Molecular Docking Simulation of Phenolics towards Tyrosinase, Phenolic Content, and Radical Scavenging Activity of Some Zingiberaceae Plant Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals
2.3. Plant Extraction
2.4. Phytochemical Screening and Total Phenolics
- Flavonoids were detected by treating the extracts with a few drops of lead acetate solution, which resulted in the formation of a yellow precipitate.
- Phenols were detected by treating the extracts with 3–4 drops of ferric chloride solution, which resulted in the formation of a dark blue color.
- Tannins were detected by treating the extracts with a 1% gelatin solution containing sodium chloride, which resulted in the formation of a white precipitate.
- Alkaloids were detected by treating the extracts with dilute hydrochloric acid and added with the Dragendorff reagent, which resulted in the formation of a red precipitate.
- Glycosides were detected by treating the extracts with sodium nitroprusside in pyridine and sodium hydroxide, which resulted in the formation of a pink to dark red color.
- Saponins were detected by treating the extracts with 20 mL of distilled water and were shaken in a graduated cylinder for 15 min. The formation of a 1 cm layer of foam indicates the presence of saponins.
- Phytosterols were detected by treating the extracts with chloroform and the Liebermann–Burchard reagent, which resulted in the formation of a brown ring at the junction.
2.5. Radical Scavenging Capacity Assay Using the DPPH Method
2.6. Liquid Chromatography–Mass Spectroscopy (LC-MS) for the Total Flavonoid Content Analysis
2.7. Molecular Docking Simulation
2.7.1. Hardware
2.7.2. Preparation of the Macromolecule
2.7.3. Preparation of the Ligand
2.7.4. Molecular Docking Simulation
3. Results
3.1. Phytochemical Screening, Total Phenolics, and Radical Scavenging Capacity
3.2. LC-MS Analysis of A. galanga Extract
3.3. Molecular Docking Simulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide research trends on medicinal plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef]
- Trugo, L.; Finglas, P.M. (Eds.) Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Spiegel, M.; Andruniów, T.; Sroka, Z. Flavones’ and flavonols’ antiradical structure-activity relationship—A quantum chemical study. Antioxidants 2020, 9, 461. [Google Scholar] [CrossRef]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Latos, M.; Zaborski, M. Influence of hydroxyl substitution on flavanone antioxidants properties. Food Chem. 2017, 215, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Amić, A.; Marković, Z.; Klein, E.; Dimitrić Marković, J.M.; Milenković, D. Theoretical study of the thermodynamics of the mechanisms underlying antiradical activity of cinnamic acid derivatives. Food Chem. 2018, 246, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Ramli, M.R.; Milow, P.; Malek, S. Diversity and traditional knowledge of medicinal plants in home gardens of Kampung Masjid Ijok, Perak, Malaysia. Biodiversitas 2021, 22, 2458–2465. [Google Scholar] [CrossRef]
- Kubo, I.; Kinst-Hori, I.; Chaudhuri, S.K.; Kubo, Y.; Sánchez, Y.; Ogura, T. Flavonols from Heterotheca inuloides: Tyrosinase inhibitory activity and structural criteria. Bioorg. Med. Chem. 2000, 8, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Nakashima, S.; Oda, Y.; Nakamura, S.; Yoshikawa, M. Melanogenesis inhibitors from the rhizomes of Alpinia officinarum in B16 melanoma cells. Bioorg. Med. Chem. 2009, 15, 6048–6053. [Google Scholar] [CrossRef]
- Huang, H.C.; Chang, S.J.; Wu, C.Y.; Ke, H.J.; Chang, T.M. [6]-Shogaol inhibits α-MSH-induced melanogenesis through the acceleration of ERK and PI3K/Akt-mediated MITF degradation. BioMed Res. Int. 2014, 2014, 842569. [Google Scholar] [CrossRef]
