Exploring Stearic-Acid-Based Nanoparticles for Skin Applications—Focusing on Stability and Cosmetic Benefits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Lipid Nanoparticles
2.3. Characterization of Lipid Nanoparticles
2.4. Stability Studies
2.4.1. Accelerated Stability
2.4.2. Long-Term Stability
2.5. In Vivo Tests
2.5.1. Skin Compatibility
2.5.2. Efficacy Assessment
2.6. Statistical Analysis
3. Results
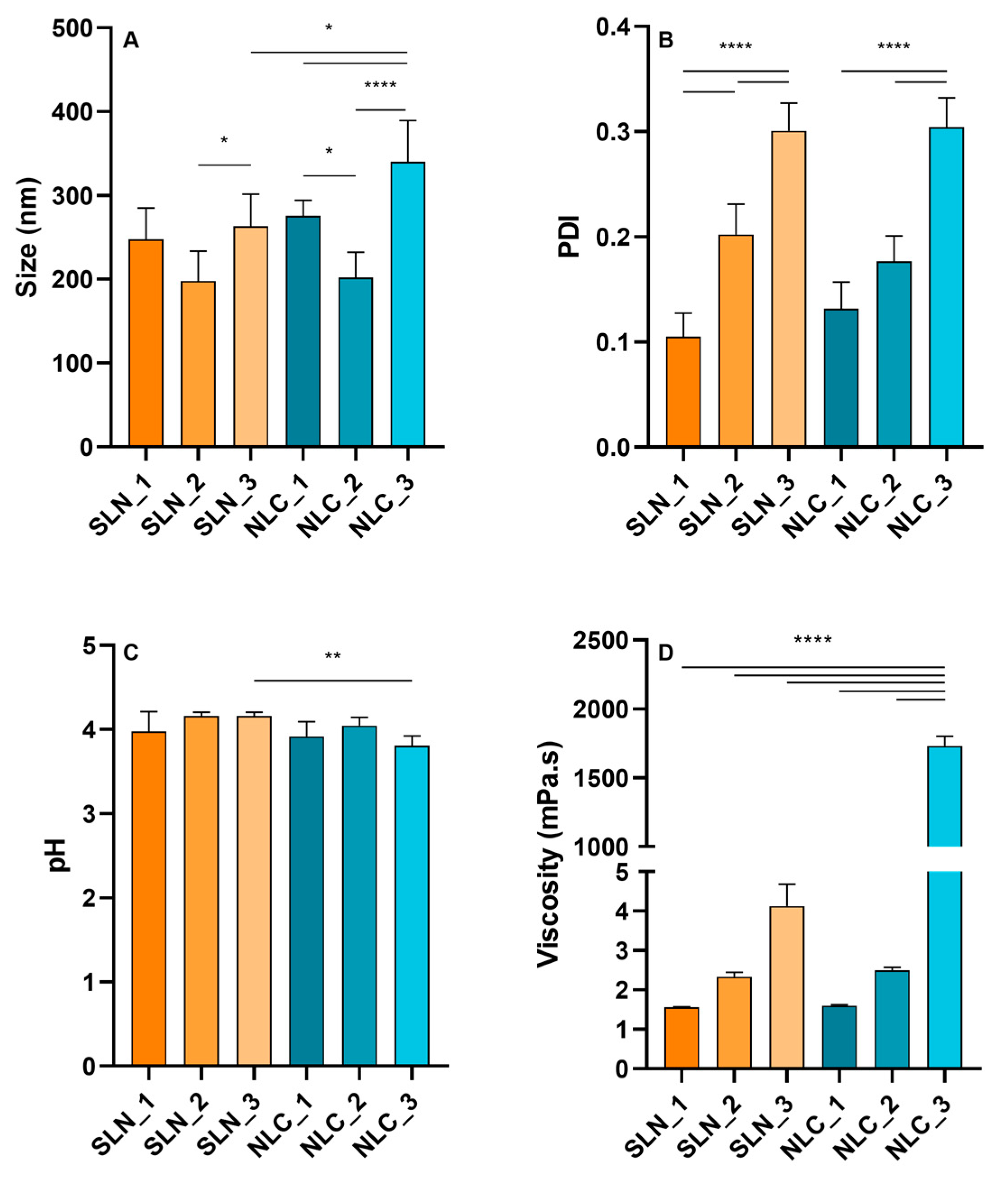
3.1. Characterization of Lipid Nanoparticles
3.2. Stability Studies
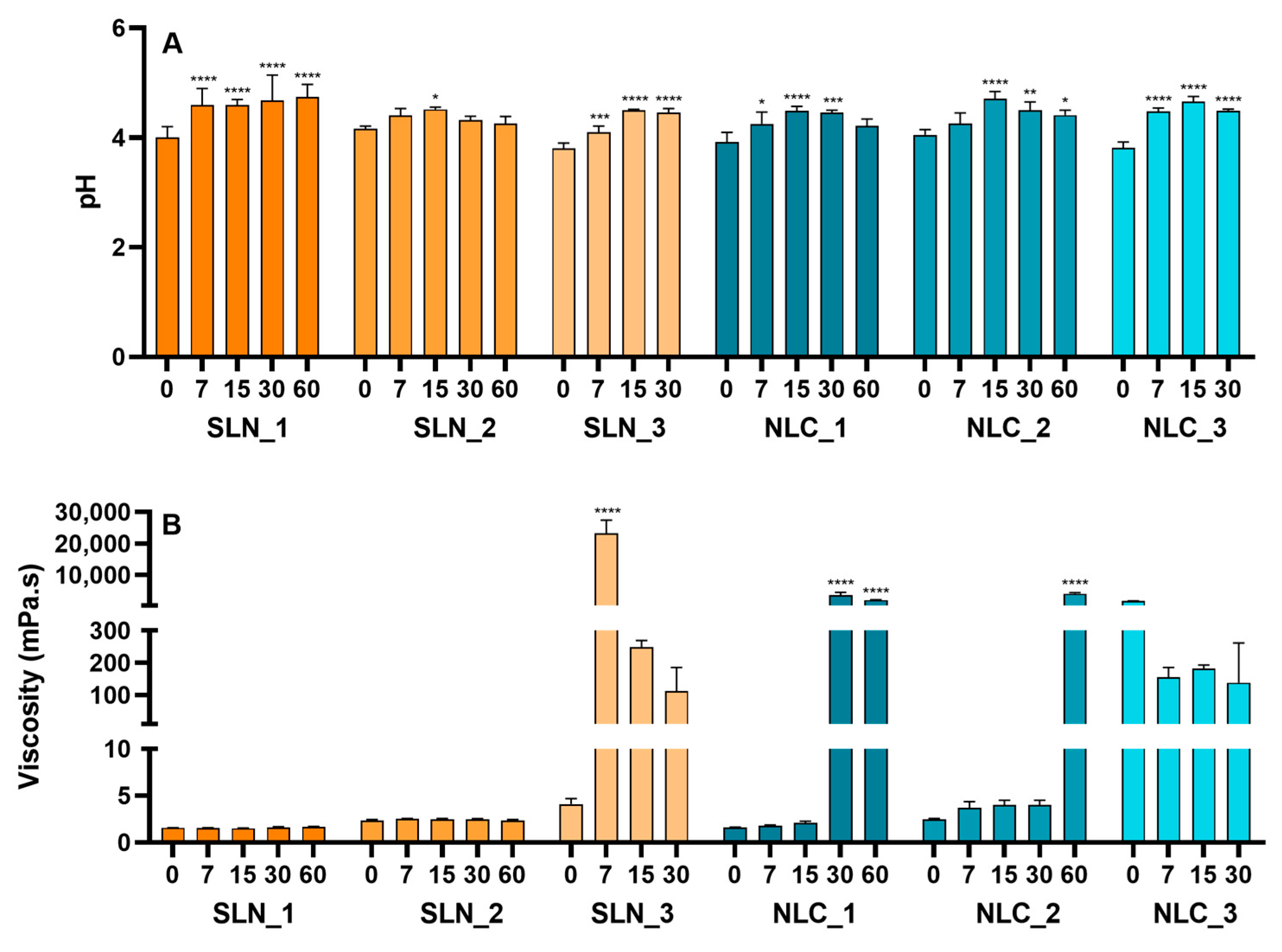
3.2.1. Accelerated Stability
3.2.2. Long-Term Stability
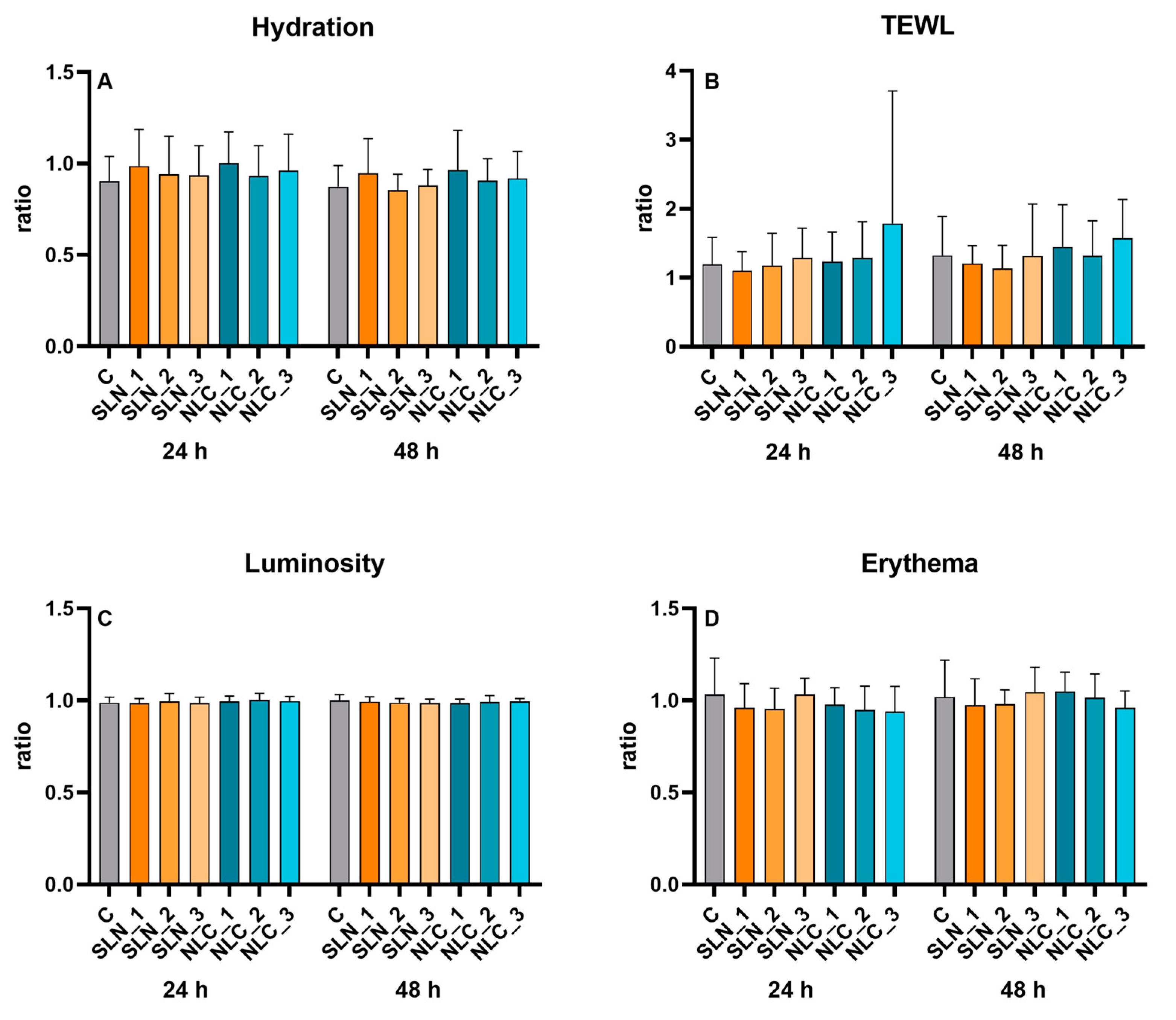
3.3. In Vivo Tests
3.3.1. Skin Compatibility
3.3.2. Skin Hydration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaboriau, H.P.; Murakami, C.S. Skin Anatomy and Flap Physiology. Otolaryngol. Clin. N. Am. 2001, 34, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Honari, G.; Andersen, R.; Maibach, H.L. Sensitive Skin Syndrome, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Nicol, N.H. Anatomy and Physiology of the Skin. Dermatol. Nurs. Dermatol. Nurses’ Assoc. 2005, 17, 62. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, K.P.; Mukherjee, S.; Chandar, P. Stratum Corneum Fatty Acids: Their Critical Role in Preserving Barrier Integrity during Cleansing. Int. J. Cosmet. Sci. 2013, 35, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Van Smeden, J.; Bouwstra, J.A. Stratum Corneum Lipids: Their Role for the Skin Barrier Function in Healthy Subjects and Atopic Dermatitis Patients. In Skin Barrier Function; Agner, T., Ed.; S. Karger AG: Basel, Switzerland, 2016; Volume 49, pp. 8–26. [Google Scholar]
- Sahle, F.F.; Gebre-Mariam, T.; Dobner, B.; Wohlrab, J.; Neubert, R.H.H. Skin Diseases Associated with the Depletion of Stratum Corneum Lipids and Stratum Corneum Lipid Substitution Therapy. Skin. Pharmacol. Physiol. 2015, 28, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.R. The Stratum Corneum: Structure and Function in Health and Disease. Dermatol. Ther. 2004, 17, 6–15. [Google Scholar] [CrossRef]
