Development and Functionality of Sinami (Oenocarpus mapora) Seed Powder as a Biobased Ingredient for the Production of Cosmetic Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Products
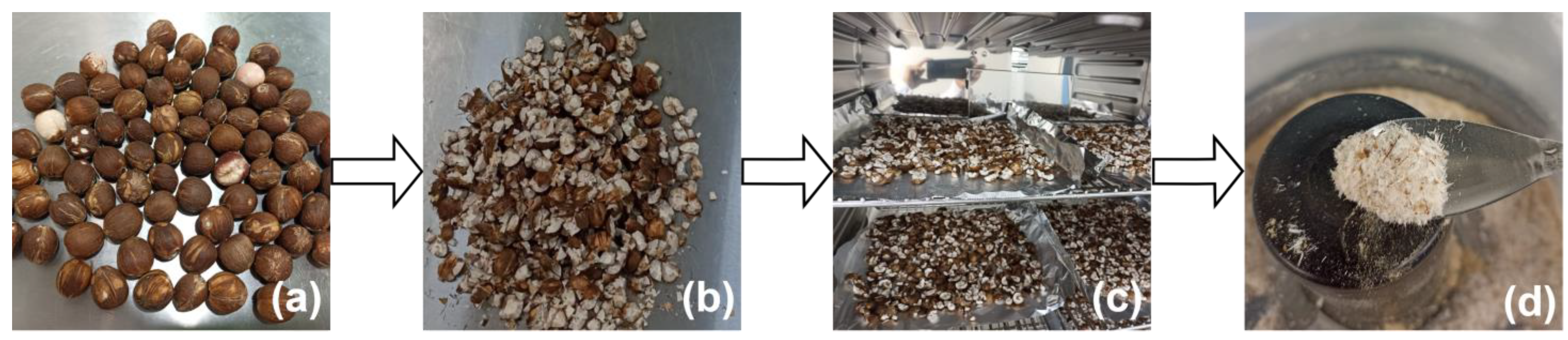
2.2. Sampling and Treatment of Seeds
2.3. Physicochemical Characterization of the Sinami Seeds
2.3.1. Proximal Composition
2.3.2. Extraction Process
2.3.3. Determination of Total Phenolic Content
2.4. Antioxidant Activity
2.4.1. DPPH Radical Scavenging Activity
2.4.2. ABTS Radical Scavenging Activity
2.5. Exfoliant Formulation
2.5.1. pH and Texture
2.5.2. Storage Stability Test
3. Results
3.1. Characterization of the Sinami Seed
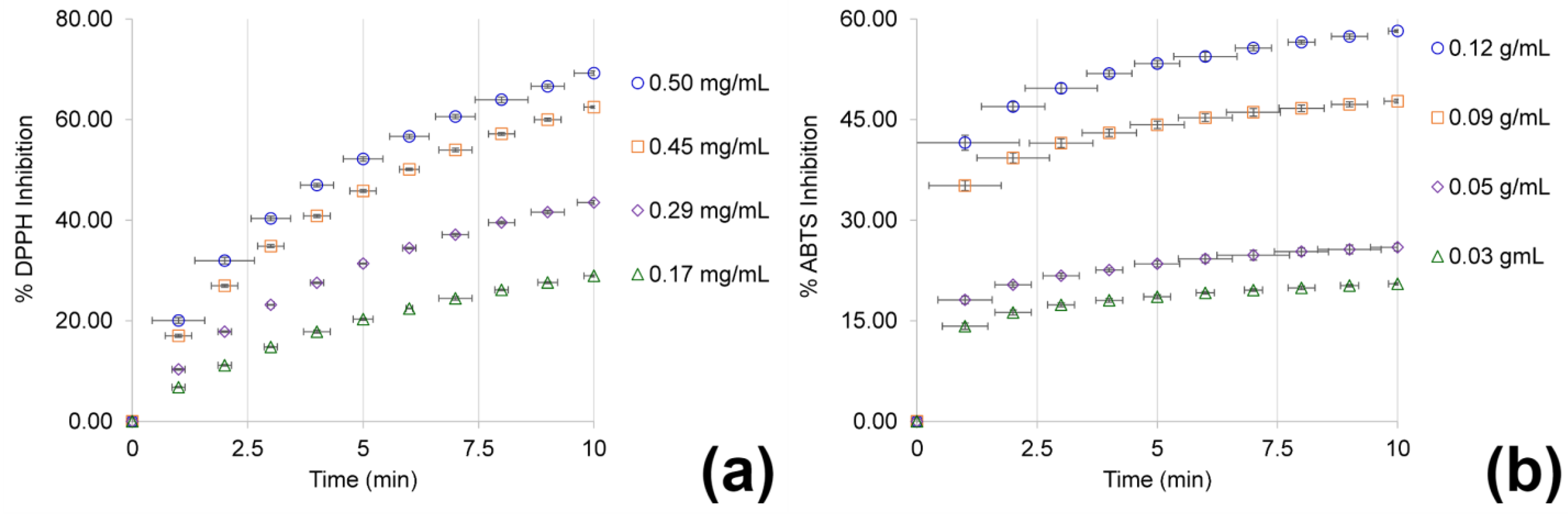
3.2. Characterization of Total Phenolic Content and Antioxidant Activity
3.3. Exfoliant Prototype Characterization
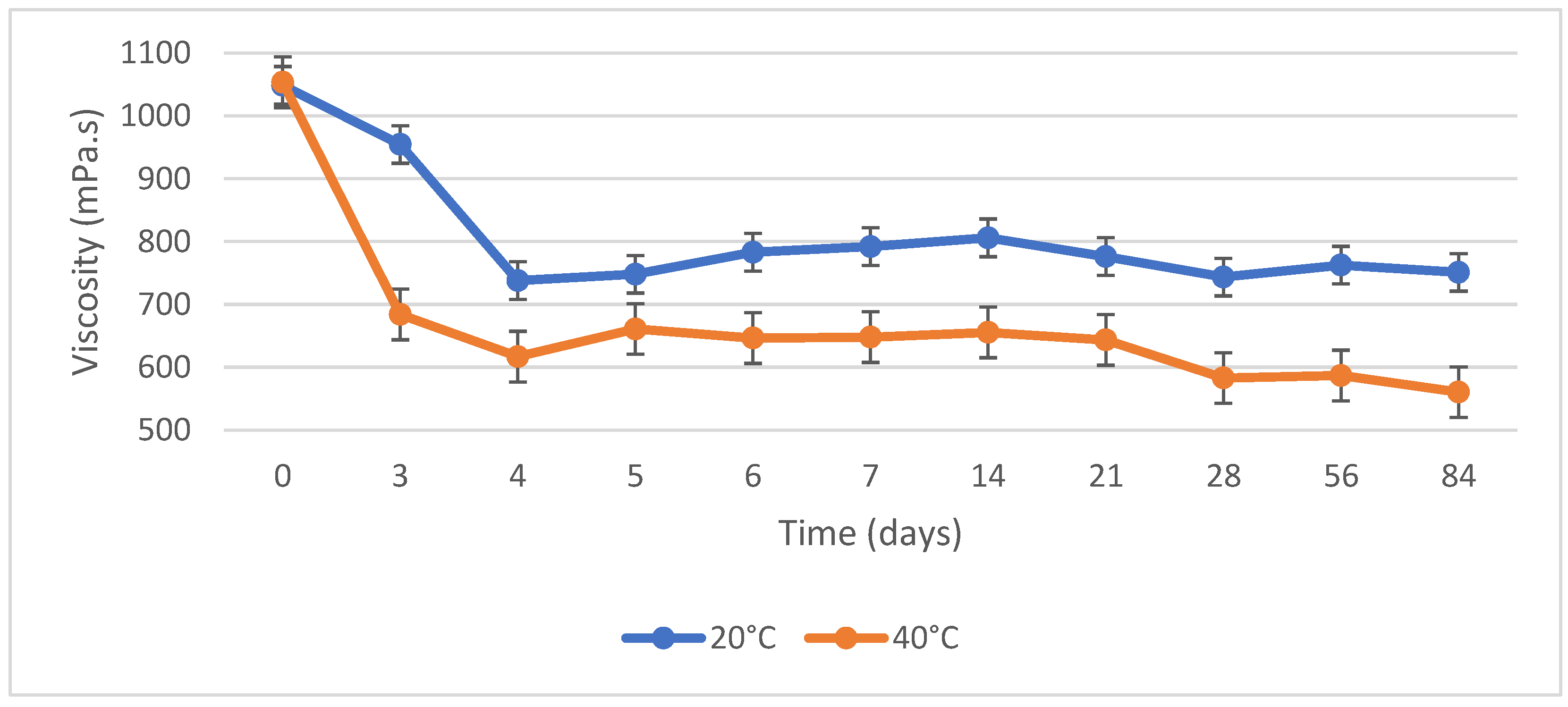
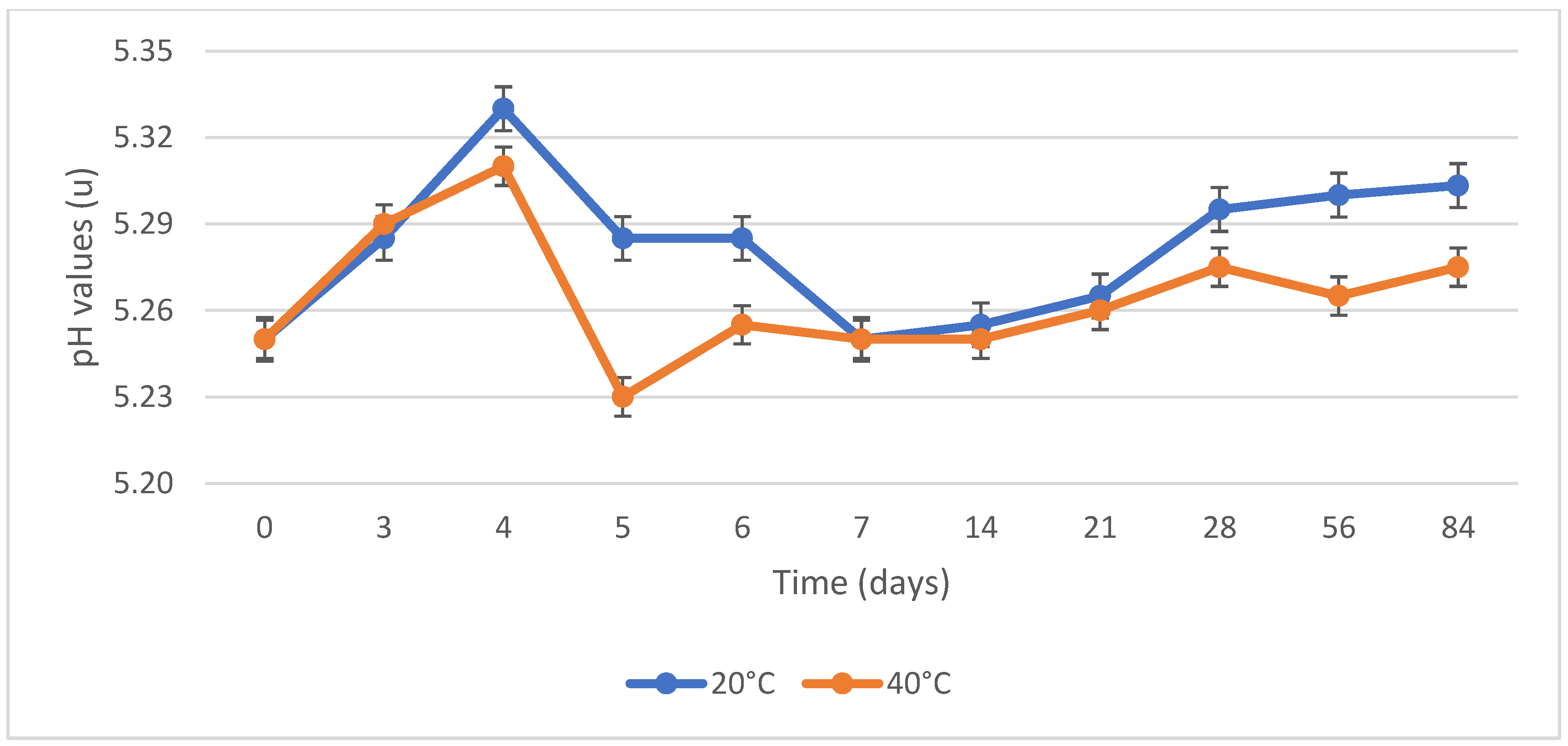
3.4. Storage Stability Test
4. Discussion
4.1. Characterization of the Sinami Seed
4.2. Characterization of Total Phenolic Content and Antioxidant Activity
4.3. Exfoliant Prototype Characterization
4.4. Storage Stability Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henderson, A.; Gloria, G.; Rodrigo, B. Field Guide to the Palms of the Americas; Princeton University Press: Princeton, NJ, USA, 1995. [Google Scholar]
