Abstract
Laser-assisted drug delivery (LADD) has gained prominence as a promising technique with the potential to enhance topical drug skin penetration and absorption. However, the effectiveness of various laser-assisted facial procedures remains unclear. This systematic review aimed to explore and summarize the evidence regarding the indications, clinical outcomes, and adverse effects of LADD performed on the face. Based on a predetermined protocol, an electronic search in MEDLINE, Scopus, the Cochrane Library, and CENTRAL electronic databases was conducted. Eligible studies comprised prospective controlled trials that explored the utility of laser-assisted techniques for topical medication facial application and reported on efficacy and/or safety. Secondary outcomes encompassed patient satisfaction. This review included 12 prospective controlled studies involving a total of 271 participants. LADD, through various laser types, topical medications, clinical protocols, and follow-up assessments, showed enhanced melasma, facial rejuvenation, scar, and periprocedural laser outcomes without increased risk of adverse effects. This review provides evidence that LADD is an effective and safe adjunct for various facial procedures. It also highlights the necessity for further high-quality studies with larger sample sizes, standardized treatment protocols, and evaluation of long-term outcomes and adverse effects in order to elucidate the potential of laser-assisted drug delivery.
1. Introduction
Laser-assisted drug delivery (LADD), the topical delivery of medications by means of a laser, is a cutting-edge technique that holds great promise in enhancing the effectiveness of drug delivery. Its application in medical and aesthetic fields is steadily increasing, owing to the advancement in laser technology, medical knowledge in terms of pathophysiology, and its reported clinical outcomes, which show a potential for improved therapeutic outcomes [1].
It is well known that the ability of a drug to penetrate the different layers of the skin can be enormously challenging. The skin layers, particularly the stratum corneum, act as a sturdy physical barrier to the environment, and its threatening factors, including topical medications, only barely penetrate the skin. Indeed, the stratum corneum is the major rate-limiting layer for drug absorption, with topical drugs having a bioavailability of only 1% to 5% [2]. As a result, various methods have been fabricated over the years to facilitate the absorption of medications applied to the skin by overcoming the skin barrier obstacle. Chemical manipulation and physical energy-based methods have been utilized to enhance drug absorption [3]. More recently, there has been a shift towards physical modulation techniques, such as electroporation, iontophoresis, lasers, microdermabrasion, microneedling, pressure, radiofrequency, and sonophoresis [4,5]. Of these methods, laser-assisted drug permeation has gained significant attention and is the focus of ongoing research and development efforts.
The importance of further investigating LADD lies in its potential to revolutionize drug delivery systems, making treatments more efficient and patient-friendly. Despite the potential benefits, the procedure is not without its challenges, and the risk of complications remains a concern. Thus, the aim of this paper was to conduct a comprehensive systematic review in order to summarize and critically evaluate the available evidence, identify current trends, and elucidate the efficacy and safety of laser-assisted drug delivery.
2. Materials and Methods
A systematic review was conducted using a predetermined protocol established according to the Cochrane Handbook’s recommendations [6]. The review adhered to the updated PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Supplementary Material, Table S1) [7]. The review protocol was registered at PROSPERO (registration no. CRD42023441751).
2.1. Search Strategy
An electronic literature search in MEDLINE (PubMed), Scopus, the Cochrane Library, and CENTRAL electronic databases was conducted from inception to June 2023. The string search “laser-assisted” and “face” was applied. No time and language restrictions were applied. This search was supplemented by a review of reference lists of potentially eligible studies and a manual search of key journals in the field of facial surgery.
2.2. Eligibility of Relevant Studies
The population of interest was adult patients undergoing laser-assisted drug delivery for non-oncological facial procedures. Studies met the following inclusion criteria: (1) prospective controlled studies of a laser-assisted drug delivery group, as part of a facial procedure, compared to a control group (either laser or topical medication application); (2) reported data on clinical outcomes and/or complications; and (3) publication in a peer-reviewed journal. We excluded studies reporting on LADD application in areas other than the face, oncological application of LADD, studies reporting on non-concomitant use of a laser with a topical agent, review articles, duplicate reports, editorials, and correspondences.
2.3. Study Selection
Two reviewers (K.S. and K.P.) independently screened retrieved database files and the full text of potentially eligible studies for relevance. Disagreement was resolved by consensus.
2.4. Data Collection and Risk of Bias Assessment
Data extraction was conducted independently by the 2 reviewers using a standardized form. Discrepancies were resolved by consensus. We extracted data, including the general study characteristics, patient demographics, LADD modalities and facial procedures applied, and outcomes of interest. The primary outcome was the efficacy of LADD in terms of measured clinical outcomes of the facial procedure performed and safety in terms of complications related to the LADD and the facial procedure performed. Secondary outcomes included patient satisfaction rates.
The quality of studies was assessed using the Cochrane risk of bias tool [6].
2.5. Data Synthesis and Analysis
We provide a narrative summary of the included studies based on the indication and the publication date. The clinical outcomes presented were further categorized, according to the follow-up time, to direct (post-treatment), early (1–4 weeks), intermediate (1–3 months), and long-term outcomes (≥6 months).