- Schmuth, M.; Blunder, S.; Dubrac, S.; Gruber, R.; Moosbrugger-Martinz, V. Epidermal Barrier in Hereditary Ichthyoses, Atopic Dermatitis, and Psoriasis. JDDG-J. Ger. Soc. Dermatol. 2015, 13, 1119–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obeidat, W.M.; Schwabe, K.; Müller, R.H.; Keck, C.M. Preservation of Nanostructured Lipid Carriers (NLC). Eur. J. Pharm. Biopharm. 2010, 76, 56–67. [Google Scholar] [CrossRef]
- Souto, E.B.; Müller, R.H. Cosmetic Features and Applications of Lipid Nanoparticles (SLN®, NLC®). Int. J. Cosmet. Sci. 2008, 30, 157–165. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Novel Drug Delivery Systems: Applications, Advantages and Disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef]
- Guimarães, K.L.; Ré, M.I. Lipid Nanoparticles as Carriers for Cosmetic Ingredients: The First (SLN) and the Second Generation (NLC). In Nanocosmetics and Nanomedicines: New Approaches for Skin Care; Springer: Berlin/Heidelberg, Germany, 2011; pp. 101–122. [Google Scholar] [CrossRef]
- Dey, S.; Datta, S.; Dasgupta, S.; Mazumder, B.; Pathak, Y.V. Lipid Nanoparticles for Topical Application of Drugs for Skin Diseases; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780323428910. [Google Scholar]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 319. [Google Scholar] [CrossRef]
- Dubey, S.K.; Dey, A.; Singhvi, G.; Pandey, M.M.; Singh, V.; Kesharwani, P. Emerging Trends of Nanotechnology in Advanced Cosmetics. Colloids Surf. B Biointerfaces 2022, 214, 112440. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.S.; Barradas, T.N.; Tavares, G.D. Advances in Nanotechnology-based Hair Care Products Applied to Hair Shaft and Hair Scalp Disorders. Int. J. Cosmet. Sci. 2022, 44, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Moddaresi, M.; Tamburic, S.; Williams, S.; Jones, S.A.; Zhao, Y.; Brown, M.B. Effects of Lipid Nanocarriers on the Performance of Topical Vehicles in Vivo. J. Cosmet. Dermatol. 2009, 8, 136–143. [Google Scholar] [CrossRef]
