Comparison of Analytical Methods for the Detection of Residual Crosslinker in Hyaluronic Acid Hydrogel Films
Abstract
:1. Introduction
2. Materials, Chemicals, and Reagents
2.1. Preparation of Hyaluronic Acid Hydrogels
2.2. Instruments Used for Analysis
2.2.1. Freeze Dryer
2.2.2. Scanning Electron Microscopy (SEM)
2.2.3. 1HNMR Spectroscopy
2.2.4. Gas Chromatography Analysis
Internal Standard Solution
Standard Solution and Calibration Curve
Sample Preparation
3. Results and Discussion
3.1. PT Description
3.2. Freeze-Drying of the Extracted Samples
3.3. SEM Scanning Electron Microscope
3.4. NMR Method Analysis
3.5. Gas Chromatography Method
3.5.1. Response Linearity
3.5.2. Precision
3.5.3. Accuracy
3.5.4. Sensitivity
3.5.5. Robustness
3.5.6. Sample Extraction in Dichloromethane (DCM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brandt, F.S.; Cazzaniga, A.; Hann, M. Cosmeceuticals: Current Trends and Market Analysis. Semin. Cutan. Med. Surg. 2011, 30, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Barel, A.O.; Paye, M.; Maibach, H.I. Handbook of Cosmetic Science and Technology; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Kiki, M.J. Biopigments of Microbial Origin and Their Application in the Cosmetic Industry. Cosmetics 2023, 10, 47. [Google Scholar] [CrossRef]
- Rodrigues, R.; Alves, R.C.; Oliveira, M.B.P.P. Exploring Olive Pomace for Skincare Applications: A Review. Cosmetics 2023, 10, 35. [Google Scholar] [CrossRef]
- Dayan, N. Skin Aging Handbook: An Integrated Approach to Biochemistry and Product Development, 3rd ed.; William Andrew: Norwich, NY, USA, 2008. [Google Scholar]
- Semenzato, A.; Costantini, A.; Meloni, M.; Maramaldi, G.; Meneghin, M.; Baratto, G. Formulating O/W Emulsions with Plant-Based Actives: A Stability Challenge for an Effective Product. Cosmetics 2018, 5, 59. [Google Scholar] [CrossRef] [Green Version]
- Chanchal, D.; Swarnlata, S. Novel Approaches in Herbal Cosmetics. J. Cosmet. Dermatol. 2008, 7, 89–95. [Google Scholar] [CrossRef]
- Rashid, F.; Albayati, M.; Dodou, K. Studies on Novel Methods for Formulating Novel Cross-Linked Hydrogel Films of Hyaluronic Acid. Cosmetics 2019, 6, 59. [Google Scholar] [CrossRef] [Green Version]
- Rashid, F.; Albayati, M.; Dodou, K. Novel Crosslinked HA Hydrogel Films for the Immediate Release of Active Ingredients. Cosmetics 2022, 10, 6. [Google Scholar] [CrossRef]
- Wende, F.J.; Gohil, S.; Mojarradi, H.; Gerfaud, T.; Nord, L.I.; Karlsson, A.; Boiteau, J.-G.; Kenne, A.H.; Sandström, C. Determination of Substitution Positions in Hyaluronic Acid Hydrogels Using NMR and MS Based Methods. Carbohydr. Polym. 2016, 136, 1348–1357. [Google Scholar] [CrossRef] [Green Version]
- Al-Sibani, M.; Al-Harrasi, A.; Neubert, R.H.H. Characterization of Linear and Chemically Cross-Linked Hyaluronic Acid Using Various Analytical Techniques Including FTIR, ESI-MS, H1 NMR, and SEM. J. Biochem. Anal. Stud. 2018, 3, 1–8. [Google Scholar] [CrossRef]
- Ström, A.; Larsson, A.; Okay, O. Preparation and Physical Properties of Hyaluronic Acid-Based Cryogels. J. Appl. Polym. Sci. 2015, 132, 1–11. [Google Scholar] [CrossRef]
- Olejnik, A. Significance of Hyaluronic Acid in Cosmetic Industry and Aesthetic Medicine. Chemik 2012, 66, 129–135. [Google Scholar]
- Lewandowska, K.; Sionkowska, A.; Grabska, S.; Michalska, M. Characterisation of Chitosan/Hyaluronic Acid Blend Films Modified by Collagen. Prog. Chem. Appl. Chitin Its Deriv. 2017, XXII, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic Acid, a Promising Skin Rejuvenating Biomedicine: A Review of Recent Updates and Pre-Clinical and Clinical Investigations on Cosmetic and Nutricosmetic Effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Huang, H. Application of Hyaluronic Acid as Carriers in Drug Delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Wong, R.S.H.; Ashton, M.; Dodou, K. Analysis of Residual Crosslinking Agent Content in UV Cross-Linked Poly(Ethylene Oxide) Hydrogels for Dermatological Application by Gas Chromatography. J. Pharm. Anal. 2016, 6, 307–312. [Google Scholar] [CrossRef] [Green Version]