3. Results
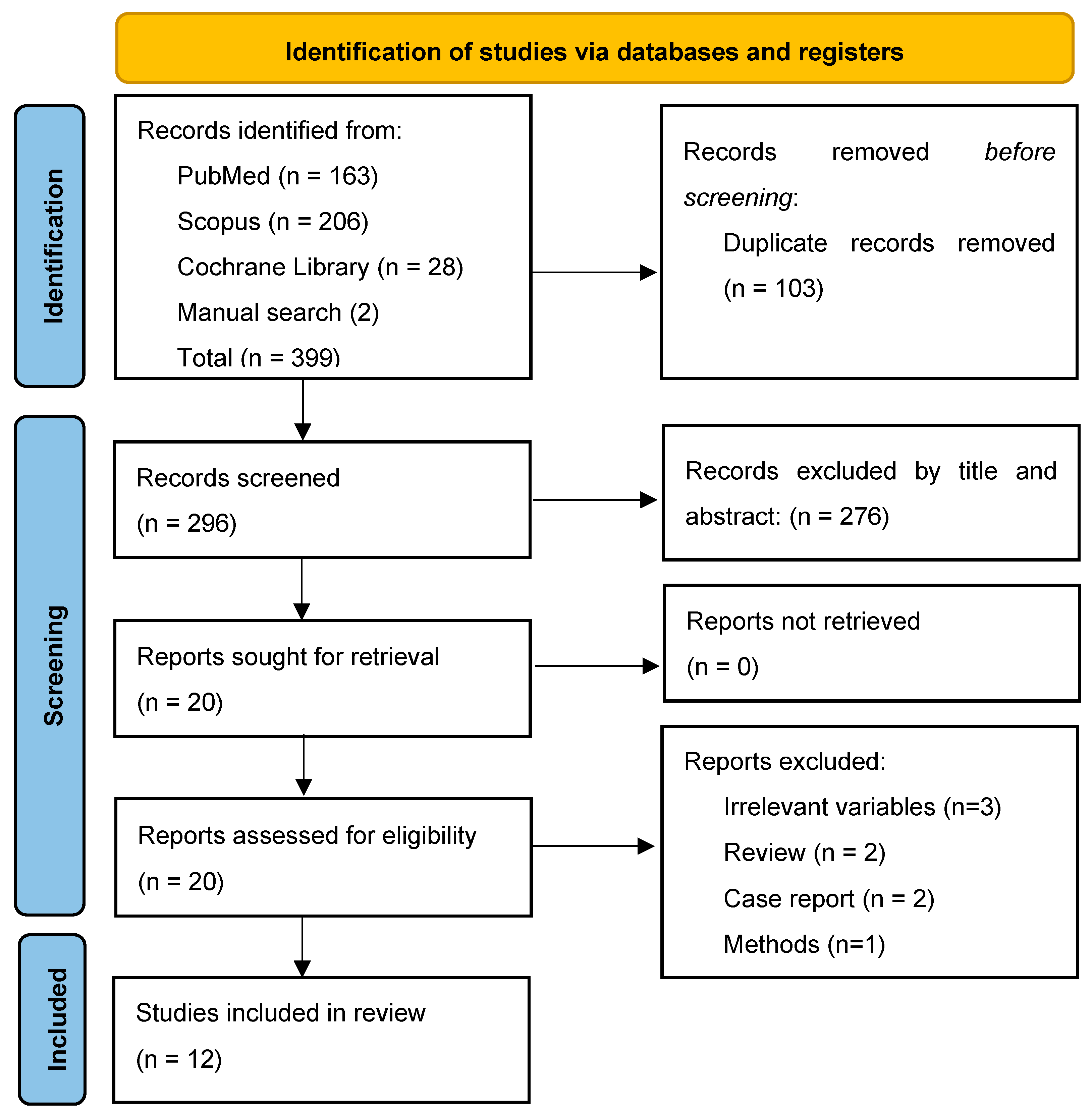
The study selection process is summarized in Figure 1. From a total of 399 records, 12 studies were eligible and included in the data analysis [8,9,10,11,12,13,14,15,16,17,18,19].
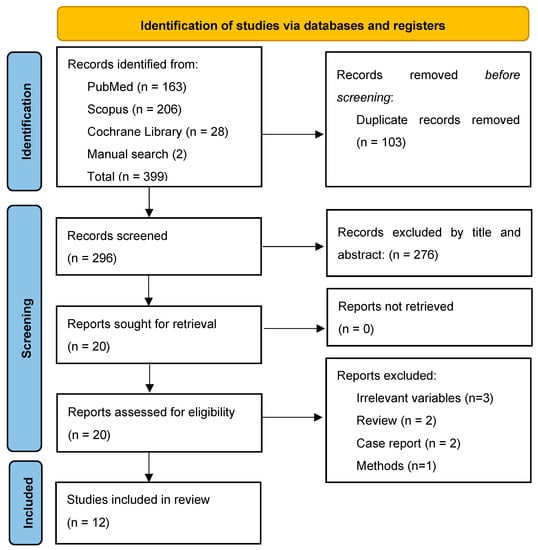
Figure 1.
PRISMA Flow Chart.
3.1. General Study Characteristics
The 12 studies included were conducted in the USA (4), Egypt (2), Indonesia (1), Thailand (1), France (1), China (1), Iraq (1), and South Korea (1). All studies but one were published after 2014. Study characteristics are summarized in Table 1. Among the twelve studies, ten were classified as randomized controlled trials (RCTs), while the remaining two were prospective studies (PSs). All studies but one were designed as intraindividual split-face trials, assessing the efficacy and safety of LADD in different time frames, ranging from one week to six months.

Table 1.
Study characteristics.
The indications for laser-assisted drug delivery were diverse, with five studies investigating the efficacy of LADD for melasma treatment, five exploring its impact on facial rejuvenation and wrinkle reduction by different applied modalities, while the remaining three studies assessing its effectiveness in post-acne scar treatment, post-laser recovery, and pre-laser local anesthesia.
The risk of bias was considered low, based on the quality of the studies (Supplementary Material, Tables S2 and S3).
3.2. Patient Characteristics
The systematic review included 271 patients; more than 94% (n = 254) were females. The age of the study samples varied, depending on the indication for the LADD application. Most participants were between 35 and 55 years old and exhibited a range of Fitzpatrick skin phototypes from I to V (mostly II to IV).
Certain exclusion criteria were applied in the studies included in this review to ensure that the study population met specific requirements. Individuals with active dermatological diseases on the face, a history of poor wound healing, keloid formation, cutaneous malignancy, autoimmune diseases, pregnancy, lactation, a baseline tan, or recent use of retinoids were excluded. Patients who demonstrated photosensitivity had photosensitizing medications or had previously undergone treatments such as oral tranexamic acid, topical bleaching agents, laser, intense pulsed light, chemical peeling, botulinum toxin, or filler treatments within the past 12 months were also excluded. Furthermore, the use of oral contraceptive pills and hormone replacement therapy was prohibited for a specific duration. Other exclusion criteria encompassed factors such as skin type VI, chronic systemic diseases, recent procedures or injections in the treatment areas, hypersensitivity to certain components, and various contraindications related to pregnancy, medication use, infection, and allergies.