- Cunha, V.M.B.; da Silva, M.P.; de Sousa, S.H.B.; Bezerra, P.D.N.; Menezes, E.G.O.; da Silva, N.J.N.; da Silva Banna, D.A.D.; Araújo, M.E.; de Carvalho Junior, R.N. Bacaba-de-Leque (Oenocarpus Distichus Mart.) Oil Extraction Using Supercritical CO2 and Bioactive Compounds Determination in the Residual Pulp. J. Supercrit. Fluids 2019, 144, 81–90. [Google Scholar] [CrossRef]
- Dos Santos, O.V.; Viana, A.A.; Soares, S.D.; Vieira, E.L.S.; Martins, M.G.; Do Nascimento, F.D.C.A.; Teixeira-Costa, B.E. Industrial Potential of Bacaba (Oenocarpus Bacaba) in Powder: Antioxidant Activity, Spectroscopic and Morphological Behavior. Food Sci. Technol. 2022, 42, 1–7. [Google Scholar] [CrossRef]
- Sosnowska, J.; Ramirez, D.; Millán, B. Palmeras Usadas Por Los Indígenas Asháninkas En La Amazonía Peruana. Rev. Peru. Biol. 2010, 17, 347–352. [Google Scholar] [CrossRef] [Green Version]
- Jaramillo-Vivanco, T.; Balslev, H.; Montúfar, R.; Cámara, R.M.; Giampieri, F.; Battino, M.; Cámara, M.; Alvarez-Suarez, J.M. Three Amazonian Palms as Underestimated and Little-Known Sources of Nutrients, Bioactive Compounds and Edible Insects. Food Chem. 2022, 372, 31273. [Google Scholar] [CrossRef]
