A Cistus incanus Extract Blocks Psychological Stress Signaling and Reduces Neurogenic Inflammation and Signs of Aging in Skin, as Shown in In-Vitro Models and a Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
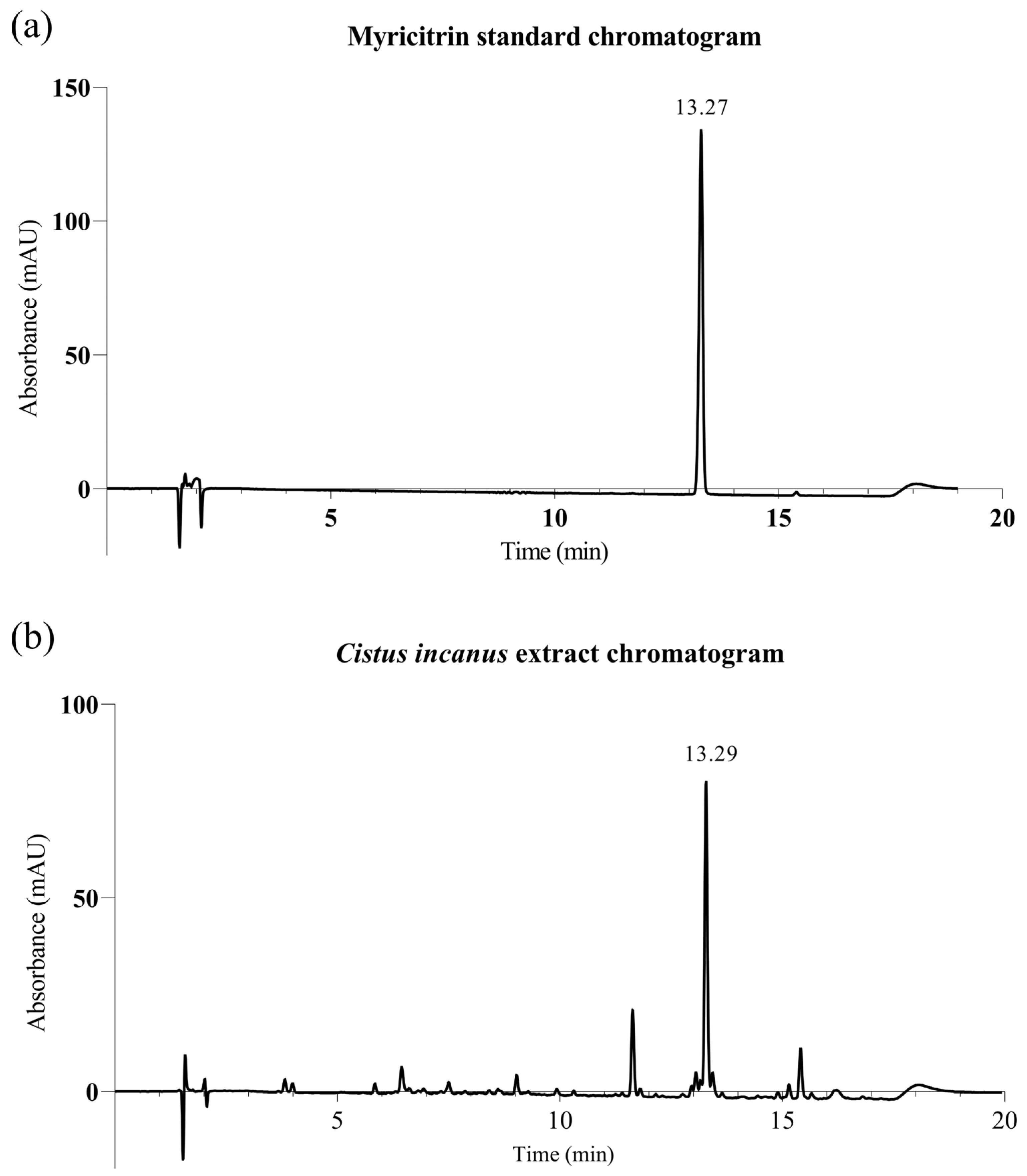
2.1. Material: Cistus Incanus Extract
2.2. Testing Protocols
2.2.1. CRH-R1 Receptor Blocking Functional Assay
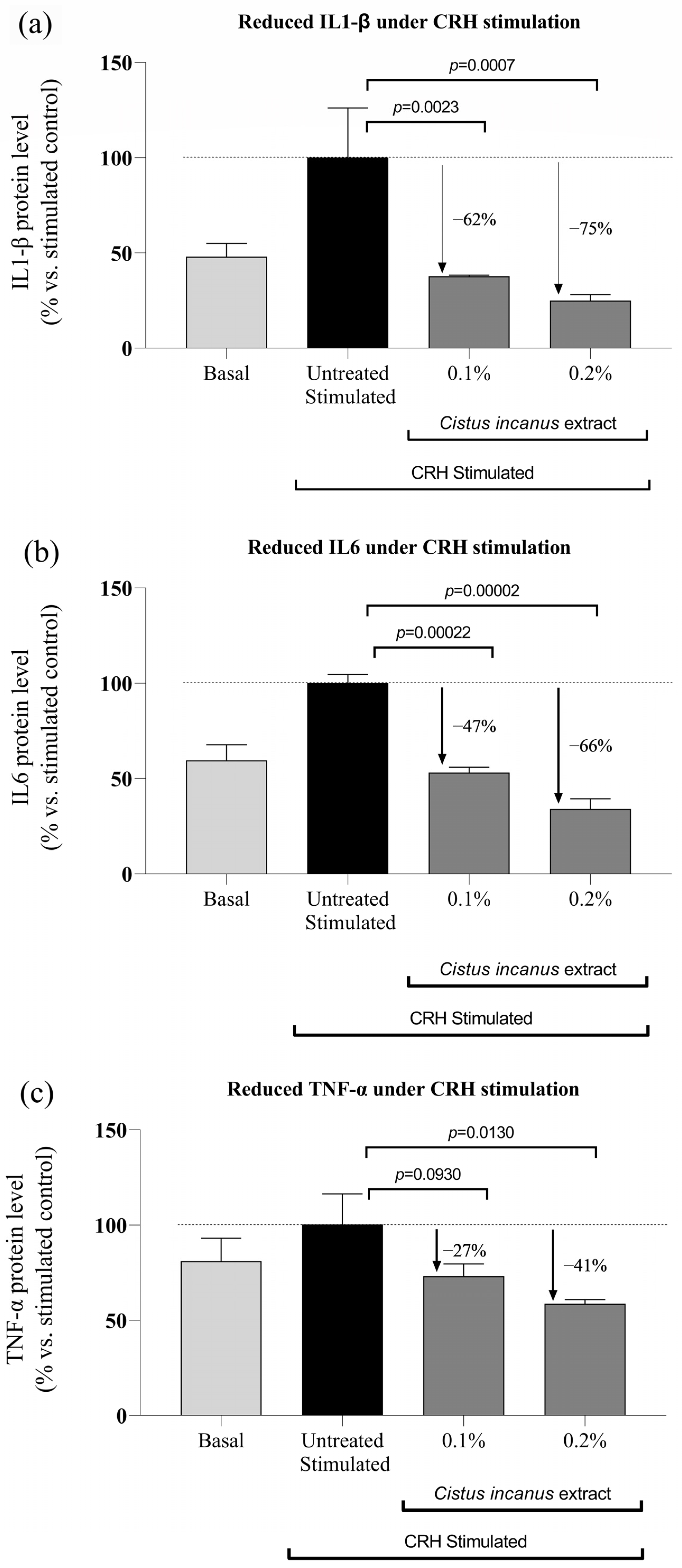
2.2.2. Reduction of Inflammatory Cytokine Markers under CRH Stimulation
2.2.3. Ex Vivo Inhibition of the NF-kB Master Inflammation Regulator
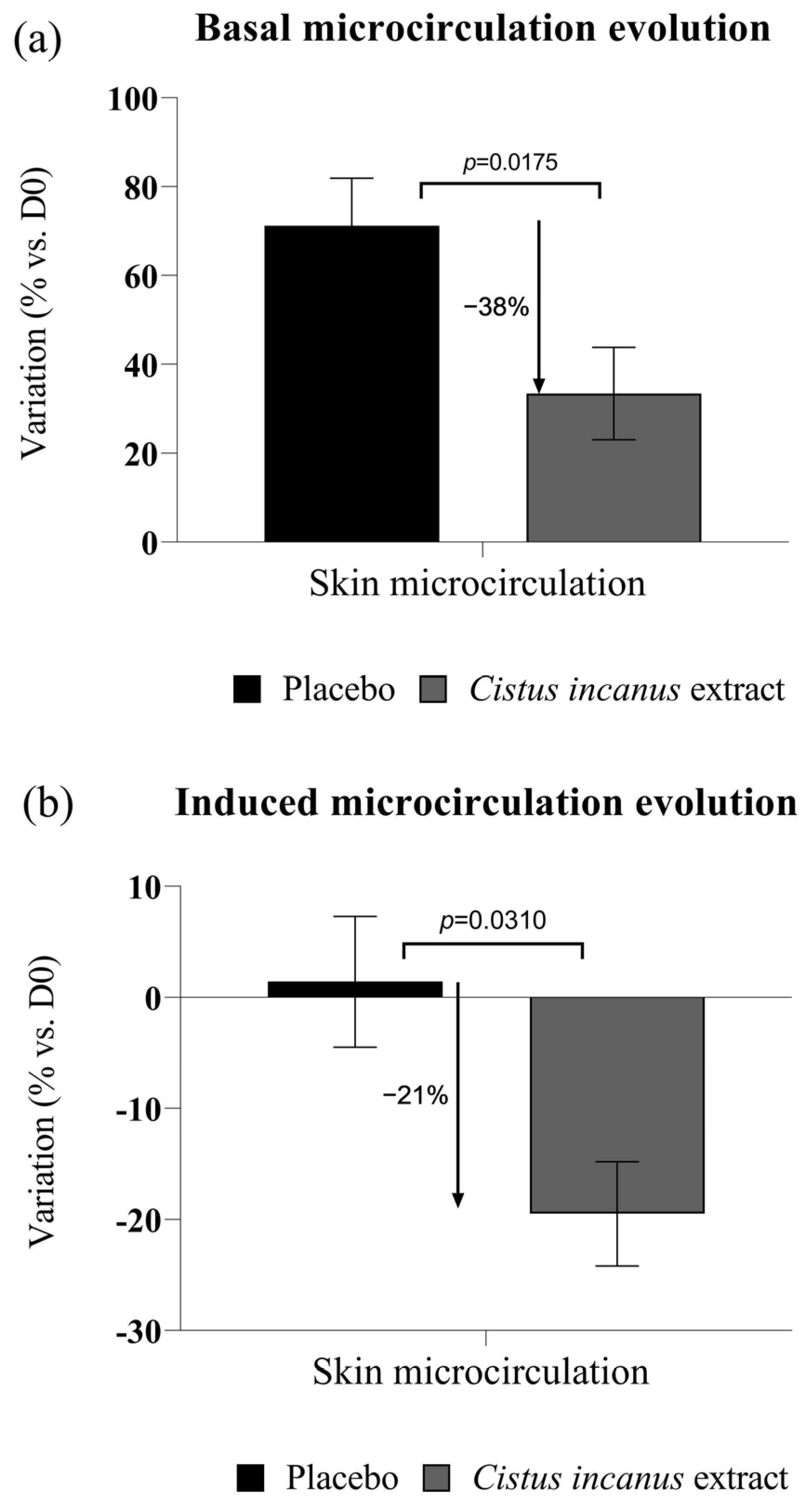
2.2.4. Clinical Trial on a Highly Stressed Population Sample
2.2.5. Statistics
3. Results
3.1. Results of In-Vitro Studies
3.1.1. CRH-R1 Receptor Blocking Functional Assay
3.1.2. In-Vitro Reduction of Inflammatory Cytokine Expression under CRH Stimulation
3.1.3. Ex-Vivo Inhibition of the NF-kB Master Inflammation Regulator
3.2. Clinical Trial Results
3.2.1. Anti-Inflammatory Effects
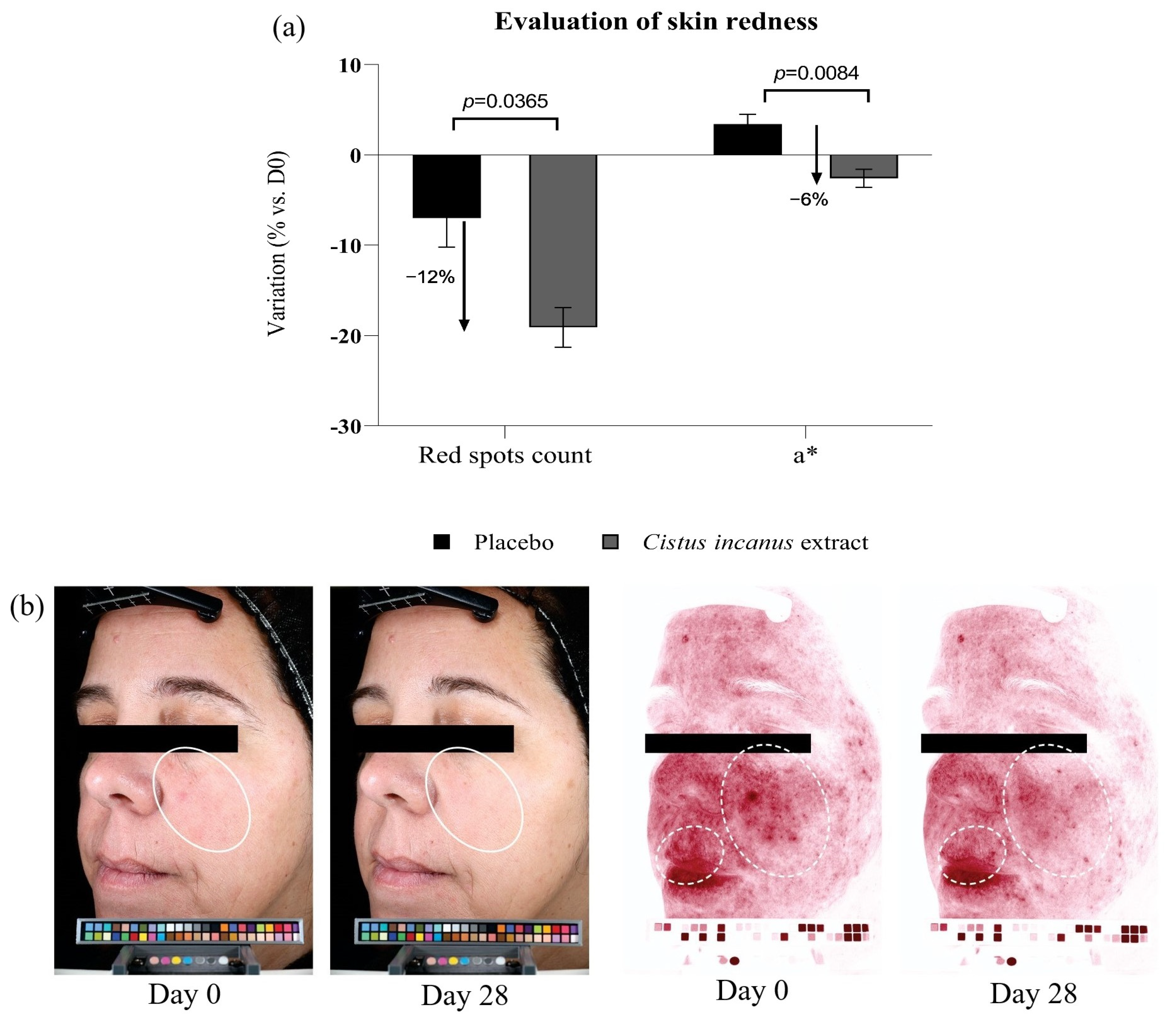
3.2.2. Skin Redness, Red Spots
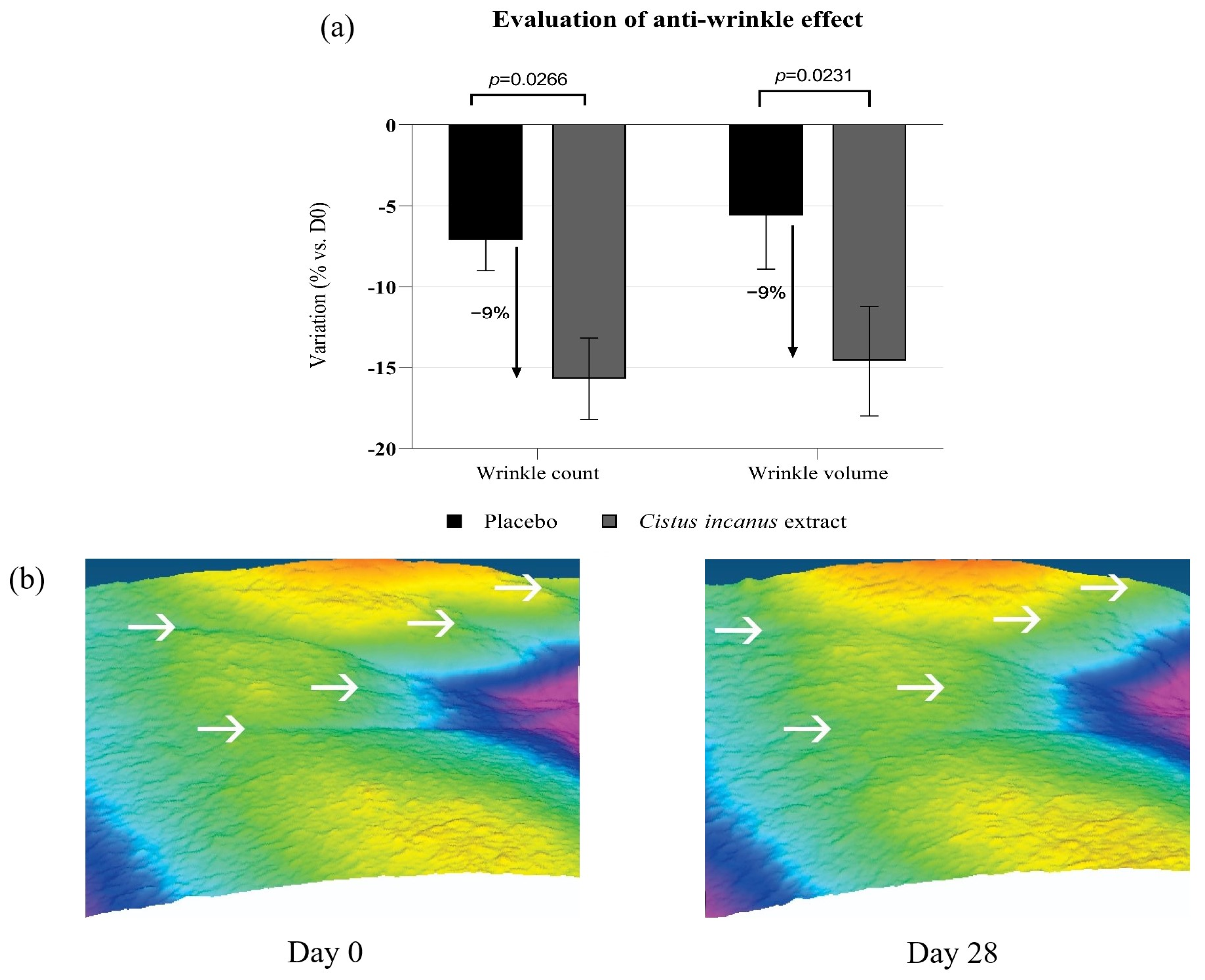
3.2.3. Anti-Aging Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dunn, J.H.; Koo, J. Psychological stress and skin aging: A review of possible mechanisms and potential therapies. Dermatol. Online J. 2013, 19, 18561. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.; Scott, K.; Vos, T.; Whiteford, H. Global prevalence of anxiety disorders: A systematic review and meta-regression. Psychol. Med. 2013, 43, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Remes, O.; Brayne, C.; van der Linde, R.; Lafortune, L. A systematic review of reviews on the prevalence of anxiety disorders in adult populations. Brain Behav. 2016, 6, e00497. [Google Scholar] [CrossRef]
- Bryant, C.; Jackson, H.; Ames, D. The prevalence of anxiety in older adults: Methodological issues and a review of the literature. J. Affect. Disord. 2008, 109, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lyga, J. Brain-skin connection: Stress, inflammation and skin aging. Inflamm. Allergy Drug Targets 2014, 13, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Pondeljak, N.; Lugovic-Mihic, L. Stress-induced Interaction of Skin Immune Cells, Hormones, and Neurotransmitters. Clin. Ther. 2020, 42, 757–770. [Google Scholar] [CrossRef]
- Zbytek, B.; Pfeffer, L.M.; Slominski, A.T. Corticotropin-releasing hormone stimulates NF-kappaB in human epidermal keratinocytes. J. Endocrinol. 2004, 181, R1–R7. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.M.; Gregg, M.; Hashemi, F.; Schott, L.; Hughes, T.K. Corticotropin Releasing Factor (CRF) Activation of NF-κB-Directed Transcription in Leukocytes. Cell. Mol. Neurobiol. 2006, 26, 1019–1034. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Pober, J.S.; Sessa, W.C. Inflammation and the blood microvascular system. Cold Spring Harb. Perspect Biol. 2014, 7, a016345. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell. Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.K.; Pang, X.; Alexacos, N.; Letourneau, R.; Theoharides, T.C. Acute immobilization stress triggers skin mast cell degranulation via corticotropin releasing hormone, neurotensin, and substance P: A link to neurogenic skin disorders. Brain. Behav. Immun. 1999, 13, 225–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robles, T.F. Stress, social support, and delayed skin barrier recovery. Psychosom. Med. 2007, 69, 807–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukas, B.; Bragagna, L.; Starzyk, K.; Labedz, K.; Stolze, K.; Novak, J. Polyphenol diversity and antioxidant activity of European Cistus creticus L. (cistaceae) compared to six further, partly sympatric Cistus species. Plants 2021, 10, 615. [Google Scholar] [CrossRef] [PubMed]
- Riehle, P.; Rusche, N.; Saake, B.; Rohn, S. Influence of the Leaf Content and Herbal Particle Size on the Presence and Extractability of Quantitated Phenolic Compounds in Cistus incanus Herbal Teas. J. Agric. Food Chem. 2014, 62, 10978–10988. [Google Scholar] [CrossRef]
- Barrajón-Catalán, E.; Fernández-Arroyo, S.; Roldán, C.; Guillén, E.; Saura, D.; Segura-Carretero, A.; Micol, V. A systematic study of the polyphenolic composition of aqueous extracts deriving from several Cistus genus species: Evolutionary relationship. Phytochem. Anal. 2011, 22, 303–312. [Google Scholar] [CrossRef]
- Lardos, A.; Prieto-Garcia, J.; Heinrich, M. Resins and gums in historical iatrosophia texts from Cyprus—A botanical and medico-pharmacological approach. Front. Pharmacol. 2011, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Tuzlacı, E.; Aymaz, P.E. Turkish folk medicinal plants. Part IV: Gönen (Balıkesir). Fitoterapia 2001, 72, 323–343. [Google Scholar] [CrossRef]
- Petereit, F.; Kolodziej, H.; Nahrstedt, A. Flavan-3-ols and proanthocyanidins from Cistus incanus. Phytochemistry 1991, 30, 981–985. [Google Scholar] [CrossRef]
- Bernacka, K.; Bednarska, K.; Starzec, A.; Mazurek, S.; Fecka, I. Antioxidant and Antiglycation Effects of Cistus x incanus Water Infusion, Its Phenolic Components, and Respective Metabolites. Molecules 2022, 27, 2432. [Google Scholar] [CrossRef]
- Zalegh, I.; Akssira, M.; Bourhia, M.; Mellouki, F.; Rhallabi, N.; Salamatullah, A.M.; Alkaltham, M.S.; Khalil Alyahya, H.; Mhand, R.A. A Review on Cistus sp.: Phytochemical and Antimicrobial Activities. Plants 2021, 10, 1214. [Google Scholar] [CrossRef] [PubMed]
