- Jerosch, J. Effects of Glucosamine and Chondroitin Sulfate on Cartilage Metabolism in OA: Outlook on Other Nutrient Partners Especially Omega-3 Fatty Acids. Int. J. Rheumatol. 2011, 2011, 969012. [Google Scholar] [CrossRef]
- Serni, U. Profile of glucosamine sulfate as an example of Slow Acting Drug in Osteoarthritis. Rev. Esp. Reumatol. 1993, 20 (Suppl. S1), 222. [Google Scholar]
- Avouac, B. Slow Acting Drugs in osteoarthritis: A step towards disease modification. Rev. Esp. Reumatol. 1993, 20 (Suppl. S1), 221–222. [Google Scholar]
- Pavelká, K.; Gatterová, J.; Olejarová, M.; Machacek, S.; Giacovelli, G.; Rovati, L.C. Glucosamine Sulfate Use and Delay of Progression of Knee Osteoarthritis. Arch. Inter. Med. 2002, 162, 2113–2123. [Google Scholar] [CrossRef] [PubMed]
- Mullerfasbender, H.; Bach, G.; Haase, W.; Rovati, L.; Sentikar, I. Glucosamine sulfate compared to ibuprofen in osteoarthritis of the knee. Osteoarthr. Cartil. 1994, 2, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D. Glucosamine therapy for knee osteoarthritis: Pharmacokinetic considerations. Expert Rev. Clin. Pharmacol. 2009, 2, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Kucharz, E.J.; Kovalenko, V.; Szántó, S.; Bruyère, O.; Cooper, C.; Reginster, J.Y. A review of glucosamine for knee osteoarthritis: Why patented crystalline glucosamine sulfate should be differentiated from other glucosamines to maximize clinical outcomes. Curr. Med. Res. Opin. 2016, 32, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Cheleschi, S.; Tenti, S.; Giannotti, S.; Veronese, N.; Reginster, J.Y.; Fioravanti, A. A Combination of Celecoxib and Glucosamine Sulfate Has Anti-Inflammatory and Chondroprotective Effects: Results from an In Vitro Study on Human Osteoarthritic Chondrocytes. Int. J. Mol. Sci. 2021, 22, 8980. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Mahmood, A.; Waghule, T.; Gorantla, S.; Kumar Dubey, S.; Alexander, A.; Singhvi, G. Revisiting techniques to evaluate drug permeation through skin. Expert Opin. Drug Deliv. 2021, 18, 1829–1842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.; Li, K.F.; Li, D.X.; Xiao, Y.M.; Fan, Y.J.; Zhang, X.D. Icariin Promotes Extracellular Matrix Synthesis and Gene Expression of Chondrocytes In Vitro. Phytother. Res. 2012, 26, 1385–1392. [Google Scholar] [CrossRef]
- Wang, Z.C.; Sun, H.J.; Li, K.H.; Fu, C.; Liu, M.Z. Icariin promotes directed chondrogenic differentiation of bone marrow mesenchymal stem cells but not hypertrophy in vitro. Exp. Med. 2014, 8, 1528–1534. [Google Scholar] [CrossRef]
- Sun, P.; Liu, Y.; Deng, X.; Yu, C.; Dai, N.; Yuan, X. An inhibitor of cathepsin K, icariin suppresses cartilage and bone degradation in mice of collagen induced arthritis. Phytomedicine 2013, 20, 975–979. [Google Scholar] [CrossRef]
- Wei, C.C.; Ping, D.Q.; You, F.T.; Qiang, C.Y.; Tao, C. Icariin Prevents Cartilage and Bone Degradation in Experimental Models of Arthritis. Mediat. Inflamm. 2016, 2016, 1–10. [Google Scholar]
- Liu, Y.J.; Feng, W.; He, D.Y.; Wang, Q.Q. Effect of icariin on bone destruction and serum RANKL/OPG levels in type II collagen-induced arthritis rats. China J. Chin. Mater. Med. 2013, 33, 1221–1225. [Google Scholar]
- Zhang, G.; Qin, L.; Shi, Y. Epimedium-derived phytoestrogen flavonoids exert beneficial effect on preventing bone loss in late postmenopausal women: A 24-month randomized, double-blind and placebocontrolled trial. J. Bone Min. Res. 2007, 22, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Choi, Y.H.; Kwon, H.; Lee, S.B.; Kim, D.H.; Sung, C.K.; Park, Y.I.; Dong, M.S. Estrogenic/antiestrogenic activities of a Epimedium koreanum extract and its major components: In vitro and in vivo studies. Food Chem. Toxicol. 2012, 50, 2751–2759. [Google Scholar] [CrossRef]