- Akombaetwa, N.; Ilangala, A.B.; Thom, L.; Memvanga, P.B.; Witika, B.A.; Buya, A.B. Current Advances in Lipid Nanosystems Intended for Topical and Transdermal Drug Delivery Applications. Pharmaceutics 2023, 15, 656. [Google Scholar] [CrossRef]
- van Smeden, J.; Boiten, W.A.; Hankemeier, T.; Rissmann, R.; Bouwstra, J.A.; Vreeken, R.J. Combined LC/MS-Platform for Analysis of All Major Stratum Corneum Lipids, and the Profiling of Skin Substitutes. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2014, 1841, 70–79. [Google Scholar] [CrossRef]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and Biopharmaceutical Aspects Influencing Skin Permeation and Role of SLN and NLC for Skin Drug Delivery. Heliyon 2022, 8, e08938. [Google Scholar] [CrossRef]
- Severino, P.; Pinho, S.C.; Souto, E.B.; Santana, M.H.A. Polymorphism, Crystallinity and Hydrophilic–Lipophilic Balance of Stearic Acid and Stearic Acid–Capric/Caprylic Triglyceride Matrices for Production of Stable Nanoparticles. Colloids Surf. B Biointerfaces 2011, 86, 125–130. [Google Scholar] [CrossRef]
- Espinosa-Olivares, M.A.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; Trejo-Márquez, M.A.; Pascual-Bustamante, S.; Ganem-Rondero, A. Nanostructured Lipid Carriers Loaded with Curcuminoids: Physicochemical Characterization, in Vitro Release, Ex Vivo Skin Penetration, Stability and Antioxidant Activity. Eur. J. Pharm. Sci. 2020, 155, 105533. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.V.; Menon, M.D.; Palshetkar, A.D.; Desai, N.D. Topical Delivery of Mupirocin Calcium Nanostructured Lipid Carriers Using a Full-Thickness Excision Wound Healing Model. J. Wound Care 2023, 32, lxiii–lxxiv. [Google Scholar] [CrossRef] [PubMed]