- Larrañeta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R.F. Synthesis and Characterization of Hyaluronic Acid Hydrogels Crosslinked Using a Solvent-Free Process for Potential Biomedical Applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wu, S.; Chen, W.; Hu, Y.; Geng, Z.; Su, J. Bone/Cartilage Targeted Hydrogel: Strategies and Applications. Bioact. Mater. 2023, 23, 156–169. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Saravanan, A.; Margel, S.; Gedanken, A.; Srinivasan, S.; Rajabzadeh, A.R. Naturally Derived Carbon Dots in Situ Confined Self-Healing and Breathable Hydrogel Monolith for Anomalous Diffusion-Driven Phytomedicine Release. ACS Appl. Bio Mater. 2022, 5, 5617–5633. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Yang, G.; Kong, H.; Guo, L.; Wei, G. Recent Advances in Protein Hydrogels: From Design, Structural and Functional Regulations to Healthcare Applications. Chem. Eng. J. 2023, 451, 138494. [Google Scholar] [CrossRef]
- Wong, R.; Ashton, M.; Dodou, K. Effect of Crosslinking Agent Concentration on the Properties of Unmedicated Hydrogels. Pharmaceutics 2015, 7, 305–319. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Chen, H. Preparation of Aroma Microcapsules with Sodium Alginate and Tetradecylallyldimethylammonium Bromide (TADAB) and Its Potential Applications in Cosmetics. Flavour Fragr. J. 2017, 33, 160–165. [Google Scholar] [CrossRef]
- Doytcheva, M. Ultraviolet-Induced Crosslinking of Solid Poly (Ethylene Oxide). J. Appl. Polym. Sci. 1997, 64, 2299–2307. [Google Scholar] [CrossRef]
- Björkner, B. The Sensitizing Capacity of Multifunctional Acrylates in the Guinea Pig. Contact Dermat. 1984, 11, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Sadamori, S.; Shigeto, N.; Hamada, T.; Okuda, K. A Method of Determining Residual Monomer in Acrylic Resin Using Methyl Ethyl Ketone. Aust. Dent. J. 1990, 35, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.H.; McPhee, V.A. Determination of Methacrylic Acid by Coulometric Titration. Anal. Chem. 1976, 48, 1820. [Google Scholar] [CrossRef]
- Ayaz, E.A.; Durkan, R.; Koroglu, A.; Bagis, B. Comparative Effect of Different Polymerization Techniques on Residual Monomer and Hardness Properties of PMMA-Based Denture Resins. J. Appl. Biomater. Funct. Mater. 2014, 12, 228–233. [Google Scholar] [CrossRef]
- Pouyani, T.; Harbison, G.S.; Prestwich, G.D. Novel Hydrogels of Hyaluronic Acid: Synthesis, Surface Morphology, and Solid-State NMR. J. Am. Chem. Soc. 1994, 116, 7515–7522. [Google Scholar] [CrossRef]
- Scott Mackenzie, D. Determining Concentration by Weight and by Volume-Application to Polymer Quenchants; Technical report; Houghton International Inc.: Valley Forge, PA, USA, 2014. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 62556, Pentaerythritol Tetraacrylate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Pentaerythritol-tetraacrylate (accessed on 25 March 2023).
- Nethercott, J.R. Skin Problems Associated with Multifunctional Acrylic Monomers in Ultraviolet Curing Inks. Br. J. Dermatol. 1978, 98, 541–552. [Google Scholar] [CrossRef]
- Unwin, J. Development of an Analysis Method to Measure Airborne Acrylate in UV-Cured Motor Vehicle Repair Coatings. Health and Safety Laboratory. 2007; pp. 1–17. Available online: http://www.hsl.gov.uk/ (accessed on 25 March 2023).
- Pentaerythritol Tetraacrylate. Toronto Research Chemicals. Available online: https://www.trc-canada.com/product-detail/?P227458 (accessed on 25 March 2023).
- Wong, R.S.H.; Dodou, K. Effect of Drug Loading Method and Drug Physicochemical Properties on the Material and Drug Release Properties of Poly (Ethylene Oxide) Hydrogels for Transdermal Delivery. Polymers 2017, 9, 286. [Google Scholar] [CrossRef]
- Available online: https://www.sigmaaldrich.com/GB/en/sds/aldrich/408905 (accessed on 25 March 2023).