- Smith, N. Oenocarpus Mapora. In Palms and People in the Amazon; Smith, N., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 421–428. [Google Scholar]
- de Oliveira, M.S.P.D.O.; Moura, E.F. Repetibilidade e Número Mínimo de Medições Para Caracteres de Cacho de Bacabi (Oenocarpus Mapora). Rev. Bras. Frutic. 2010, 32, 1173–1179. [Google Scholar] [CrossRef] [Green Version]
- Domingues, A.F.N.; Carvalho, A.V.; de Barros, C.R. Caracterização Físico-Química Da Polpa de Bacabi (Oenocarpus Mapora H. Karsten). In Boletim de Pesquisa e Desenvolvimento; Embrapa: Brasília, Brazil, 2014. [Google Scholar]
- Neves, L.T.B.C.; Campos, D.C.D.S.; Mendes, J.K.S.; Urnhani, C.O.; de Araújo, K.G.M. Quality of Fruits Manually Processed of Açaí (Euterpe Oleracea MART.) and BACABA (Oenocarpus Bacaba MART.). Rev. Bras. Frutic. 2015, 37, 729–738. [Google Scholar] [CrossRef] [Green Version]
- Ocampo-Duran, Á.; Fernández-Lavado, A.P.; Castro-Lima, F. Aceite de La Palma de Seje Oenocarpus Bataua Mart. Por Su Calidad Nutricional Puede Contribuir a La Conservación y Uso Sostenible de Los Bosques de Galería En La Orinoquia Colombiana. Orinoquia 2013, 17, 215–229. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, A.M.; Casimiro-Gonzales, S.; Gómez-Coca, R.B.; Moreda, W.; Best, I.; Cajo-Pinche, M.I.; Loja, J.F.; Ibañez, E.; Cifuentes, A.; Ramos-Escudero, F. Comparison