- Zanoli, P.; Benelli, A.; Zavatti, M.; Rivasi, M.; Baraldi, C.; Baraldi, M. Improved sexual behavior in male rats treated with a Chinese herbal extract: Hormonal and neuronal implications. Asian J. Androl. 2008, 10, 937–945. [Google Scholar] [CrossRef]
- Yang, A.; Yu, C.; Lu, Q.; Li, H.; Li, Z.; He, C. Mechanism of Action of Icariin in Bone Marrow Mesenchymal Stem Cells. Stem Cells Int. 2019, 4, 5747298. [Google Scholar] [CrossRef]
- Calamia, V.; Mateos, J.; Fernández-Puente, P.; Lourido, L.; Rocha, B.; Fernández-Costa, C.; Montell, E.; Vergés, J.; Ruiz-Romero, C.; Blanco, F.J. A pharmacoproteomic study confirms the synergistic effect of chondroitin sulfate and glucosamine. Sci. Rep. 2014, 4, 5069. [Google Scholar] [CrossRef]
- Malzfeldt, E.; Lehmann, P.; Goerz, G.; Lippold, B.C. Influence of drug solubility in the vehicle on clinical efficacy of ointments. Arch. Dematol. Res. 1989, 281, 193–197. [Google Scholar] [CrossRef]
- Jin, J.; Wang, H.; Hua, X.; Chen, D.; Huang, C.; Chen, Z. An outline for the pharmacological effect of icariin in the nervous system. Eur. J. Pharmacol. 2019, 842, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lu, Y.R.; Kou, N.; Hu, M.J.; Wang, Q.S.; Cui, Y.L. Intranasal delivery of icariin via a nanogel-thermoresponsive hydrogel compound system to improve its antidepressant-like activity. Int. J. Pharm. 2020, 586, 119550. [Google Scholar] [CrossRef]
- Bassleer, C.; Henrotin, Y.; Franchimont, P. In-vitro evaluation of drugs proposed as chondroprotective agents. Int. J. Tissue React. 1992, 14, 231–241. [Google Scholar]
- Setnikar, I.; Cereda, R.; Pacini, M.A.; Revel, L. Antireactive properties of glucosamine sulfate. Arzneimittelforschung 1991, 41, 157–161. [Google Scholar] [PubMed]
- Setnikar, I.; Pacini, M.A.; Revel, L. Antiarthritic effects of glucosamine sulfate studied on animal models. Arzneimittelforschung 1991, 41, 542–545. [Google Scholar]
- Noack, W.; Fischer, M.; FSrster, K.K.; Rovati, L.C.; Setnikar, I. Glucosamine sulfate in osteoarthritis of the knee. Osteoarthr. Cart 1994, 2, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Rovati, L.C.; Setnikar, I.; FSrster, K.K.; Reichelt, A.; Noack, W. Glucosamine sulfate in gonarthrosis: Efficacy in placebo controlled studies. Rev Esp Reum. 1993, 20 (Suppl. S1), 72. [Google Scholar]
- Giacovelli, G.; Rovati, L.C. Clinical efficacy of glucosamine sulfate in osteoarthritis of the spine. Rev. Esp. Reum. 1993, 20 (Suppl. S1), 96. [Google Scholar]
- Silva, J.A.; Apolinário, A.C.; Souza, M.S.R.; Damasceno, B.P.G.L.; Medeiros, A.C.D. Cutaneous Administration of Drugs: Challenges and Strategies for the Development of Trans-dermal Formulations. Rev. De Ciências Farm. Básica 2010, 31, 125–131. [Google Scholar]
- Persiani, S.; Rotini, R.; Trisolino, G.; Rovati, L.C.; Locatelli, M.; Paganini, D.; Antonioli, D.; Roda, A. Synovial and plasma glucosamine concentrations in osteoarthritic patients following oral crystalline glucosamine sulphate at therapeutic dose. Osteoarthr. Cartil. 2007, 15, 764–772. [Google Scholar] [CrossRef]
- Persiani, S.; Roda, E.; Rovati, L.C.; Locatelli, M.; Giacovelli, G.; Roda, A. Glucosamine oral bioavailability and plasma pharmacokinetics after increasing doses of crystalline glucosamine sulfate in man. Osteoarthr. Cartil. 2005, 13, 1041–1049. [Google Scholar] [CrossRef]
- Lee, C.W.; Li, Z.; Patel, K.; Olobo, J.O.; Lee, E.J.D.; Goh, L.B. The Transdermal Profiles of Mediflex™ Glucosamine Cream in Mouse and Man. Available online: https://docplayer.net/31056811-The-transdermal-profiles-of-mediflex-tm-glucosamine-cream-in-mouse-and-man.html (accessed on 10 February 2023).