- Kasongo, W.A.; Pardeike, J.; Müller, R.H.; Walker, R.B. Selection and Characterization of Suitable Lipid Excipients for Use in the Manufacture of Didanosine-Loaded Solid Lipid Nanoparticles and Nanostructured Lipid Carriers. J. Pharm. Sci. 2011, 100, 5185–5196. [Google Scholar] [CrossRef] [PubMed]
- Wagemaker, T.A.L.; Rijo, P.; Rodrigues, L.M.; Maia Campos, P.M.B.G.; Fernandes, A.S.; Rosado, C. Integrated Approach in the Assessment of Skin Compatibility of Cosmetic Formulations with Green Coffee Oil. Int. J. Cosmet. Sci. 2015, 37, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.M.F. Preparação e Caracterização de Nanotransportadores (Nanocápsulas, Nanoesferas, Lipossomas e Transportadores Lipídicos Nanoestruturados) sem Substância Ativa; Escola Superior de Tecnologia e Gestão, Instituto Politécnico de Bragança: Bragança, Portugal, 2013. [Google Scholar]
- Han, F.; Li, S.; Yin, R.; Liu, H.; Xu, L. Effect of Surfactants on the Formation and Characterization of a New Type of Colloidal Drug Delivery System: Nanostructured Lipid Carriers. Colloids Surf. A 2008, 315, 210–216. [Google Scholar] [CrossRef]
- Schäfer-Korting, M.; Mehnert, W.; Korting, H.C. Lipid Nanoparticles for Improved Topical Application of Drugs for Skin Diseases. Adv. Drug Deliv. Rev. 2007, 59, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, B.; Siddik, Z.H.; Nagoor, N.H. Optimization of Nanostructured Lipid Carriers: Understanding the Types, Designs, and Parameters in the Process of Formulations. J. Nanopart. Res. 2020, 22, 141. [Google Scholar] [CrossRef]
- De Caro, V.; Giannola, L.I.; Di Prima, G. Solid and Semisolid Innovative Formulations Containing Miconazole-Loaded Solid Lipid Microparticles to Promote Drug Entrapment into the Buccal Mucosa. Pharmaceutics 2021, 13, 1361. [Google Scholar] [CrossRef] [PubMed]