- Saifulazmi, N.F.; Rohani, E.R.; Harun, S.; Bunawan, H.; Hamezah, H.S.; Nor Muhammad, N.A.; Azizan, K.A.; Ahmed, Q.U.; Fakurazi, S.; Mediani, A.; et al. A Review with Updated Perspectives on the Antiviral Potentials of Traditional Medicinal Plants and Their Prospects in Antiviral Therapy. Life 2022, 12, 1287. [Google Scholar] [CrossRef] [PubMed]
- Rebensburg, S.; Helfer, M.; Schneider, M.; Koppensteiner, H.; Eberle, J.; Schindler, M.; Gurtler, L.; Brack-Werner, R. Potent in vitro antiviral activity of Cistus incanus extract against HIV and Filoviruses targets viral envelope proteins. Sci. Rep. 2016, 6, 20394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuchta, A.; Konopacka, A.; Waleron, K.; Viapiana, A.; Wesołowski, M.; Dąbkowski, K.; Ćwiklińska, A.; Mickiewicz, A.; Śledzińska, A.; Wieczorek, E.; et al. The effect of Cistus incanus herbal tea supplementation on oxidative stress markers and lipid profile in healthy adults. Cardiol. J. 2021, 28, 534–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mocan, A.; Fernandes, Â.; Calhelha, R.C.; Gavrilas, L.; Ferreira, I.C.F.R.; Ivanov, M.; Sokovic, M.; Barros, L.; Babota, M. Bioactive Compounds and Functional Properties of Herbal Preparations of Cystus creticus L. Collected from Rhodes Island. Front. Nutr. 2022, 9, 881210. [Google Scholar] [CrossRef]