- Chigurupati, S.; Al-Murikhy, A.; Almahmoud, S.A.; Almoshari, Y.; Saber Ahmed, A.; Vijayabalan, S.; Ghazi Felemban, S.; Raj Palanimuthu, V. Molecular docking of phenolic compounds and screening of antioxidant and antidiabetic potential of Moringa oleifera ethanolic leaves extract from Qassim region, Saudi Arabia. Saudi J. Biol. Sci. 2022, 29, 854–859. [Google Scholar] [CrossRef]
- Lekmine, S.; Benslama, O.; Kadi, K.; Martín-García, A.I.; Yilmaz, M.A.; Akkal, S.; Boumegoura, A.; Alhomida, A.S.; Ola, M.S.; Ali, A. LC/MS-MS analysis of phenolic compounds in Hyoscyamus albus L. extract: In vitro antidiabetic activity, in silico molecular docking, and in vivo investigation against STZ-induced diabetic mice. Pharmaceuticals 2023, 16, 1015. [Google Scholar] [CrossRef] [PubMed]
- Kurnia, D.; Putri, S.A.; Tumilaar, S.G.; Zainuddin, A.; Dharsono, H.D.A.; Amin, M.F. In silico study of antiviral activity of polyphenol compounds from Ocimum basilicum by molecular docking, ADMET, and drug-likeness analysis. Adv. Appl. Bioinform. Chem. 2023, 16, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Baruah, I.; Kashyap, C.; Guha, A.K.; Borgohain, G. Insights into the interaction between polyphenols and β-lactoglobulin through molecular docking, MD simulation, and QM/MM approach. CS Omega 2022, 7, 23083–23095. [Google Scholar]
- Lešnik, S.; Bren, U. Mechanistic insights into biological activities of polyphenolic compounds from rosemary obtained by inverse molecular docking. Foods 2022, 11, 67. [Google Scholar] [CrossRef]
- Lescano, C.H.; de Lima, F.F.; Mendes-Silvério, C.B.; Justo, A.F.O.; da Silva Baldivia, D.; Vieira, C.P.; Sanjinez-Argandoña, E.J.; Cardoso, C.A.L.; Mónica, F.Z.; de Oliveira, I.P. Effect of polyphenols from Campomanesia adamantium on platelet aggregation and inhibition of cyclooxygenases: Molecular docking and in vitro analysis. Front. Pharmacol. 2018, 9, 617. [Google Scholar] [CrossRef]
- Raal, A.; Meos, A.; Hinrikus, T.; Heinämäki, J.; Romāne, E.; Gudienė, V.; Jak Tas, V.; Koshovyi, O.; Kovaleva, A.; Fursenco, C.; et al. Dragendorff’s reagent: Historical perspectives and current status of a versatile reagent introduced over 150 years ago at the University of Dorpat, Tartu, Estonia. Die Pharm. 2020, 75, 299–306. [Google Scholar]
- Adu, J.K.; Amengor, C.D.K.; Kabiri, N.; Orman, E.; Patamia, S.A.G.; Okra, B.K. Validation of a simple and robust Liebermann–Burchard colorimetric method for the assay of cholesterol in selected milk products in Ghana. Int. J. Food Sci. 2019, 2019, 9045938. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Scarano, P.; Tartaglia, M.; Zuzolo, D.; Prigioniero, A.; Guarino, C.; Sciarrillo, R. Recovery and valorization of bioactive and functional compounds from the discarded of Opuntia ficus-indica (L.) Mill. fruit peel. Agronomy 2022, 12, 388. [Google Scholar] [CrossRef]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and extraction: A review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Nakamura, M.; Ra, J.-H.; Jee, Y.; Kim, J.-S. Impact of different partitioned solvents on chemical composition and bioavailability of Sasa quelpaertensis Nakai leaf extract. J. Food Drug Anal. 2017, 25, 316–326. [Google Scholar] [CrossRef]
- Ayele, D.T.; Akele, M.L.; Melese, A.T. Analysis of total phenolic contents, flavonoids, antioxidant and antibacterial activities of Croton macrostachyus root extracts. BMC Chem. 2022, 16, 30. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its main pharmacological activity and potential application in clinical medicine. Oxid. Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef] [PubMed]