- Mura, P.; Maestrelli, F.; D’Ambrosio, M.; Luceri, C.; Cirri, M. Evaluation and Comparison of Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) as Vectors to Develop Hydrochlorothiazide Effective and Safe Pediatric Oral Liquid Formulations. Pharmaceutics 2021, 13, 437. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for Topical, Dermal, and Transdermal Drug Delivery. Expert. Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef]
- Souto, E.B.; Almeida, A.J.; Müller, R.H. Lipid Nanoparticles (SLN®, NLC®) for Cutaneous Drug Delivery: Structure, Protection and Skin Effects. J. Biomed. Nanotechnol. 2007, 3, 317–331. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, F.; Liu, W.; Wan, J.; Wan, C.; Xu, H.; Lam, C.W.; Yang, X. Nanostructured Lipid Carriers as a Novel Oral Delivery System for Triptolide: Induced Changes in Pharmacokinetics Profile Associated with Reduced Toxicity in Male Rats. Int. J. Nanomed. 2014, 9, 1049–1063. [Google Scholar] [CrossRef] [Green Version]
- Iriventi, P.; Gupta, N.V. Topical Delivery of Curcumin and Caffeine Mixture-Loaded Nanostructured Lipid Carriers for Effective Treatment of Psoriasis. Pharmacogn. Mag. 2020, 16, 206–217. [Google Scholar] [CrossRef]
- Tichota, D.M.; Silva, A.C.; Sousa Lobo, J.M.; Amaral, M.H. Design, Characterization, and Clinical Evaluation of Argan Oil Nanostructured Lipid Carriers to Improve Skin Hydration. Int. J. Nanomed. 2014, 9, 3855–3864. [Google Scholar] [CrossRef] [Green Version]
- Savić, V.; Ilić, T.; Nikolić, I.; Marković, B.; Čalija, B.; Cekić, N.; Savić, S. Tacrolimus-Loaded Lecithin-Based Nanostructured Lipid Carrier and Nanoemulsion with Propylene Glycol Monocaprylate as a Liquid Lipid: Formulation Characterization and Assessment of Dermal Delivery Compared to Referent Ointment. Int. J. Pharm. 2019, 569, 118624. [Google Scholar] [CrossRef] [PubMed]
- Jannin, V.; Pochard, E.; Chambin, O. Influence of Poloxamers on the Dissolution Performance and Stability of Controlled-Release Formulations Containing Precirol® ATO 5. Int. J. Pharm. 2006, 309, 6–15. [Google Scholar] [CrossRef]