3.3. LADD Characteristics
Tranexamic acid and kojic acid for melasma were the most frequently evaluated topical agents (four trials), followed by hydroquinone (one trial), autologous platelet-rich plasma (PRP) (one trial), hyaluronic acid (HA) (one trial), poly-L-lactic acid (PLLA) (one trial), botulinum toxin A (BoNT A) (one trial), vitamins C and E (one trial), lidocaine (one trial), and amniotic membrane stem cell (AMSC) metabolite product (one trial).
The lasers utilized were the fractional carbon dioxide (five studies); the Erbium-doped Yttriu Aluminum Garnet (Er:YAG) (four studies); and the fractional Thulium, the Q-Switched Neodymium-doped Yttrium Aluminum Garnet (Nd:YAG), and the picosecond Alexandrite (one study each) lasers.
The fractional CO2 laser was utilized mostly for facial rejuvenation, while the Er:YAG was used for melasma treatment. However, the Er:YAG was also used for facial rejuvenation and to enhance analgesia before a facial laser treatment. On the other hand, melasma was also treated by means of a Q-Switched Nd:YAG, a Thulium 1927 nm, and a picosecond Alexandrite 755 nm laser. The different laser setups are depicted in Table 1. Different laser modes were used for different facial procedures, with this diversity also characterizing the same indications and lasers between the studies.
3.4. Outcomes
The reported outcomes from the studies included in this review were analyzed and thus are presented in Table 2.

Table 2.
Outcomes of the studies included in the systematic review.
3.4.1. Melasma
The effectiveness of laser-assisted drug delivery in treating melasma was evaluated in five studies. Li et al. performed a hemi-face RCT of a novel picosecond alexandrite 755 nm laser for topical delivery of 2.5 mL tranexamic acid 10% (intervention) or saline (control) [9]. Both approaches demonstrated a significant decrease in hemi-MASI (melasma area and severity index) scores during follow-up periods of 1, 3, and 6 months. The MASI score difference between the two sides was significant, though at 1- and 3- but not the 6-month follow-up.
Al-Dhalimi et al. evaluated the effect of Er:YAG laser-assisted delivery of kojic acid (versus kojic acid cream alone) in melasma [11]. The study protocol involved six sessions at two-week intervals, with clinical assessment at 3 months. Both sides showed significant improvement compared to baseline in terms of MASI scores, but there was no significant difference between the two groups at 3 months. However, the combined treatment exhibited a significantly higher physician global assessment score, indicating better response rates.
In another prospective study, Park et al. assessed the efficacy of Q-switched Nd-YAG laser-assisted drug delivery compared to laser treatment alone [12]. One month after the last session (five in total, at 2-week intervals), the test side, treated with a combination of topical mixture of tranexamic acid (TXA) 3%, Kojic acid 1%, and Niacinamide 5%, demonstrated a significantly greater improvement in the hemi-MASI score than the control side treated with laser alone.
Similarly, Wanitphakdeedecha et al. evaluated the thylium 1927 nm fractional laser-assisted topical tranexamic acid 1.2% (compared to normal saline solution) delivery [13]. Both sides showed significant improvement in modified MASI and melanin index (MI) scores compared to baseline at all time points from the first week to six months. However, a statistically significant difference between sides, favoring LADD, was seen only for MI at the 6-month assessment.
Finally, Badawi et al. compared the effectiveness of Er:YAG laser-assisted hydroquinone (HQ) delivery to hydroquinone alone in 30 female melasma patients [16]. Six sessions at 2-week intervals were performed, with outcome evaluation at 2 weeks and recurrence rates at 14 weeks after the end of treatment. Both modalities yielded a significant improvement in MASI scores compared to baseline at 2 weeks. The intervention side showed significantly better MASI scores and results in decreasing the degree of pigmentation compared to the HQ-only treated side. In fact, a rate of >50% improvement was achieved in 73.4% and 40% of Er:YAG + HQ and HQ sides, respectively.
Patient self-evaluation of LADD efficacy for melasma treatment, as assessed in the reviewed clinical studies, provided valuable insights into treatment satisfaction and perceived outcomes. Daily diaries, visual analog scale (VAS) scores, and questionnaires were employed in these studies. Overall, satisfaction rates followed similar patterns to the investigator-evaluated outcomes and clinical measurements, mainly by MASI scores. Indeed, statistically significant satisfaction rates favoring the LADD hemi-face were reported in all studies. These satisfaction rates typically exceeded the relevant clinical outcomes and were maintained at all time points assessed.
3.4.2. Facial Rejuvenation and Rhytides
Five clinical studies evaluated the efficacy of LADD for facial rejuvenation. The RCT of Li et al., apart from melasma, photographically assessed four clinical indications of photoaging by a quartile scale [9]. Following the three treatment sessions, dyschromia and skin texture improvements were observed for both modalities at all time points, compared to the baseline, while no concomitant significant improvement in laxity and rhytids was noted.
Benzaquen et al. conducted an RCT by performing one session of fractional CO2 laser-assisted hyaluronic acid (HA) (compared to normal saline) delivery to evaluate facial skin remodeling at three months follow-up [10]. The study showed a greater but not significant improvement of facial skin rejuvenation parameters, namely skin texture, firmness, radiance, and fine lines, at the LADD side.
The RCT of Widianingsih et al. evaluated the outcomes of Er:YAG laser-assisted amniotic membrane stem cell (AMSC) metabolite product delivery following three sessions at 4-week intervals [14]. At 3 months follow-up, a slightly better but not significant result in the AMSC compared to the saline side was observed in terms of pores, wrinkles, pigmentation, and skin tone.
The RCT of Mahmoud et al. evaluated 10 patients following one session of fractional CO2 laser-assisted botulinum toxin A (or normal saline) delivery for periorbital wrinkles [18]. Superior clinical efficacy for dynamic rhytids was reported at 1 month after treatment on the LADD side. However, no statistical difference on either side compared to the baseline was revealed for static rhytids. This clinical trial was the first to support the delivery of macromolecules through fractionated channels.