- Abdi, K.; Mandegary, A.; Amini, M.; Bagheri, M.; Gerami-Panah, F. Determination of Residual Methylmethacrylate Monomerin Denture Base Resins by Gas Chromatography. Iran. J. Pharm. Res. 2005, 4, 227–232. [Google Scholar] [CrossRef]
- Craig, A.P.; Fields, C.C.; Simpson, J.V. Development of a Gas Chromatography-Mass Spectrometry Method for the Quantification of Glucaric Acid Derivatives in Beverage Substrates. Int. J. Anal. Chem. 2014, 2014, 402938. [Google Scholar] [CrossRef] [PubMed]
- Bernal, E.; Guo, X. Limit of Detection and Limit of Quantification Determination in Gas Chromatography. Adv. Gas Chromatogr. 2014, 3, 57–63. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency. Note for Guidance on Validation of Analytical Procedures: Text and Methodology; CPMP/ICH/381/95; European Medicines Agency: Amsterdam, The Netherlands, 1995.
- Armbruster, D.A.; Tillman, M.D.; Hubbs, L.M. Limit of Detection (LQD)/Limit of Quantitation (LOQ): Comparison of the Empirical and the Statistical Methods Exemplified with GC-MS Assays of Abused Drugs. Clin. Chem. 1994, 40, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Krupčík, J.; Májek, P.; Gorovenko, R.; Blaško, J.; Kubinec, R.; Sandra, P. Considerations on the Determination of the Limit of Detection and the Limit of Quantification in One-Dimensional and Comprehensive Two-Dimensional Gas Chromatography. J. Chromatogr. A 2015, 1396, 117–130. [Google Scholar] [CrossRef] [PubMed]
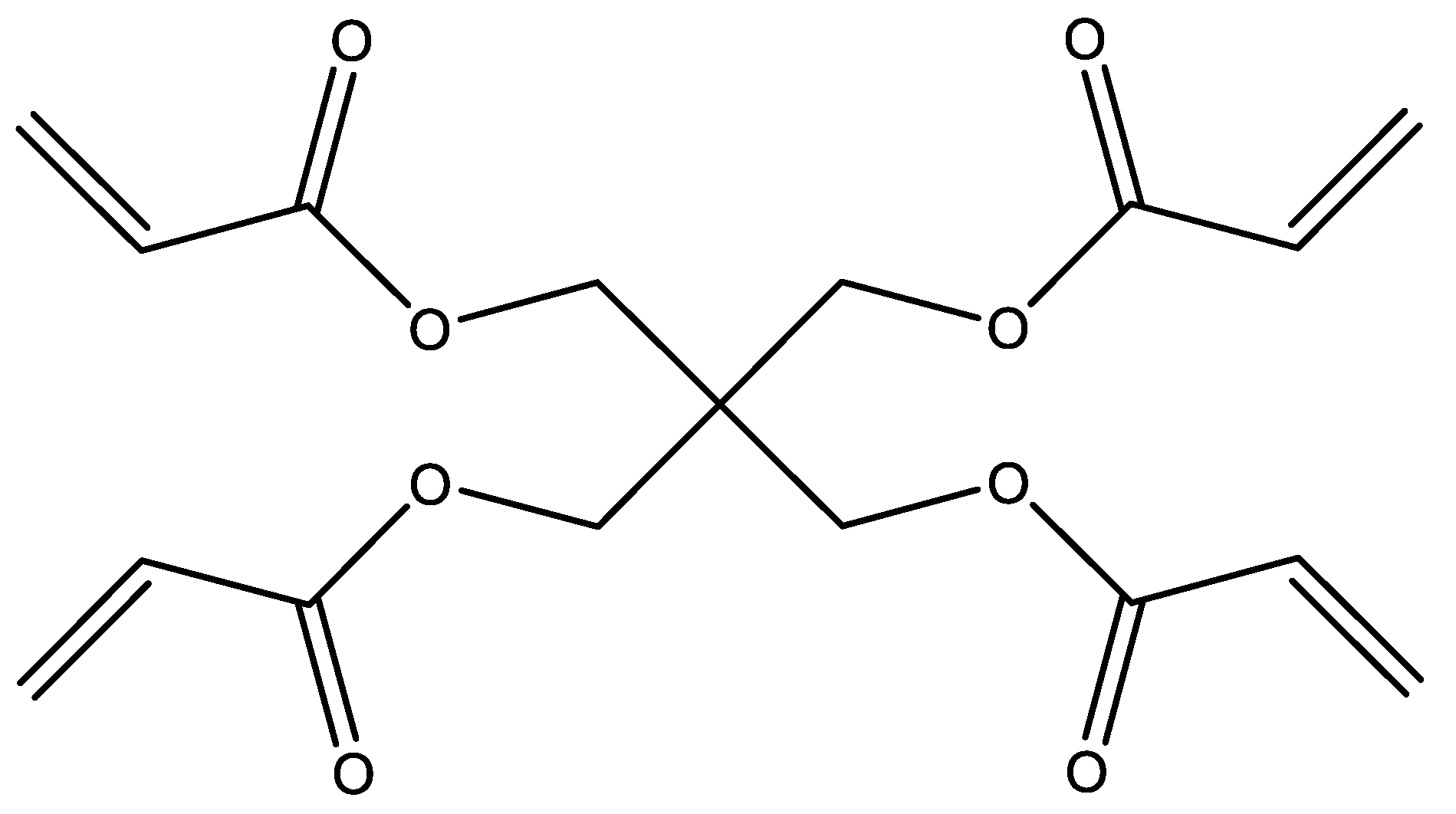




| Hydrogel Films Names | % w/w of the PT in HA | Ratio of PT to HA (%) | The % w/w of PT in the Film |
|---|---|---|---|
| M20 | 20% | (1:5) | 1.00% |
| M25 | 25% | (1:4) | 1.25% |
| M40 | 40% | (2:5) | 2.00% |
| M50 | 50% | (1:2) | 2.55% |
| M75 | 75% | (3:4) | 3.83% |
| M100 | 100% | (1:1) | 5.00% |
| Samples | PT Concentration in GC Analysis % m/m ± SD | ||
|---|---|---|---|
| Sample 1 | Not freeze-dried | 0.00028 | ±0.75 |
| Sample 2 | 0.00031 | ±0.62 | |
| Sample 3 | 0.00035 | ±0.59 | |
| Mean (0.0003) | |||
| Sample 4 | Freeze-dried | 0.00275 | ±1.02 |
| Sample 5 | 0.00409 | ±2.50 | |
| Sample 6 | 0.00305 | ±1.02 | |
| Mean (0.0033) |