of Four Oil Extraction Methods for Sinami Fruit (Oenocarpus Mapora H. Karst): Evaluating Quality, Polyphenol Content and Antioxidant Activity. Foods 2022, 11, 1518. [Google Scholar] [CrossRef]
- Cardona, J.E.; Carrillo, M.P.; Mosquera, D.M.; Gutierrez, R.H.; Hernandez, M.S. EVALUACIÓN DE MÉTODOS DE EXTRACCIÓN DEL ACEITE DE MILPESILLOS (Oenocarpus Mapora). Vitae 2012, 19, 183–185. [Google Scholar]
- Ferrer Cutire, F.C.; Casimiro Gonzales, S.; Ramos Escudero, F.; Muñoz, A.M. VALORIZACIÓN DE TORTA RESIDUAL DE ACEITE DE SINAMI (Oenocarpus Mapora H. Karst) OBTENIDA DEL PRENSADO POR TORNILLO COMO UN RECURSO DE VALOR AGREGADO. Rev. De La Soc. Química Del Perú 2022, 88, 78–89. [Google Scholar] [CrossRef]
- Guzmán, C.; Rojas, M.A.; Aragón, M. Cosmetics Optimization of Ultrasound-Assisted Emulsification of Emollient Nanoemulsions of Seed Oil of Passiflora Edulis Var. Edulis. Cosmetics 2020, 8, 1. [Google Scholar] [CrossRef]
- Behalpade, S.; Gajbhiye, S. Review Article: Skin Care With Exfoliation Process. Int. J. Corrent Sci. 2022, 12, 372–379. [Google Scholar]
- Dehaven, C. Mechanisms of Exfoliation, Science of Skincare. Available online: https://www.isclinical.com/media/WhitePapers/pdf/WhitePaper_MechanismsOfExfoliation_Jan2015_1_.pdf (accessed on 9 February 2023).
- Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Melo, P.S.; Selani, M.M.; Gonçalves, R.H.; de Oliveira Paulino, J.; Massarioli, A.P.; de Alencar, S.M. Açaí Seeds: An Unexplored Agro-Industrial Residue as a Potential Source of Lipids, Fibers, and Antioxidant Phenolic Compounds. Ind. Crops Prod. 2021, 161, 113204. [Google Scholar] [CrossRef]
- Arrieta-Escobar, J.A.; Bernardo, F.P.; Orjuela, A.; Camargo, M.; Morel, L. Incorporation of Heuristic Knowledge in the Optimal Design of Formulated Products: Application to a Cosmetic Emulsion. Comput. Chem. Eng. 2019, 122, 265–274. [Google Scholar] [CrossRef]
- Azconia, L. Exfoliación. Farm. Prof. 2006, 20, 53–59. [Google Scholar]
- Sun, J.Z.; Parr, J.W. Formulating Scrubs. Cosmet. Toilet. Mag. 2003, 118, 35–40. [Google Scholar]
- Blaak, J.; Staib, P. The Relation of PH and Skin Cleansing. Curr. Probl. Dermatol. 2018, 54, 132–142. [Google Scholar] [CrossRef]
- Lubrizol. Quick Start Guide; Lubrizol: Wickliffe, OH, USA, 2012. [Google Scholar]
- Linares-Devia, N.; Arrieta-Escobar, J.; Baena, Y.; Orjuela, A.; Osorio, C. Development and Characterization of Emulsions Containing Ground Seeds of Passiflora Species as Biobased Exfoliating Agents. Cosmetics 2022, 9, 15. [Google Scholar] [CrossRef]
- Barboza, N.L.; Cruz, J.M.D.A.; Corrêa, R.F.; Lamarão, C.V.; Lima, A.R.; Inada, N.M.; Sanches, E.A.; de Araújo Bezerra, J.; Campelo, P.H. Buriti (Mauritia Flexuosa L. f.): An Amazonian Fruit with Potential Health Benefits. Food Res. Int. 2022, 159, 111654. [Google Scholar] [CrossRef]
- Piotrowska, A.; Czerwińska-Ledwig, O.; Serdiuk, M.; Serdiuk, K.; Pilch, W. Composition of Scrub-Type Cosmetics from the Perspective of Product Ecology and Microplastic Content. Toxicol. Environ. Health Sci. 2020, 12, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Montúfar, R.; Laffargue, A.; Pintaud, J.C.; Hamon, S.; Avallone, S.; Dussert, S. Oenocarpus Bataua Mart. (Arecaceae): Rediscovering a Source of High Oleic Vegetable Oil from Amazonia. J. Am. Oil Chem. Soc. 2010, 87, 167–172. [Google Scholar] [CrossRef]
- Da Cruz Rodrigues, A.M.; Darnet, S.; Da Silva, L.H.M. Fatty Acid Profiles and Tocopherol Contents of Buriti (Mauritia Flexuosa), Patawa (Oenocarpus Bataua), Tucuma (Astrocaryum Vulgare), Mari (Poraqueiba Paraensis) and Inaja (Maximiliana Maripa) Fruits. J. Braz. Chem. Soc. 2010, 21, 2000–2004. [Google Scholar] [CrossRef]
- de Cól, C.D.; Tischer, B.; Hickmann Flôres, S.; Rech, R. Foam-Mat Drying of Bacaba (Oenocarpus Bacaba): Process Characterization, Physicochemical Properties, and Antioxidant Activity. Food Bioprod. Process. 2021, 126, 23–31. [Google Scholar] [CrossRef]
- Vásquez-Ocmín, P.G.; Alvarado, L.F.; Solís, V.S.; Torresb, R.P.; Mancini-Filhob, J. Chemical Characterization and Oxidative Stability of the Oils from Three Morphotypes of Mauritia Flexuosa L.f, from the Peruvian Amazon. Grasas Y Aceites 2010, 61, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Surayah Osman, N.; Ayuna Mohamed Khamil, I.; Sapawe, N. Proximate Analysis of Animal Feed Pellet Formulated from Sunflower Shell Waste. Mater. Today Proc. 2019, 19, 1796–1802. [Google Scholar] [CrossRef]
- de Cássia Braga Arruda, J.; da Fonseca, L.A.B.; Pinto, L.C.P.; de Oliveira Pinheiro, H.C.; Monteiro, B.T.O.; Manno, M.C.; de Souza Lima, K.R.; de Lima, A.R. Açaí Seed Bran in the Feed of Slow-Growth Broilers. Acta Amazon. 2018, 48, 298–303. [Google Scholar] [CrossRef]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Dias-Audibert, F.L.; Delafiori, J.; de Souza, F.G.; Catharino, R.R.; do Sacramento, C.K.; Pastore, G.M. Chemical Characterization of Eugenia Stipitata: A Native Fruit from the Amazon Rich in Nutrients and Source of Bioactive Compounds. Food Res. Int. 2021, 139, 109904. [Google Scholar] [CrossRef]
- Martínez-Valverde, I.; Periago, M.J.; Ros, G. Significado Nutricional de Los Compuestos Fenólicos de La Dieta. Arch. Latinoam. Nutr. 2000, 50, 5–18. [Google Scholar]
- Gimeno, E. Compuestos Fenólicos. Un Análisis de Sus Beneficios Para La Salud. Offarm 2004, 23, 80–84. [Google Scholar]
- Granato, D.; Santos, J.S.; Maciel, L.G.; Nunes, D.S. Chemical Perspective and Criticism on Selected Analytical Methods Used to Estimate the Total Content of Phenolic Compounds in Food Matrices. TrAC Trends Anal. Chem. 2016, 80, 266–279. [Google Scholar] [CrossRef]
- Navarro-Valdez, K.; Capillo-Herrera, N.; Calixto-Cotos, M.R.; Santisteban-Rojas, O.P. Extraction and Microencapsulation of Antioxidant Compounds from Oenocarpus Bataua Mart Seed. Sci. Agropecu. 2020, 11, 547–554. [Google Scholar] [CrossRef]