Finally, the single-arm study of Ibrahim et al. assessed upper lip rejuvenation following three sessions at 2-month intervals with the fractional CO2 laser-assisted PLLA delivery [15]. A significant reduction in wrinkle severity was observed, with a progressive decrease of 26.4% after the first, 42.8% after the second, and 47.0% after the third treatment, as calculated by computer-generated image analysis. Similarly, both blinded raters successfully identified the pre/post-third treatment photographs on the first pass, and the participants rated the rhytids as “much improved”.
Patient self-evaluation of LADD efficacy for facial rejuvenation was also reported in the aforementioned studies. Benzaquen et al. found that skin radiance exhibited the most significant improvement in patients’ evaluation, while Ibrahim et al. showed that patients’ self-rated median scores were significantly improved [10,15]. Similarly, significantly higher satisfaction rates on the LADD hemi-face were measured by Mahmoud et al. and Li et al. at the 1-month follow-up [9,18].
3.4.3. Other Applications
Laser-assisted drug delivery (LADD) has been investigated in various other applications, such as the treatment of atrophic scars, pre-laser analgesia, and post-laser enhanced recovery, offering valuable insights into its potential benefits. Gawdat et al. conducted an RCT by dividing 30 patients with atrophic acne scars into two groups [19]. In the first group, fractional CO2 laser was followed by intradermal (ID) PRP on one side and intradermal saline on the other, while in the second group, the same fractional laser treatment was followed either by ID PRP or topical PRP (LADD side). Three monthly sessions were performed, and the outcomes were assessed at 6 months. The combination types of treatment (both topical and ID PRP) demonstrated significantly better clinical improvement, which was also confirmed by the optical coherence tomography measurements. Although no significant differences in the improvement grade and downtime in the PRP-treated areas were revealed, the LADD was associated with significantly lower pain scores than the ID PRP administration. Patient assessment aligned with the physician assessment, supporting the reliability of the presented outcomes.
In an RCT, Waibel et al. evaluated the postprocedural recovery in terms of wound healing by comparing carbon dioxide fractional laser-assisted Vitamin C, E, and Ferulic acid (or vehicle) delivery [17]. Although the results were not statistically significant, a trend towards decreased edema on postoperative day 7 and decreased erythema on days 3 to 5 on the LADD side compared to the vehicle was shown. In that respect, the patients could resume work and social activities on day 5 instead of the typical 7–10 days downtime after ablative fractional laser treatments. To further explore the underlying mechanisms of wound healing mediated by the delivered active serum, several molecular pathways analyses were performed, which showed a significant reduction in the bFGF expression only on the control side at 5 days and 3 months post-treatment. Considering the direct positive wound healing effect of bFGF, the authors correlated their findings with the bFGF adequate synthesis and regulation by laser serum delivery.
Finally, in a hemi-face RCT, Yun et al. investigated the efficacy of Er:YAG laser-assisted anesthesia with lidocaine 5% cream before laser resurfacing, revealing significantly lower pain scores compared to lidocaine cream alone during both passes and especially after the second pass.
3.5. Complications
The analysis of complications from the included studies showed that LADD is not associated with a higher risk of complications, either from the laser, the topical agent delivery, or the combination treatment performed. Most studies referenced only mild and transient laser-related adverse effects (e.g., pain, erythema, crusting). The identified complications were sporadic, including irritation, erythema, dryness, scaling, post-inflammatory hyperpigmentation (PIH), edema, crust formation, tingling, stinging sensation, mild pain, and acne eruption. The low incidence of these adverse effects, without statistically significant differences between the treatment modalities applied, supports the safety of LADD.
4. Discussion
The current era of evidence-based medicine is characterized by better medical and surgical outcomes based on the accumulated data from well-conducted trials, systematic reviews, and meta-analyses of these studies [20,21,22]. This evidence has a direct impact on the quality and safety of the provided treatments and also on patient recovery and satisfaction [23,24,25]. Based on these principles, we aimed to summarize and comprehensively evaluate the efficacy and safety of laser-assisted drug delivery in various non-oncological facial applications. The findings from the analysis of twelve relevant studies of high methodological quality indicated that LADD holds promise in improving the outcomes following various facial procedures, such as treatment of melasma, atrophic scars, facial rejuvenation, enhancing recovery after laser, and local anesthesia before laser treatment.
Interestingly, the positive effect of laser-assisted delivery of topical agents for the same indication was achieved by different combinations of laser devices and topical agents, compared to monotherapy either with a laser or a topical medication. The principal role in these effects is the synergism between the laser and the topical substance. The commonly used fractional ablative lasers, by means of selective epidermal damage, create spatially distributed columns of microscopic treatment zones, which act as conduits through which the topical agents bypass the stratum corneum and the other epidermal barrier mechanisms towards the deeper tissues [26]. Consequently, the percutaneous penetration of topically applied compounds is enhanced, enabling their increased diffusion and absorption.
The efficacy and safety of LADD should be evaluated, considering the characteristics of the laser device and its settings, the characteristics of the topical agent applied, and the patient characteristics. Starting with the laser device, the incurred channel attributes, in terms of number, depth, size, and the surrounding coagulation zone (CZ) due to thermal damage, should be properly configured. Laser density defines the number of channels, and thus, by increasing laser density up to approximately 5%, the topical agent absorption will be proportionally increased without further effect, presumably due to tissue saturation [27]. Similarly, channel size, as defined by spot size, will affect the amount of compound absorbed. Channel depth, which determines the level of substance penetration, depends on the laser fluence or energy settings. However, hydrophobic substances, such as lidocaine or imiquimod, show a depth-independent uptake due to the hydrophilic dermis [1,28,29]. Therefore, hydrophilic medications are expected to penetrate deeper, which can ameliorate treatment efficacy but also endanger safety due to exaggerated local and systemic responses [30]. The coagulation zones around the laser channel enhance absorption, which partly explains the superior effect of LADD compared to non-thermal modalities, such as microneedling, while it may lead to an increased risk of local and systemic reactions [31,32,33]. Notably, CZ size depends on the laser-induced thermal damage; thus, it is associated with the risk of adverse events, such as blistering and scarring. Consequently, laser settings must be configured wisely in order to maximize efficacy while mitigating periprocedural risk. In this review, the above requirements were practically confirmed by using settings of low fluence and pulse energy, a spot size of 7–10 mm, a frequency of 4–5 Hz, and a density of 5%.