| Selected PT Concentrations (%m/m) | Peak Area Ratio % (Mean ±SD) Intra-Day Precision | RSD% | Peak Area Ratio % (Mean ±SS) Inter-Day Precision | RSD% |
|---|---|---|---|---|
| 0.000032 | 1.1062 ± (0.006) | 0.54 | 1.193 ± (0.056) | 4.72 |
| 0.00052 | 17.365 ± (0.439) | 2.53 | 15.278 ± (0.283) | 1.85 |
| 0.00833 | 264.41 ± (6.035) | 2.28 | 256.15 ± (2.541) | 0.99 |
| mean | (1.78)% | (2.52)% |
| Selected PT Concentrations Added % m/m | Blank Sample Concentration % m/m | Recovered Concentration % m/m | % Recovery (Accuracy) | %RSD |
|---|---|---|---|---|
| 0.004 | 0.009 | 0.0158 | 105.49 ± 1.26 | 1.24 |
| 0.006 | 0.009 | 0.0184 | 102.38 ± 0.65 | 0.64 |
| 0.009 | 0.009 | 0.0126 | 97.31 ± 3.26 | 3.20 |
| mean | 101.73 | 1.69 | ||
| Sensitivity Parameters | PT Concentration % m/m | Signal of Noise N/S ± (SD) |
|---|---|---|
| LOD | 0.000032 | 4.2 ± 0.15 |
| LOQ | 0.00013 | 15.7 ± 0.41 |
| Different Parameters | PT Concentration %m/m | Peak Area Ratio Mean ± SD | % RSD |
|---|---|---|---|
| No variation applied | 0.00416 | 0.377 ± 0.002 | 0.54 |
| 0.0020 | 0.215 ± 0.004 | 2.03 | |
| 0.0010 | 0.095 ± 0.001 | 0.96 | |
| Detector temperature (+5) | 0.00416 | 0.303 ± 0.006 | 1.93 |
| 0.0020 | 0.164 ± 0.003 | 1.93 | |
| 0.0010 | 0.075 ± 0.001 | 1.25 | |
| Detector temperature −(5) | 0.00416 | 0.297 ± 0.007 | 2.36 |
| 0.0020 | 0.179 ± 0.004 | 0.83 | |
| 0.0010 | 0.084 ± 0.002 | 1.82 | |
| Oven temperature (+5) | 0.00416 | 0.307 ± 0.007 | 2.13 |
| 0.0020 | 0.184 ± 0.004 | 1.90 | |
| 0.0010 | 0.082 ± 0.001 | 1.80 | |
| Oven temperature (−5) | 0.00416 | 0.301 ± 0.001 | 0.43 |
| 0.0020 | 0.178 ± 0.001 | 0.56 | |
| 0.0010 | 0.075 ± 0.001 | 1.27 | |
| Flow rate (+10) | 0.00416 | 0.319 ± 0.005 | 1.67 |
| 0.0020 | 0.596 ± 0.008 | 1.36 | |
| 0.0010 | 0.081 ± 0.001 | 1.41 | |
| Flow rate (−10) | 0.00416 | 0.300 ± 0.006 | 2.02 |
| 0.0020 | 0.677 ± 0.023 | 3.39 | |
| 0.0010 | 0.281 ± 0.005 | 1.72 | |
| Mean | (1.59) |
| Samples | PT Concentration (Chloroform Extraction) % m/m | PT Concentration (DCM Extraction) % m/m |
|---|---|---|
| M25 | 0.002703 | 0.002700 |
| M25 | 0.005 | 0.00056 |
| M25 | 0.0013 | 0.00045 |
| SD ± (0.001) | SD ± (0.001) | |
| M50 | 0.0011 | 0.001 |
| M50 | 0.00166 | 0.00168 |
| M50 | 0.002 | 0.002 |