- Soong, Y.Y.; Barlow, P.J. Antioxidant Activity and Phenolic Content of Selected Fruit Seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Contreras-Calderón, J.; Calderón-Jaimes, L.; Guerra-Hernández, E.; García-Villanova, B. Antioxidant Capacity, Phenolic Content and Vitamin C in Pulp, Peel and Seed from 24 Exotic Fruits from Colombia. Food Res. Int. 2011, 44, 2047–2053. [Google Scholar] [CrossRef]
- Corrêa, P.G.; Moura, L.G.S.; Amaral, A.C.F.; do Amaral Souza, F.D.C.; Aguiar, J.P.L.; Aleluia, R.L.; de Andrade Silva, J.R. Chemical and Nutritional Characterization of Ambelania Duckei (Apocynaceae) an Unexplored Fruit from the Amazon Region. Food Res. Int. 2023, 163, 112290. [Google Scholar] [CrossRef]
- Fidelis, M.; do Carmo, M.A.V.; da Cruz, T.M.; Azevedo, L.; Myoda, T.; Miranda Furtado, M.; Boscacci Marques, M.; Sant’Ana, A.S.; Inês Genovese, M.; Young Oh, W.; et al. Camu-Camu Seed (Myrciaria Dubia)–From Side Stream to an Antioxidant, Antihyperglycemic, Antiproliferative, Antimicrobial, Antihemolytic, Anti-Inflammatory, and Antihypertensive Ingredient. Food Chem. 2020, 310, 125909. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Methods to Measure the Antioxidant Activity of Phytochemicals and Plant Extracts. J. Agric. Food Chem. 2018, 66, 3324–3329. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, Natural Sources, Extraction and Analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Benoit, C.; Virginie, C.; Boris, V. The Use of NADES to Support Innovation in the Cosmetic Industry, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 97. [Google Scholar]
- Amrani, A.; Bouakline, H.; Elkabous, M.; Brahmi, M.; Karzazi, Y.; El Bachiri, A.; Tahani, A. Ceratonia Siliqua L Seeds Extract: Experimental Analysis and Simulation Study. Mater. Today Proc. 2022, 72, 3705–3711. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Carvalho-Silva, L.B.; Morales, J.P.; Malta, L.G.; Muramoto, M.T.; Ferreira, J.E.M.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Maróstica Junior, M.R.; Pastore, G.M. Evaluation of the Antioxidant, Antiproliferative and Antimutagenic Potential of Araçá-Boi Fruit (Eugenia Stipitata Mc Vaugh-Myrtaceae) of the Brazilian Amazon Forest. Food Res. Int. 2013, 50, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Netto, A.B. Tropical Fruits as Natural, Exceptionally Rich, Sources of Bioactive Compounds. Int. J. Fruit Sci. 2018, 18, 231–242. [Google Scholar] [CrossRef]
- Yamaguchi, K.K.L.; Lamarão, C.V.; Aranha, E.S.P.; Souza, R.O.S.; Oliveira, P.D.A.; Vasconcellos, M.C.; Lima, E.S.; Veiga-Junior, V.F. HPLC-DAD Profile of Phenolic Compounds, Cytotoxicity, Antioxidant and Anti-Inflammatory Activities of the Amazon Fruit Caryocar Villosum. Quim. Nova 2017, 40, 483–490. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic Compounds. In Bioactive Compounds: Health Benefits and Potential Applications; Woodhead Publishing: Sawston, UK, 2019; pp. 33–50. [Google Scholar]
- Neri-Numa, I.A.; de Carvalho-Silva, L.B.; Macedo Ferreira, J.E.; Tomazela Machado, A.R.; Malta, L.G.; Tasca Gois Ruiz, A.L.; de Carvalho, J.E.; Pastore, G.M. Preliminary Evaluation of Antioxidant, Antiproliferative and Antimutagenic Activities of Pitomba (Talisia Esculenta). LWT 2014, 59, 1233–1238. [Google Scholar] [CrossRef] [Green Version]
- da Fonseca Machado, A.P.; de Paula do Nascimento, R.; da Rocha Alves, M.; Reguengo, L.M.; Junior, M.R.M. Brazilian Tucumã-Do-Amazonas (Astrocaryum Aculeatum) and Tucumã-Do-Pará (Astrocaryum Vulgare) Fruits: Bioactive Composition, Health Benefits, and Technological Potential. Food Res. Int. 2022, 151, 110902. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.M.; Jan, N.; Wani, T.A.; Ahmad, M.; Masoodi, F.A.; Gani, A. Optimization of Antioxidant Activity and Total Polyphenols of Dried Apricot Fruit Extracts (Prunus Armeniaca L.) Using Response Surface Methodology. J. Saudi Soc. Agric. Sci. 2017, 16, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, O.D.; do Nascimento, W.M.O.; Cruz, F.J.R.; Gurgel, E.S.C. Seed Anatomy and Histochemistry of Myrciaria Dubia (Kunth) McVaugh, an Amazonian Myrtaceace. Flora 2021, 280, 151847. [Google Scholar] [CrossRef]
- Kelleppan, V.T.; Butler, C.S.G.; Williams, A.P.; Vidallon, M.L.P.; Giles, L.W.; King, J.P.; Sokolova, A.V.; de Campo, L.; Pearson, G.R.; Tabor, R.F.; et al. Components of Cocamidopropyl Betaine: Surface Activity and Self-Assembly of Pure Alkyl Amidopropyl Betaines. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130435. [Google Scholar] [CrossRef]
- Lochhead, R.Y. Basic Physical Sciences for the Formulation of Cosmetic Products; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Insuquimica ALKOPON CN (TEXAPON 70%)–OXITENO–URUGUAY–Galón x 4 Kg. Available online: https://tienda.insuquimica.com/producto/alkopon-cn-texapon-70-oxiteno-uruguay-galon-x-4-kg/ (accessed on 2 February 2023).