A recent review of preclinical development and clinical applications of LADD concluded that ablative fractional lasers are highly effective and safe to facilitate topical drug delivery with little restriction on the physicochemical properties of medications [34]. These properties, though, are also substantial to the treatment efficacy. The molecular weight, hydrophilicity, and solubility dictate the ability, depth, and speed of skin penetration [1]. Liquid solutions and gels penetrate laser channels more readily than cream formulations, thus affecting the tissue response in terms of clinical but also side effects [35]. This systematic review of controlled studies supports the efficacy of LADD compared to monotherapy, irrespective of substance properties. Indeed, anesthesia induced by Er:YAG-assisted lidocaine cream delivery was superior to cream alone for facial rejuvenation with laser [8]. In addition, the review showed that the safety profile of the delivered substances was not affected by LADD, as the reported side effects were essentially laser-related.
Patient and skin characteristics should also be accounted for regarding LADD efficacy. The anatomical site, cutaneous integrity and enzymatic activity, vasculature, acidity and hydration, occlusion, and age of the patient affect transdermal drug penetration [36,37]. It is logical that injured or inflamed skin absorbs topical compounds more readily than intact skin. Interestingly, modifications in local vascular supply by topical brimonidine cream, epinephrine solution, or pulsed dye laser enhance the LADD of 5-fluorouracil due to its increased concentration and prolonged dwell time in the skin [36]. In this review, we focused on facial applications of LADD, which enhanced the homogeneity of the studies and outcomes. Patient and skin characteristics (i.e., Fitzpatrick skin type) were not significantly different between the groups of each trial, which further strengthens the presented evidence.
Although there is a variety of indications for LADD, the recently published evidence-based clinical practice guidelines strongly recommend LADD only for the treatment of actinic keratoses, actinic cheilitis, and Bowen disease [38,39,40,41]. In general, an ablative laser pretreatment with CO2 or Er:YAG was followed by photodynamic therapy with methyl aminolevulinate or aminolevulinic acid. Higher laser depth parameters have shown significantly higher efficacy and lower recurrence rates at 12 months and 5 years than conventional methyl aminolevulinate photodynamic therapy for the treatment of actinic keratosis and Bowen disease [38,40,41,42]. In this review of facial non-oncological applications of LADD, the treatment of melasma, scars, facial rejuvenation, and laser procedure optimization were the main indications. For melasma, LADD with tranexamic acid, kojic acid, or hydroquinone demonstrated effectiveness up to 6 months post-treatment. However, direct comparisons between LADD and monotherapy, in terms of MASI/mMASI scores, supported superior outcomes only up to 3 months [9,11,12,13,16]. The patient satisfaction rates typically exceeded the relevant clinical outcomes and were maintained at all time points assessed. These results align with the meta-analysis of five RCTs, which showed that laser modalities combined with TXA significantly decreased the MASI/mMASI scores, while no serious adverse events were observed, except mild erythema and burning pain [43]. The efficacy of LADD will probably render the intradermal tranexamic acid injections alone or combined with Q-switched Nd-YAG laser, though effective, obsolete due to the associated pain from injection despite topical anesthetic cream used [44].
Regarding facial rejuvenation, this review supported the use of fractional lasers for the delivery of various topical cosmeceuticals, including poly-L-lactic acid, hyaluronic acid, and BTX-A. These outcomes are similar to the review by Muskat et al. regarding LADD and treatment of rhytids, as well as scars [45]. LADD, with either ablative or nonablative laser types, was evaluated in combination with corticosteroids, botulinum toxin-A (BTX-A), 5-fluorouracil, 5-aminolevulinic acid photodynamic therapy, stem cells, platelet-rich plasma, and prostaglandin analogs for the treatment of scars. Various clinical outcomes, with reduced adverse effects rates, were revealed [45]. Similarly, the findings of the systematic review by Truong et al., which evaluated LADD efficacy for the treatment of hypertrophic scars and keloids, were limited by the quality and heterogeneity in participants, methodology, and outcome assessment of the included RCTs [46]. Considering that our review included only one study regarding post-acne atrophic scars, more high-quality LADD studies in the context of scar treatment are needed.
This plethora of options for lasers and topical preparations, indications for LADD, and relative accessibility to this modality enable widespread adoption. The aspect of safety should be thus emphasized, as with any novel intervention. In a systematic review of LADD safety and adverse effects, Ng et al. highlighted the multiple adverse effects that clinicians should consider prior to carrying out LADD [47]. Common findings, such as erythema, edema, pain, and crusting, and more severe adverse effects, such as allergic reactions, infection, scarring, and hyperpigmentation, were noted. These adverse effects are mostly related to the lasers themselves; thus, the laser type and its function settings should be carefully adjusted based on the LADD pathophysiology, as mentioned above [48]. All studies included in this review quantified the proportion of participants that experienced adverse effects, which confirmed the safety of LADD (compared to controls), also reporting no systemic or life-threatening side effects. This vigilance regarding the adverse effects emphasizes the importance of patient safety, especially in facial and cosmetic procedures [49,50].
This systematic review addresses the effect of the LADD on various facial procedures. Among its strengths is the rigorous methodology used, analyzing a fairly homogeneous sample of patients undergoing different facial procedures assisted by lasers, thus providing outcomes generalizable to a broader population and insights into the potential versatility of LADD in clinical practice. In addition, the comparison groups studied had similar baseline characteristics, thus limiting potential bias from known confounding factors to the primary outcomes of interest. This was also confirmed by the risk of bias assessment performed.