- Kong, M.; Hashim, K.B.; Lin, P.; Coestesquis, G.; Xu, A.Y.; Lebes, F.; Ting, C.M. Penetration of Topical Glucosamine Sulfate into the Synovial Fluid of Patients with Knee Osteoarthritis: A Nonrandomized, OpenLabel, Single Dose, Bioavailability Study. J. Biosci. Med. 2019, 7, 76–90. [Google Scholar] [CrossRef]
- Huskisson, E.C. Glucosamine and Chondroitin for Osteoarthritis. J. Int. Med. Res. 2008, 36, 6. [Google Scholar] [CrossRef] [PubMed]
- Leite, C.B.S.; Coehlo, J.M.; Muehlmann, L.A.; de Azevedo, R.B.; Sousa, M.H. Skin Delivery of Glucosamine and Chondroitin Sulphates—A Perspective on the Conservative Treatment for Osteoarthritis of the Knee. J. Biosci. Med. 2017, 5, 11–20. [Google Scholar] [CrossRef]
- Miki, R.; Ichitsuka, Y.; Yamada, T.; Kimura, S.; Egawa, Y.; Seki, T.; Morimoto, Y. Development of a membrane impregnated with a poly(dimethylsiloxane)/poly(ethylene glycol) copolymer for a high-throughput screening of the permeability of drugs, cosmetics, and other chemicals across the human skin. Eur. J. Pharm. Sci. 2015, 66, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Flaten, G.E.; Palac, Z.; Engesland, A.; Filipović-Grčić, J.; Vanić, Ž.; Škalko-Basnet, N. In vitro skin models as a tool in optimization of drug formulation. Eur. J. Pharm. Sci. 2015, 75, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, L.J.; Kaplan, L.N.; Hamadeh, M.J.; Grigoriu, A.C.; Baron, M. Sulfate could mediate the therapeutic effect of glucosamine sulfate. Metabolism. 2001, 50, 767–770. [Google Scholar] [CrossRef]
- Cordoba, F.; Nimni, M.E. Chondroitin sulfate and other sulfate containing chondroprotective agents may exhibit their effects by overcoming a deficiency of sulfur amino acids. Osteoarthr. Cartil. 2003, 11, 228–230. [Google Scholar] [CrossRef]
- Kanwischer, M.; Kim, S.Y.; Bian, S.; Kwon, K.A.; Kim, J.S.; Kim, D.D. Evaluation of the Physicochemical Stability and Skin Permeation of Glucosamine Sulfate. Drug Dev. Ind. Pharm. 2005, 31, 91–97. [Google Scholar] [CrossRef] [PubMed]
- FDA. Guidance for Industry. Nonsterile semisolid dosage forms, scale-up and postapproval changes: Chemistry, manufacturing, and controls; in vitro release testing and in vivo bioequivalence documentation. Cent. Drug Eval. Res. 1997, CMC 7, 19–25.