- Butani, D.; Yewale, C.; Misra, A. Topical Amphotericin B Solid Lipid Nanoparticles: Design and Development. Colloids Surf. B Biointerfaces 2016, 139, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, G.J.; Dufour, E.K. Nano-Sized Cosmetic Formulations or Solid Nanoparticles in Sunscreens: A Risk to Human Health? Arch. Toxicol. 2012, 86, 1063–1075. [Google Scholar] [CrossRef]
- Eiras, F.; Amaral, M.H.; Silva, R.; Martins, E.; Lobo, J.M.S.; Silva, A.C. Characterization and Biocompatibility Evaluation of Cutaneous Formulations Containing Lipid Nanoparticles. Int. J. Pharm. 2017, 519, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.H.; Basri, M.; Ismail, R.; Lau, H.L.N.; Tejo, B.A.; Kanthimathi, M.S.; Hassan, H.A.; Choo, Y.M. Effect of Compositions in Nanostructured Lipid Carriers (NLC) on Skin Hydration and Occlusion. Int. J. Nanomed. 2013, 8, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Purnamawati, S.; Indrastuti, N.; Danarti, R.; Saefudin, T. The Role of Moisturizers in Addressing Various Kinds of Dermatitis: A Review. Clin. Med. Res. 2017, 15, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Sarhadi, S.; Gholizadeh, M.; Moghadasian, T.; Golmohammadzadeh, S. Moisturizing Effects of Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) Using Deionized and Magnetized Water by In Vivo and In Vitro Methods. Iran. J. Basic. Med. Sci. 2020, 23, 337–343. [Google Scholar] [CrossRef]
- Jeon, H.S.; Seo, J.E.; Kim, M.S.; Kang, M.H.; Oh, D.H.; Jeon, S.O.; Jeong, S.H.; Choi, Y.W.; Lee, S. A Retinyl Palmitate-Loaded Solid Lipid Nanoparticle System: Effect of Surface Modification with Dicetyl Phosphate on Skin Permeation In Vitro and Anti-Wrinkle Effect In Vivo. Int. J. Pharm. 2013, 452, 311–320. [Google Scholar] [CrossRef]
- El-Housiny, S.; Shams Eldeen, M.A.; El-Attar, Y.A.; Salem, H.A.; Attia, D.; Bendas, E.R.; El-Nabarawi, M.A. Fluconazole-Loaded Solid Lipid Nanoparticles Topical Gel for Treatment of Pityriasis Versicolor: Formulation and Clinical Study. Drug Deliv. 2018, 25, 78–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Formulations | Stearic Acid (% w/v) | Precirol® ATO 5 (% w/v) | Capryol® 90 (% w/v) | Tween® 80 (% w/v) |