While this meta-analysis provides valuable insights into the outcomes and complications of LADD, there are certain limitations that should be acknowledged. The main limitation is the relatively small number of studies included in the analysis. While aiming to evaluate the LADD’s effect on facial procedures while mitigating the potential risk of bias on the review outcomes, a rather specific review question and corresponding search strategy were adopted. The heterogeneity in the included study designs regarding methodology, treatment protocols, and follow-up duration was another limitation that may influence the overall findings. Although this review comprised only high-quality RCTs, such variation precluded data pooling, quantitative synthesis, or subgroup analyses. Considering that LADD is a novel modality that has only recently emerged as an adjunct for various skin procedures, this heterogeneity is anticipated. The review’s reliance on relatively short follow-up studies may not fully capture the long-term efficacy and safety outcomes of LADD.
Further research is warranted to address these limitations and enhance the understanding of LADD in facial treatments. Future studies should employ standardized treatment protocols, larger sample sizes, and longer follow-up periods to generate more robust evidence. This is particularly important when the applied medications may increase cellular turnover, such as proliferative anti-ageing peptides; induce DNA damage, such as chemotherapeutics; or promote the proliferation of aberrant cells due to growth factors applied. Although this review and the available evidence do not support such implications, the long-term risk following LADD of various compounds remains unknown.
5. Conclusions
In conclusion, this systematic review presents the indications and highlights the efficacy of laser-assisted drug delivery in enhancing the outcomes of various topical medications applied on the face. A clinical benefit of LADD was unanimously revealed without an increased risk for potential adverse effects. In clinical practice, caution should be exercised when performing LADD, focusing on laser type, its function settings, and the properties of topical medication delivered through the skin. Further research is needed to refine treatment protocols, optimize patient selection, and establish guidelines for adverse effects mitigation, which will enhance the safety and efficacy of laser-assisted drug delivery in routine practice.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cosmetics10050122/s1, Table S1: PRISMA Checklist; Table S2: Risk of bias (RoB2) graph; Table S3: Summary graph of RoB2.
Author Contributions
Conceptualization, K.S.; methodology, K.S. and K.P.; software, K.S.; validation, K.S. and K.P.; formal analysis, K.S.; investigation, K.S. and K.P.; resources, K.S.; data curation, K.S. and K.P.; writing—original draft preparation, K.S. and K.P.; writing—review and editing, all authors; visualization, K.S.; supervision, K.S.; project administration, K.P.; funding acquisition, K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wenande, E.; Anderson, R.R.; Haedersdal, M. Fundamentals of Fractional Laser-Assisted Drug Delivery: An in-Depth Guide to Experimental Methodology and Data Interpretation. Adv. Drug Deliv. Rev. 2020, 153, 169–184. [Google Scholar] [CrossRef]
- Elias, P.M.; Menon, G.K. Structural and Lipid Biochemical Correlates of the Epidermal Permeability Barrier. In Advances in Lipid Research; Elsevier: Amsterdam, The Netherlands, 1991; Volume 24, pp. 1–26. ISBN 978-0-12-024924-4. [Google Scholar]
- Chuang, S.-Y.; Lin, Y.-K.; Lin, C.-F.; Wang, P.-W.; Chen, E.-L.; Fang, J.-Y. Elucidating the Skin Delivery of Aglycone and Glycoside Flavonoids: How the Structures Affect Cutaneous Absorption. Nutrients 2017, 9, 1304. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R.; Mandru, A. Enhancement of Skin Permeability with Thermal Ablation Techniques: Concept to Commercial Products. Drug Deliv. Transl. Res. 2021, 11, 817–841. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Yun, P.L.; Tachihara, R.; Anderson, R.R. Efficacy of Erbium:Yttrium-Aluminum-Garnet Laser-Assisted Delivery of Topical Anesthetic. J. Am. Acad. Dermatol. 2002, 47, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, C.; Zhang, H.; Li, L.; Song, Y. Efficacy and Safety of 755-Nm Picosecond Alexandrite Laser with Topical Tranexamic Acid versus Laser Monotherapy for Melasma and Facial Rejuvenation: A Multicenter, Randomized, Double-Blinded, Split-Face Study in Chinese Patients. Lasers Med. Sci. 2022, 37, 2879–2887. [Google Scholar] [CrossRef]
- Benzaquen, M.; Fongue, J.; Pauly, V.; Collet-Villette, A.-M. Laser-Assisted Hyaluronic Acid Delivery by Fractional Carbon Dioxide Laser in Facial Skin Remodeling: A Prospective Randomized Split-Face Study in France. Lasers Surg. Med. 2021, 53, 1166–1172. [Google Scholar] [CrossRef]
- Al-Dhalimi, M.A.; Yasser, R.H. Evaluation of the of the Efficacy of Fractional Erbium-Doped Yttrium Aluminum Garnet Laser-Assisted Drug Delivery of Kojic Acid in the Treatment of Melasma; A Split Face, Comparative Clinical Study. J. Cosmet. Laser Ther. 2021, 23, 65–71. [Google Scholar] [CrossRef]