- Bährle-Rapp, M. Cocamidopropyl Betaine. In Springer Lexikon Kosmetik und Körperpflege; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2007; p. 611. [Google Scholar]
- Hunter, J.E.; Fowler, J.F. Safety to Human Skin of Cocamidopropyl Betaine: A Mild Surfactant for Personal-Care Products. J. Surfactants Deterg. 1998, 1, 235–239. [Google Scholar] [CrossRef]
- Venkataramani, D.; Tsulaia, A.; Amin, S. Fundamentals and Applications of Particle Stabilized Emulsions in Cosmetic Formulations. Adv. Colloid. Interface Sci. 2020, 283, 102234. [Google Scholar] [CrossRef]
- Zięba, M.; Małysa, A.; Klimaszewska, E.; Jagiełło, O.; Gruszczyńska, M.; Gajowiak, M. The Impact of Storage Temperature on the Quality of Liquid Bath Cosmetic Products. Stud. Oeconomica Posnaniensia 2017, 5, 59–72. [Google Scholar] [CrossRef]
- Goussard, V.; Duprat, F.; Ploix, J.L.; Dreyfus, G.; Nardello-Rataj, V.; Aubry, J.M. A New Machine-Learning Tool for Fast Estimation of Liquid Viscosity. Application to Cosmetic Oils. J. Chem. Inf. Model. 2020, 60, 2012–2023. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzaman, N.; Yusop, S.M. Determination of Stability of Cosmetic Formulations Incorporated with Water-Soluble Elastin Isolated from Poultry. J. King Saud. Univ. Sci. 2021, 33, 101519. [Google Scholar] [CrossRef]
- Some, I.T.; Bogaerts, P.; Hanus, R.; Hanocq, M.; Dubois, J. Improved Kinetic Parameter Estimation in PH-Profile Data Treatment. Int. J. Pharm. 2000, 198, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Berthele, H.; Sella, O.; Lavarde, M.; Mielcarek, C.; Pense-Lheritier, A.M.; Pirnay, S. Determination of the Influence of Factors (Ethanol, PH and a w) on the Preservation of Cosmetics Using Experimental Design. Int. J. Cosmet. Sci. 2014, 36, 54–61. [Google Scholar] [CrossRef]



| Functionality | Ingredient | INCI Name |
|---|---|---|
| Emulsion stabilizing and viscosity controlling | Carbopol ultrez 20 | Acrylates/C10–30 |
| Surfactant—emulsifying | Alkopon | Sodium laureth sulfate |
| Surfactant—emollient | Cocamidopropyl | Cocamidopropyl betaine |
| Surfactant—emulsifying | Lathanol powder | Sodium lauryl sulfoacetate |
| Surfactant—emulsifying | Hostapon sci 85 p | Sodium cocoyl glycinate |
| Humectant and solvent | Glycerin | Glycerin |
| Preservative | Procide cg | Methylchloroisothiazolinone |
| Viscosity controlling | Table salt | Sodium chloride |
| Preservative | Poliquaternium-7 | Poliquaternium-7 |
| pH control | Triethanolamine | triethanolamine |
| Phase | N° | Ingredient | INCI Name | Functionality | % |
|---|---|---|---|---|---|
| A | 001 | Aqua | Aqua | Driver | 81.97 |
| 002 | Carbopol Ultrez 20 | Acrylates/C10–30 | Viscosity controlling | 1.00 | |
| B | 003 | Alkopon | Sodium laureth sulfate | Surfactant—emulsifying | 7.00 |
| 004 | Cocamidopropyl Betaina | Cocamidopropyl Betaina | Surfactant—emollient | 1.50 | |
| C | 005 | Glycerin | Glycerin | Humectant and solvent | 2.00 |
| 006 | Prodice CG | Methylchloroisothiazolinone | Preservative | 0.10 | |
| 007 | Vanilla essence | - | Masking agent | 0.30 | |
| D | 008 | Red dye | CI 75470- | Cosmetic colorant | 0.18 |