- EMA. Guideline on Quality of Transdermal Patches; European Medicines Agency: London, UK, 2014; Volume 24. [Google Scholar]
- Argoff, C.E.; Gloth, F.M. Topical Nonsteroidal Anti-Inflammatory Drugs for Management of Osteoarthritis in Long-Term Care Patients. Ther. Clin. Risk Manag. 2011, 7, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.F.; Rouse, J.J.; Sanderson, F.D.; Eccleston, G.M. The relevance of polymeric synthetic membranes in topical formulation assessment and drug diffusion study. Arch. Pharmacal Res. 2012, 35, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Ameri, M.; Lewis, H.; Lehman, P. Effect of Skin Model on In Vitro Performance of an Adhesive Dermally Applied Microarray Coated with Zolmitriptan. J. Pharm. 2018, 3, 7459124–7459130. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.A.M.; Seiller, M.; Grossiord, J.L.; Marty, J.P.; Wepierre, J. Vehicle influence on in vitro release of metronidazole: Role of w/o/w multiple emulsion. Int. J. Pharm. 1994, 109, 251–259. [Google Scholar] [CrossRef]
- Lin, H.M.; Lin, L.F.; Sun, M.Y.; Liu, J.; Wu, Q. Topical Delivery of Four Neuroprotective Ingredients by Ethosome-Gel: Synergistic Combination for Treatment of Oxaliplatin-Induced Peripheral Neuropathy. Int. J. Nanomed. 2020, 15, 3251–3266. [Google Scholar] [CrossRef]





| Prepared Formulations | ||
|---|---|---|
| INGREDIENT | CREAM (wt.%) | GEL (wt.%) |
| Water | up to 100.00 | up to 100.00 |
| Epimedium L. extract (Species: Epimedium Koreanum) | 70.00 | 70.00 |
| Propylene glycol | 3.00 | 3.00 |
| D-Glucosamine sulfate potassium chloride salt | 10.00 | 10.00 |
| Xanthan gum | 0.20 | - |
| Sodium polyacrylate | - | 3.00 |
| Vitis vinifera L. seed oil | 3.00 | - |
| Glyceryl stearate | 3.00 | - |
| Stearyl alcohol | 2.50 | - |
| Ceteareth 25 | 2.00 | - |
| Butyrospermum parkii (shea) butter | 1.00 | - |
| PEG-40 hydrogenated castor oil | - | 1.00 |
| D-Camphor | 0.50 | 0.50 |
| D-Menthol | 0.20 | 0.20 |
| Alcohol denat. | 1.00 | 1.00 |
| Rosemary essential oil | 0.50 | 0.50 |
| Marjoram essential oil | 0.20 | 0.20 |
| Cinnamon essential oil | 0.05 | 0.05 |
| Clove essential oil | 0.20 | 0.20 |
| Dehydroacetic acid (and) Benzyl alcohol | 1.00 | 1.00 |
| Time (min) | A (% v/v) | B (% v/v) |
|---|---|---|
| 0 | 75 | 25 |
| 1 | 75 | 25 |
| 15 | 55 | 45 |
| 16 | 55 | 45 |
| 17 | 10 | 90 |
| 22 | 10 | 90 |
| 23 | 75 | 25 |
| 28 | 75 | 25 |
| Formulation | Color | Homogeneity and Consistency | pH | Viscosity [mPa × s] | Density |
|---|---|---|---|---|---|
| cream 1_1 sample | off-white | creamy, excellent homogenous, no visible impurities | 6.32 | 37.800 | 0.792 |
| cream 1_2 sample | off-white | creamy, excellent homogenous, no visible impurities | 6.29 | 38.200 | 0794 |
| cream 1_3 sample | off-white | creamy, excellent homogenous, no visible impurities | 6.33 | 37.800 | 0.791 |
| gel 1_1 sample | slightly brown | nontransparent gel, homogenous, no visible impurities | 5.19 | 33.600 | 0.987 |
| gel 1_2 sample | slightly brown | nontransparent gel, homogenous, no visible impurities | 5.17 | 34.250 | 0.984 |
| gel 1_3 sample | slightly brown | nontransparent gel, homogenous, no visible impurities | 5.18 | 33.950 | 0.989 |