- Park, S.J.; Park, J.W.; Seo, S.J.; Park, K.Y. Evaluating the Tolerance and Efficacy of Laser-Assisted Delivery of Tranexamic Acid, Niacinamide, and Kojic Acid for Melasma: A Single Center, Prospective, Split-Face Trial. Dermatol. Ther. 2022, 35, e15287. [Google Scholar] [CrossRef]
- Wanitphakdeedecha, R.; Sy-Alvarado, F.; Patthamalai, P.; Techapichetvanich, T.; Eimpunth, S.; Manuskiatti, W. The Efficacy in Treatment of Facial Melasma with Thulium 1927-Nm Fractional Laser-Assisted Topical Tranexamic Acid Delivery: A Split-Face, Double-Blind, Randomized Controlled Pilot Study. Lasers Med. Sci. 2020, 35, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Widianingsih, N.P.S.; Setyaningrum, T.; Prakoeswa, C.R.S. The Efficacy and Safety of Fractional Erbium Yag Laser Combined with Topical Amniotic Membrane Stem Cell (AMSC) Metabolite Product for Facial Rejuvenation: A Controlled, Split-Face Study. Dermatol. Rep. 2019, 11, S1. [Google Scholar] [CrossRef]
- Ibrahim, O.; Ionta, S.; Depina, J.; Petrell, K.; Arndt, K.A.; Dover, J.S. Safety of Laser-Assisted Delivery of Topical Poly-l-Lactic Acid in the Treatment of Upper Lip Rhytides: A Prospective, Rater-Blinded Study. Dermatol. Surg. 2019, 45, 968–974. [Google Scholar] [CrossRef]
- Badawi, A.M.; Osman, M.A. Fractional Erbium-Doped Yttrium Aluminum Garnet Laser-Assisted Drug Delivery of Hydroquinone in the Treatment of Melasma. Clin. Cosmet. Investig. Dermatol. 2018, 11, 13–20. [Google Scholar] [CrossRef]
- Waibel, J.S.; Mi, Q.-S.; Ozog, D.; Qu, L.; Zhou, L.; Rudnick, A.; Al-Niaimi, F.; Woodward, J.; Campos, V.; Mordon, S. Laser-Assisted Delivery of Vitamin C, Vitamin E, and Ferulic Acid Formula Serum Decreases Fractional Laser Postoperative Recovery by Increased Beta Fibroblast Growth Factor Expression. Lasers Surg. Med. 2016, 48, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, B.H.; Burnett, C.; Ozog, D. Prospective Randomized Controlled Study to Determine the Effect of Topical Application of Botulinum Toxin A for Crow’s Feet after Treatment with Ablative Fractional CO2 Laser. Dermatol. Surg. 2015, 41, S75–S81. [Google Scholar] [CrossRef]
- Gawdat, H.I.; Hegazy, R.A.; Fawzy, M.M.; Fathy, M. Autologous Platelet Rich Plasma: Topical Versus Intradermal After Fractional Ablative Carbon Dioxide Laser Treatment of Atrophic Acne Scars. Dermatol. Surg. 2014, 40, 152–161. [Google Scholar] [CrossRef]
- Seretis, K.; Goulis, D.; Demiri, E.C.; Lykoudis, E.G. Prevention of Seroma Formation Following Abdominoplasty: A Systematic Review and Meta-Analysis. Aesthetic Surg. J. 2017, 37, 316–323. [Google Scholar] [CrossRef]
- Seretis, K.; Boptsi, A.; Boptsi, E.; Lykoudis, E.G. The Efficacy of Wide-Awake Local Anesthesia No Tourniquet (WALANT) in Common Plastic Surgery Operations Performed on the Upper Limbs: A Case-Control Study. Life 2023, 13, 442. [Google Scholar] [CrossRef]
- Demiri, E.; Koliakos, G.; Goulis, D.G.; Seretis, K. Weight Reduction Following Abdominoplasty: A Systematic Review. Plast. Reconstr. Surg. 2013, 132, 314e–316e. [Google Scholar] [CrossRef]
- Seretis, K. The Efficacy of Local Anesthesia for Postoperative Pain Control in Breast Augmentation Surgery: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Aesthetic Plast. Surg. 2022, 46, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Seretis, K.; Bounas, N. The Efficacy of Different Nerve Blocks on Postoperative Pain and Sequelae in Patients Undergoing Abdominoplasty: A Network Meta-Analysis. Aesthet Surg. J. 2023, 43, NP325–NP336. [Google Scholar] [CrossRef] [PubMed]
- Seretis, K.; Bounas, N.; Papaspyrou, F. Antibiotic Prophylaxis in Reduction Mammaplasty: A Network Meta-Analysis. Aesthetic Plast. Surg. 2023, 47, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, O.; Wenande, E.; Hogan, S.; Arndt, K.A.; Haedersdal, M.; Dover, J.S. Challenges to Laser-Assisted Drug Delivery: Applying Theory to Clinical Practice. Lasers Surg. Med. 2018, 50, 20–27. [Google Scholar] [CrossRef]
- Haak, C.S.; Christiansen, K.; Erlendsson, A.M.; Taudorf, E.H.; Thaysen-Petersen, D.; Wulf, H.C.; Haedersdal, M. Ablative Fractional Laser Enhances MAL-Induced PpIX Accumulation: Impact of Laser Channel Density, Incubation Time and Drug Concentration. J. Photochem. Photobiol. B 2016, 159, 42–48. [Google Scholar] [CrossRef]
- Oni, G.; Brown, S.A.; Kenkel, J.M. Can Fractional Lasers Enhance Transdermal Absorption of Topical Lidocaine in an in Vivo Animal Model? Lasers Surg. Med. 2012, 44, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Taudorf, E.H.; Lerche, C.M.; Erlendsson, A.M.; Philipsen, P.A.; Hansen, S.H.; Janfelt, C.; Paasch, U.; Anderson, R.R.; Haedersdal, M. Fractional Laser-Assisted Drug Delivery: Laser Channel Depth Influences Biodistribution and Skin Deposition of Methotrexate. Lasers Surg. Med. 2016, 48, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.L.; Ortner, V.K.; Haedersdal, M.; Olesen, U.H. Optical Imaging Visualizes a Homogeneous and Horizontal Band-Like Biodistribution of Large- and Small-Size Hydrophilic Compounds Delivered by Ablative Fractional Laser. Pharmaceutics 2022, 14, 1537. [Google Scholar] [CrossRef]
- Haak, C.S.; Hannibal, J.; Paasch, U.; Anderson, R.R.; Haedersdal, M. Laser-Induced Thermal Coagulation Enhances Skin Uptake of Topically Applied Compounds. Lasers Surg. Med. 2017, 49, 582–591. [Google Scholar] [CrossRef]