| SD ± (0.0005) | SD ± (0.0008) |
| Hydrogel Film Samples | PT Concentration % m/m with NMR | PT Concentration % m/m with GC | Acceptance of the PT Concentration ˂ 0.008%m/m |
|---|---|---|---|
| M20 (1) | 0.0017 | 0.0017 | Accepted |
| M20 (2) | 0.0021 | 0.0021 | Accepted |
| M20 (3) | 0.0051 | 0.0041 | Accepted |
| mean | 0.0030 ± (0.001) | 0.0026 ± (0.001) | |
| M25 (1) | 0.0015 | 0.0013 | Accepted |
| M25 (2) | 0.0016 | 0.0015 | Accepted |
| M25 (3) | 0.0046 | 0.004 | Accepted |
| mean | 0.0026 ± (0.001) | 0.0023 ± (0.001) | |
| M40 (1) | 0.0026 | 0.0002 | Accepted |
| M40 (2) | 0.0018 | 0.00015 | Accepted |
| M40 (3) | 0.0008 | 0.00053 | Accepted |
| Mean | 0.0017 ± (0.0009) | 0.0006 ± (0.0004) | |
| M50 (1) | 0.0018 | 0.0019 | Accepted |
| M50 (2) | 0.0019 | 0.0015 | Accepted |
| M50 (3) | 0.0014 | 0.0015 | Accepted |
| Mean | 0.0017 ± (0.0008) | 0.0016 ± (0.0002) | |
| M75 (1) | 0.0069 | 0.0058 | Accepted |
| M75 (2) | 0.0085 | 0.0049 | Rejected |
| M75 (3) | 0.0083 | 0.0073 | Rejected |
| Mean | 0.0079 ± (0.0009) | 0.006 ± (0.001) | |
| M100 (1) | 0.0506 | 0.0301 | Rejected |
| M100 (2) | 0.0679 | 0.0355 | Rejected |
| M100 (3) | 0.031 | 0.0189 | Rejected |
| Mean | 0.0498 ± (0.018) | 0.028 ± (0.008) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, F.; Childs, S.; Dodou, K. Comparison of Analytical Methods for the Detection of Residual Crosslinker in Hyaluronic Acid Hydrogel Films. Cosmetics 2023, 10, 70. https://doi.org/10.3390/cosmetics10030070
Rashid F, Childs S, Dodou K. Comparison of Analytical Methods for the Detection of Residual Crosslinker in Hyaluronic Acid Hydrogel Films. Cosmetics. 2023; 10(3):70. https://doi.org/10.3390/cosmetics10030070
Chicago/Turabian StyleRashid, Fatimah, Stephen Childs, and Kalliopi Dodou. 2023. "Comparison of Analytical Methods for the Detection of Residual Crosslinker in Hyaluronic Acid Hydrogel Films" Cosmetics 10, no. 3: 70. https://doi.org/10.3390/cosmetics10030070
APA StyleRashid, F., Childs, S., & Dodou, K. (2023). Comparison of Analytical Methods for the Detection of Residual Crosslinker in Hyaluronic Acid Hydrogel Films. Cosmetics, 10(3), 70. https://doi.org/10.3390/cosmetics10030070