- Dimcheva, V.; Karsheva, M. Cistus incanus from Strandja Mountain as a Source of Bioactive Antioxidants. Plants 2018, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Petereit, F.; Kolodziej, H.; Nahrstedt, A. Proanthocyanidins and bio genetically related dihydroflavonols from Cistus incanus L. In Plant Polyphenols: Synthesis, Properties, Significance; Hemingway, R.W., Laks, P.E., Eds.; Springer: Boston, MA, USA, 1992; pp. 729–737. [Google Scholar]
- Gori, A.; Ferrini, F.; Marzano, M.C.; Tattini, M.; Centritto, M.; Baratto, M.C.; Pogni, R.; Brunetti, C. Characterization and Antioxidant Activity of Crude Extract and Polyphenolic Rich Fractions from C. incanus Leaves. Int. J. Mol. Sci. 2016, 17, 1344. [Google Scholar] [CrossRef]
- Yang, Y.L.; Liu, M.; Cheng, X.; Li, W.H.; Zhang, S.S.; Wang, Y.H.; Du, G.H. Myricitrin blocks activation of NF-κB and MAPK signaling pathways to protect nigrostriatum neuron in LPS-stimulated mice. J. Neuroimmunol. 2019, 337, 577049. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, S.P.; Nguyen, M.; Yow, T.T.; Chu, C.; Johnston, G.A.R.; Hanrahan, J.R.; Chebib, M. The flavonoid glycosides, myricitrin, gossypin and naringin exert anxiolytic action in mice. Neurochem. Res. 2009, 34, 1867–1875. [Google Scholar] [CrossRef]
- Qi, S.; Feng, Z.; Li, Q.; Qi, Z.; Zhang, Y. Myricitrin Modulates NADPH Oxidase-Dependent ROS Production to Inhibit Endotoxin-Mediated Inflammation by Blocking the JAK/STAT1 and NOX2/p47phox Pathways. Oxidative Med. Cell. Longev. 2017, 2017, 9738745. [Google Scholar] [CrossRef]