- Bay, C.; Lerche, C.M.; Ferrick, B.; Philipsen, P.A.; Togsverd-Bo, K.; Haedersdal, M. Comparison of Physical Pretreatment Regimens to Enhance Protoporphyrin IX Uptake in Photodynamic Therapy: A Randomized Clinical Trial. JAMA Dermatol. 2017, 153, 270–278. [Google Scholar] [CrossRef]
- Nieboer, M.J.; Meesters, A.A.; Almasian, M.; Georgiou, G.; de Rie, M.A.; Verdaasdonk, R.M.; Wolkerstorfer, A. Enhanced Topical Cutaneous Delivery of Indocyanine Green after Various Pretreatment Regimens: Comparison of Fractional CO2 Laser, Fractional Er:YAG Laser, Microneedling, and Radiofrequency. Lasers Med. Sci. 2020, 35, 1357–1365. [Google Scholar] [CrossRef]
- Zhao, Y.; Voyer, J.; Li, Y.; Kang, X.; Chen, X. Laser Microporation Facilitates Topical Drug Delivery: A Comprehensive Review about Preclinical Development and Clinical Application. Expert Opin. Drug Deliv. 2023, 20, 31–54. [Google Scholar] [CrossRef]
- Meesters, A.A.; Bakker, M.M.; de Rie, M.A.; Wolkerstorfer, A. Fractional CO2 Laser Assisted Delivery of Topical Anesthetics: A Randomized Controlled Pilot Study. Lasers Surg. Med. 2016, 48, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Wenande, E.; Gundavarapu, S.C.; Tam, J.; Bhayana, B.; Thomas, C.N.; Farinelli, W.A.; Vakoc, B.J.; Anderson, R.R.; Haedersdal, M. Local Vasoregulative Interventions Impact Drug Concentrations in the Skin after Topical Laser-Assisted Delivery. Lasers Surg. Med. 2022, 54, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, N.J.; Grégoire, S.; Cubberley, R.; Duplan, H.; Eilstein, J.; Ellison, C.; Lester, C.; Fabian, E.; Fernandez, J.; Géniès, C.; et al. Measurement of the Penetration of 56 Cosmetic Relevant Chemicals into and through Human Skin Using a Standardized Protocol. J. Appl. Toxicol. 2020, 40, 403–415. [Google Scholar] [CrossRef]
- Seo, J.-W.; Kim, H.-J.; Song, K.-H. A Comparison of the Efficacy of Ablative Fractional Laser–Assisted Photodynamic Therapy According to Ablative Depth for Actinic Keratosis: A Single-Blinded, Randomized, Comparative, Prospective Study. J. Am. Acad. Dermatol. 2019, 81, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Labadie, J.G.; Ibrahim, S.A.; Worley, B.; Kang, B.Y.; Rakita, U.; Rigali, S.; Arndt, K.A.; Bernstein, E.; Brauer, J.A.; Chandra, S.; et al. Evidence-Based Clinical Practice Guidelines for Laser-Assisted Drug Delivery. JAMA Dermatol. 2022, 158, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-R.; Seo, J.-W.; Kim, H.-J.; Song, K.-H. A Comparison of the Efficacy of Ablative Fractional Laser-Assisted Photodynamic Therapy According to the Density of the Ablative Laser Channel in the Treatment of Actinic Keratosis: A Prospective, Randomized, Controlled Trial. J. Am. Acad. Dermatol. 2021, 85, 750–752. [Google Scholar] [CrossRef]
- Kim, H.-J.; Song, K.-H. Ablative Fractional Laser–Assisted Photodynamic Therapy Provides Superior Long-Term Efficacy Compared with Standard Methyl Aminolevulinate Photodynamic Therapy for Lower Extremity Bowen Disease. J. Am. Acad. Dermatol. 2018, 79, 860–868. [Google Scholar] [CrossRef]
- Vrani, F.; Sotiriou, E.; Lazaridou, E.; Vakirlis, E.; Sideris, N.; Kirmanidou, E.; Apalla, Z.; Lallas, A.; Ioannides, D. Short Incubation Fractional CO 2 Laser-Assisted Photodynamic Therapy vs. Conventional Photodynamic Therapy in Field-Cancerized Skin: 12-Month Follow-up Results of a Randomized Intraindividual Comparison Study. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 79–83. [Google Scholar] [CrossRef]
- Feng, J.; Shen, S.; Song, X.; Xiang, W. Efficacy and Safety of Laser-Assisted Delivery of Tranexamic Acid for the Treatment of Melasma: A Systematic Review and Meta-analysis. J. Cosmet. Laser Ther. 2022, 24, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Hawwam, S.A.; Ismail, M.; El-Attar, Y.A. Split-Face Comparative Study between Intradermal Tranexamic Acid Injection Alone versus Intradermal Tranexamic Acid Injection Combined with Q-Switched Nd:YAG Laser in Melasma Treatment: Dermoscopic and Clinical Evaluation. Lasers Med. Sci. 2022, 37, 2193–2201. [Google Scholar] [CrossRef] [PubMed]
- Muskat, A.; Kost, Y.; Balazic, E.; Cohen, J.L.; Kobets, K. Laser-Assisted Drug Delivery in the Treatment of Scars, Rhytids, and Melasma: A Comprehensive Review of the Literature. Aesthetic Surg. J. 2023, 43, NP181–NP198. [Google Scholar] [CrossRef] [PubMed]
- Truong, K.; Prasidha, I.; Wain, T. A Systematic Review of Randomised Controlled Trials Investigating Laser Assisted Drug Delivery for the Treatment of Keloid and Hypertrophic Scars. Lasers Med. Sci. 2022, 37, 47–59. [Google Scholar] [CrossRef]
- Ng, W.H.S.; Smith, S.D. Laser-Assisted Drug Delivery: A Systematic Review of Safety and Adverse Events. Pharmaceutics 2022, 14, 2738. [Google Scholar] [CrossRef]
- Prohaska, J.; Hohman, M.H. Laser Complications. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Oni, G.; Rasko, Y.; Kenkel, J. Topical Lidocaine Enhanced by Laser Pretreatment: A Safe and Effective Method of Analgesia for Facial Rejuvenation. Aesthetic Surg. J. 2013, 33, 854–861. [Google Scholar] [CrossRef]
- Haedersdal, M.; Erlendsson, A.M.; Paasch, U.; Anderson, R.R. Translational Medicine in the Field of Ablative Fractional Laser (AFXL)-Assisted Drug Delivery: A Critical Review from Basics to Current Clinical Status. J. Am. Acad. Dermatol. 2016, 74, 981–1004. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).