| 009 | Yellow dye | CI 19140 | Cosmetic colorant | 0.12 | |
| 010 | Brilliant Blue dye | CI 42090- | Cosmetic colorant | 0.03 | |
| 011 | Triethanolamine | Triethanolamine | pH control | 0.80 | |
| 012 | Sinami seed powder | - | Abrasive agent | 5.00 |
| Parameter 1 | Oenocarpus mapora | Euterpe oleracea (Reference) |
|---|---|---|
| Moisture (%) 3 | 25.1 ± 0.19 | 7.91 ± 0.01 |
| Lipids (%) 3 | 0.44 ± 0.03 | 2.75 ± 0.01 |
| Crude protein (N × 5.3) (%) 3 | 3.43 ± 0.05 | 4.89 ± 0.03 |
| Ash (%) 2,3 | 1.01 ± 0.01 | 1.36 ± 0.01 |
| Total fiber (%) 3 | 9.94 ± 0.46 | - |
| Sample | TPC (mg/g) | DPPH IC50 (mg/mL) | ABTS IC50 (mg/mL) |
|---|---|---|---|
| Oenocarpus mapora | 12.3 ± 0.25 | 0.34 ± 0.001 | 0.10 ± 0.0002 |
| Ingredients/Parameters | Prototype 1 | Prototype 2 | Prototype 3 | Prototype 4 |
|---|---|---|---|---|
| Water | 86.80 | 66.25 | 76.70 | 81.97 |
| Acrylates/C10–30 | 0.40 | 0.45 | 1.00 | 1.00 |
| Sodium cocoyl glycinate | 4.00 | 4.00 | - | - |
| Sodium laureth sulfate | - | 5.00 | 5.00 | 7.00 |
| Sodium lauryl sulfoacetate | - | 5.00 | 2.50 | - |
| Cocamidopropyl betaine | - | - | - | 1.50 |
| Glycerin | 2.00 | 2.00 | 2.00 | 2.00 |
| Methylchloroisothiazolinone | 0.10 | 0.10 | 0.10 | 0.10 |
| Triethanolamine | 0.40 | 0.40 | 0.80 | 0.80 |
| Sinami seed powder | 5.00 | 7.00 | 10.00 | 5.00 |
| Color visualization | White/milky | White/milky | White/milky | Transparent |
| pH value | 4.55 | 6.29 | 6.48 | 5.25 |
| Viscosity (mPa.s) | 890 | 920 | 1240 | 1050 |
| Sample | Common Name | TPC (mg GAE/g) | Reference |
|---|---|---|---|
| Oenocarpus mapora | Sinami | 12.3 ± 0.25 | - |
| Oenocarpus bataua | Ungurahui | 452.8 | [37] |
| Euterpe oleracea Mart. | Açaí | 64.6 | [18] |
| Artocarpus heterophyllus | Jackfruit | 27.7 | [38] |
| Mauritia flexuosa | Aguaje | 65.5 | [39] |
| Ambelania duckei | - | 374.9 | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Orejon, F.L.; Huaman, J.; Lozada, P.; Ramos-Escudero, F.; Muñoz, A.M. Development and Functionality of Sinami (Oenocarpus mapora) Seed Powder as a Biobased Ingredient for the Production of Cosmetic Products. Cosmetics 2023, 10, 90. https://doi.org/10.3390/cosmetics10030090
Romero-Orejon FL, Huaman J, Lozada P, Ramos-Escudero F, Muñoz AM. Development and Functionality of Sinami (Oenocarpus mapora) Seed Powder as a Biobased Ingredient for the Production of Cosmetic Products. Cosmetics. 2023; 10(3):90. https://doi.org/10.3390/cosmetics10030090
Chicago/Turabian StyleRomero-Orejon, Frank L., Jorge Huaman, Patricia Lozada, Fernando Ramos-Escudero, and Ana María Muñoz. 2023. "Development and Functionality of Sinami (Oenocarpus mapora) Seed Powder as a Biobased Ingredient for the Production of Cosmetic Products" Cosmetics 10, no. 3: 90. https://doi.org/10.3390/cosmetics10030090
APA StyleRomero-Orejon, F. L., Huaman, J., Lozada, P., Ramos-Escudero, F., & Muñoz, A. M. (2023). Development and Functionality of Sinami (Oenocarpus mapora) Seed Powder as a Biobased Ingredient for the Production of Cosmetic Products. Cosmetics, 10(3), 90. https://doi.org/10.3390/cosmetics10030090