- Chen, R.; Lewis, K.A.; Perrin, M.H.; Vale, W.W. Expression cloning of a human corticotropin-releasing-factor receptor. Proc. Natl. Acad. Sci. USA 1993, 90, 8967–8971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M.; Strzępek-Gomółka, M.; Antosiewicz, B. Characterization of Cistus × incanus L. and Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attaguile, G.; Russo, A.; Campisi, A.; Savoca, F.; Acquaviva, R.; Ragusa, N.; Vanella, A. Antioxidant activity and protective effect on DNA cleavage of extracts from Cistus incanus L. and Cistus monspeliensis L. Cell Biol. Toxicol. 2000, 16, 83–90. [Google Scholar] [CrossRef] [PubMed]




| INCI/Chemical Name | % in Formula—Active | % in Formula—Placebo |
|---|---|---|
| Water | 84.35 | 84.85 |
| Butylene glycol | 4.00 | 4.00 |
| Dipropylene glycol | 1.00 | 1.00 |
| Hexylene glycol | 1.00 | 1.00 |
| Polysorbate 20 | 1.00 | 1.00 |
| Hydrogenated polydecene | 1.50 | 1.50 |
| Cyclomethicone | 4.00 | 4.00 |
| Cistus incanus extract | 1.00 | 0.00 |
| Glycerol | 0.00 | 0.50 |
| Carbomer | 0.80 | 0.80 |
| Triethanolamine | 0.70 | 0.70 |
| Phenoxyethanol | 0.40 | 0.40 |
| Methyl paraben | 0.15 | 0.15 |
| EDTA | 0.10 | 0.10 |
| FD&C Blue 1 | 0.0000000 | 0.0000010 |
| FD&C red 40 | 0.0000000 | 0.0000075 |
| FD&C yellow 5 | 0.0000000 | 0.0000250 |