|---|---|---|---|---|
| SLN_1 | 5 | - | - | 2 |
| SLN_2 | - | 5 | - | 2 |
| SLN_3 | 2.5 | 2.5 | - | 2 |
| NLC_1 | 4.17 | - | 0.83 | 2 |
| NLC_2 | - | 4.17 | 0.83 | 2 |
| NLC_3 | 2.08 | 2.08 | 0.83 | 2 |
| Formulation | Before Gradual Heating | After Gradual Heating | ||
|---|---|---|---|---|
| pH | Viscosity (mPa.s) | pH | Viscosity (mPa.s) | |
| SLN_1 | 4.11 ± 0.07 | 1.56 ± 0.02 | 4.3 ± 0.3 | 1.56 ± 0.02 |
| SLN_2 | 4.20 ± 0.02 | 2.3 ± 0.1 | 4.33 ± 0.04 | 2.5 ± 0.1 |
| SLN_3 | 3.85 ± 0.08 | 6 ± 3 | 3.6 ± 0.1 * | 3.1 ± 0.3 |
| NLC_1 | 4.07 ± 0.02 | 1.60 ± 0.03 | 4.2 ± 0.1 | 2.05 ± 0.02 |
| NLC_2 | 4.11 ± 0.06 | 2.49 ± 0.08 | 4.3 ± 0.1 | 3.9 ± 0.7 |
| NLC_3 | 3.7 ± 0.1 | 1730 ± 70 | 3.99 ± 0.06 | 619 ± 269 **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira-Leite, C.; Bom, M.; Ribeiro, A.; Almeida, C.; Rosado, C. Exploring Stearic-Acid-Based Nanoparticles for Skin Applications—Focusing on Stability and Cosmetic Benefits. Cosmetics 2023, 10, 99. https://doi.org/10.3390/cosmetics10040099
Pereira-Leite C, Bom M, Ribeiro A, Almeida C, Rosado C. Exploring Stearic-Acid-Based Nanoparticles for Skin Applications—Focusing on Stability and Cosmetic Benefits. Cosmetics. 2023; 10(4):99. https://doi.org/10.3390/cosmetics10040099
Chicago/Turabian StylePereira-Leite, Catarina, Mariana Bom, Andria Ribeiro, Cíntia Almeida, and Catarina Rosado. 2023. "Exploring Stearic-Acid-Based Nanoparticles for Skin Applications—Focusing on Stability and Cosmetic Benefits" Cosmetics 10, no. 4: 99. https://doi.org/10.3390/cosmetics10040099
APA StylePereira-Leite, C., Bom, M., Ribeiro, A., Almeida, C., & Rosado, C. (2023). Exploring Stearic-Acid-Based Nanoparticles for Skin Applications—Focusing on Stability and Cosmetic Benefits. Cosmetics, 10(4), 99. https://doi.org/10.3390/cosmetics10040099