| 2 g of glucosamine sulfate/100 g of product | |||||||||
| Cream | Gel | Ointment | |||||||
| Time [h] | average [µg/cm2] | SD [µg/cm2] | [%] | average [µg/cm2] | SD [µg/cm2] | [%] | average [µg/cm2] | SD [µg/cm2] | [%] |
| 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1 | 45 | 11.70 | 2.25 | 132.00 | 18.50 | 6.60 | 0.00 | 0.00 | 0.00 |
| 2 | 104 | 10.40 | 5.20 | 152.50 | 10.90 | 7.63 | 12.00 | 5.50 | 0.60 |
| 3 | 236 | 21.30 | 11.80 | 213.50 | 17.70 | 10.68 | 64.50 | 19.70 | 3.23 |
| 4 | 335 | 18.50 | 16.75 | 229.00 | 10.20 | 11.45 | 84.00 | 21.70 | 4.20 |
| 5 | 355 | 24.90 | 17.75 | 294.50 | 9.70 | 14.73 | 107.50 | 14.70 | 5.38 |
| 5 g of glucosamine sulfate/100 g of product | |||||||||
| Cream | Gel | Ointment | |||||||
| Time [h] | average [µg/cm2] | SD [µg/cm2] | [%] | average [µg/cm2] | SD [µg/cm2] | [%] | average [µg/cm2] | SD [µg/cm2] | [%] |
| 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1 | 156.00 | 10.80 | 3.10 | 326.10 | 7.60 | 6.50 | 25.70 | 12.10 | 0.50 |
| 2 | 385.20 | 3.10 | 7.70 | 472.10 | 6.10 | 9.40 | 39.00 | 15.90 | 0.80 |
| 3 | 535.60 | 61.80 | 10.70 | 575.20 | 15.30 | 11.50 | 208.40 | 54.10 | 4.20 |
| 4 | 1046.40 | 34.00 | 20.90 | 810.70 | 10.70 | 16.20 | 270.90 | 80.70 | 5.40 |
| 5 | 1260.90 | 49.10 | 25.20 | 989.70 | 11.50 | 19.80 | 345.20 | 51.00 | 6.90 |
| 10 g of glucosamine sulfate/100 g of product | |||||||||
| Cream | Gel | Ointment | |||||||
| Time [h] | average [µg/cm2] | SD [µg/cm2] | [%] | average [µg/cm2] | SD [µg/cm2] | [%] | average [µg/cm2] | SD [µg/cm2] | [%] |
| 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1 | 422.20 | 27.40 | 4.20 | 974.30 | 108.60 | 9.70 | 42.40 | 11.80 | 0.40 |
| 2 | 1083.10 | 9.60 | 10.80 | 1137.30 | 29.40 | 11.40 | 82.70 | 4.10 | 0.80 |
| 3 | 2479.50 | 20.50 | 24.80 | 1589.00 | 93.20 | 15.90 | 299.90 | 82.60 | 3.00 |
| 4 | 3179.20 | 61.20 | 31.80 | 1713.90 | 15.40 | 17.10 | 468.80 | 24.00 | 4.70 |
| 5 | 3838.10 | 39.50 | 38.40 | 2173.10 | 36.60 | 21.70 | 603.10 | 36.10 | 6.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pikosz, K.; Nowak, I.; Feliczak-Guzik, A. Potential of Icariin–Glucosamine Combination in the Treatment of Osteoarthritis by Topical Application: Development of Topical Formulation and In Vitro Permeation Study. Cosmetics 2023, 10, 36. https://doi.org/10.3390/cosmetics10010036
Pikosz K, Nowak I, Feliczak-Guzik A. Potential of Icariin–Glucosamine Combination in the Treatment of Osteoarthritis by Topical Application: Development of Topical Formulation and In Vitro Permeation Study. Cosmetics. 2023; 10(1):36. https://doi.org/10.3390/cosmetics10010036
Chicago/Turabian StylePikosz, Katarzyna, Izabela Nowak, and Agnieszka Feliczak-Guzik. 2023. "Potential of Icariin–Glucosamine Combination in the Treatment of Osteoarthritis by Topical Application: Development of Topical Formulation and In Vitro Permeation Study" Cosmetics 10, no. 1: 36. https://doi.org/10.3390/cosmetics10010036
APA StylePikosz, K., Nowak, I., & Feliczak-Guzik, A. (2023). Potential of Icariin–Glucosamine Combination in the Treatment of Osteoarthritis by Topical Application: Development of Topical Formulation and In Vitro Permeation Study. Cosmetics, 10(1), 36. https://doi.org/10.3390/cosmetics10010036