| TOTAL | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Havas, F.; Cohen, M.; Krispin, S.; Loing, E.; Attia-Vigneau, J. A Cistus incanus Extract Blocks Psychological Stress Signaling and Reduces Neurogenic Inflammation and Signs of Aging in Skin, as Shown in In-Vitro Models and a Randomized Clinical Trial. Cosmetics 2023, 10, 4. https://doi.org/10.3390/cosmetics10010004
Havas F, Cohen M, Krispin S, Loing E, Attia-Vigneau J. A Cistus incanus Extract Blocks Psychological Stress Signaling and Reduces Neurogenic Inflammation and Signs of Aging in Skin, as Shown in In-Vitro Models and a Randomized Clinical Trial. Cosmetics. 2023; 10(1):4. https://doi.org/10.3390/cosmetics10010004
Chicago/Turabian StyleHavas, Fabien, Moshe Cohen, Shlomo Krispin, Estelle Loing, and Joan Attia-Vigneau. 2023. "A Cistus incanus Extract Blocks Psychological Stress Signaling and Reduces Neurogenic Inflammation and Signs of Aging in Skin, as Shown in In-Vitro Models and a Randomized Clinical Trial" Cosmetics 10, no. 1: 4. https://doi.org/10.3390/cosmetics10010004
APA StyleHavas, F., Cohen, M., Krispin, S., Loing, E., & Attia-Vigneau, J. (2023). A Cistus incanus Extract Blocks Psychological Stress Signaling and Reduces Neurogenic Inflammation and Signs of Aging in Skin, as Shown in In-Vitro Models and a Randomized Clinical Trial. Cosmetics, 10(1), 4. https://doi.org/10.3390/cosmetics10010004