- Panchal, H.; Shah, M.B. Development and validation of a rapid LC-MS/MS method for simultaneous determination of kaempferol and quercetin in Thespesia populnea Extract. J. AOAC Int. 2017, 100, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.; Cao, S.; Feng, L.; Yin, X.; Wang, W.; Zhang, X.; Ni, J. Simultaneous analysis of quercetin and naringenin in rat plasma by liquid chromatography–tandem mass spectrometry: Application to a pharmacokinetic study after oral administration. J. Chromatogr. Sci. 2016, 54, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.; Bastola, T.; Tripathi, J.; Tripathi, M.; Rokaya, R.K.; Dhakal, B.; Rabin, D.C.; Bhandari, R.; Poudel, A. Estimation of total quercetin and rutin content in Malus domestica of Nepalese origin by HPLC method and determination of their antioxidative activity. J. Food Qual. 2020, 2020, 8853426. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, I.S.; Ur Rehman, S.; Na, C.-S.; Yoo, H.H. HPLC Determination of bioactive flavonoids in Hovenia dulcis fruit extracts. J. Chromatogr. Sci. 2016, 54, 130–135. [Google Scholar]
- Ang, L.F.; Yam, M.F.; Fung, Y.T.; Kiang, P.K.; Darwin, Y. HPLC method for simultaneous quantitative detection of quercetin and curcuminoids in traditional Chinese medicines. J. Pharmacopunct. 2014, 17, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Paje, L.A.; Choi, W.H.; Cho, E.J.; Kim, H.Y.; Jacinto, S.D.; Lee, S. Validation of an optimized HPLC/UV method for the quantification of flavonoids in lotus. Appl. Biol. Chem. 2020, 63, 84. [Google Scholar] [CrossRef]
- Yao, X.; Zhou, G.; Tang, Y.; Li, Z.; Su, S.; Qian, D.; Duan, J.A. A UPLC-MS/MS method for qualification of quercetin-3-O-β-D-glucopyranoside-(4→1)-α-L-rhamnoside in rat plasma and application to pharmacokinetic studies. Molecules 2013, 18, 3050–3059. [Google Scholar] [CrossRef]
- Gbylik-Sikorska, M.; Gajda, A.; Burmańczuk, A.; Grabowski, T.; Posyniak, A. Development of a UHPLC-MS/MS method for the determination of quercetin in milk and its application to a pharmacokinetic study. J. Vet. Res. 2019, 63, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Sendovski, M.; Kanteev, M.; Ben-Yosef, V.S.; Adir, N.; Fishman, A. First structures of an active bacterial tyrosinase reveal copper plasticity. J. Mol. Biol. 2011, 405, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Firmansyah, D.; Sumiwi, S.A.; Saptarini, N.M.; Levita, J. Molecular interaction of curcumin, demethoxycurcumin, bisdemethoxycurcumin, and turmerone of Curcuma longa with tyrosinase and tyrosinase-related protein-1. Rasayan J. Chem. 2021, 14, 2298–2303. [Google Scholar] [CrossRef]
- Megantara, S.; Wathoni, N.; Mohammed, A.F.A.; Suhandi, C.; Ishmatullah, M.H.; Putri, M.F.F.D. In silico study: Combination of α-mangostin and chitosan conjugated with trastuzumab against human epidermal growth factor receptor 2. Polymers 2022, 14, 2747. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, M.; Bandy, B. Mechanisms of flavonoid protection against myocardial ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2009, 46, 309–317. [Google Scholar] [CrossRef]
- Sulaiman, C.T.; Balachandran, I. Total phenolics and total flavonoids in selected Indian medicinal plants. Indian. J. Pharm. Sci. 2012, 74, 258–260. [Google Scholar] [CrossRef]
- Oki, T.; Masuda, M.; Furuta, S.; Nishibia, Y.; Terahara, N.; Suda, I. Involvement of anthocyanins and other phenolic compounds in the radical-scavenging activity of purple-fleshed sweet potato cultivars. Food Chem. Toxicol. 2002, 67, 1752–1756. [Google Scholar] [CrossRef]
- Nampoothiri, S.V.; Esakkidurai, T.; Pitchumani, K. Identification and quantification of phenolic compounds in Alpinia galanga and Alpinia calcarata and its relation to free radical quenching properties: A comparative study. J. Herbs Spices Med. Plants 2015, 21, 140–147. [Google Scholar] [CrossRef]
- Burapan, S.; Kim, M.; Paisooksantivatana, Y.; Eser, B.E.; Han, J. Thai curcuma species: Antioxidant and bioactive compounds. Foods 2020, 9, 1219. [Google Scholar] [CrossRef]
- Aljobair, M.O. Chemical composition, antimicrobial properties, and antioxidant activity of galangal rhizome. Food Sci. Technol. 2022, 42, e45622. [Google Scholar] [CrossRef]
- Suzery, M.; Ningrum, A.N.; Nudin, B.; Mulyani, N.S.; Cahyono, B. Determination of quercetin and rutin in red galangal rhizomes (Alpinia purpurata) and white galangal (Alpinia galanga) with high-performance liquid chromatography method. IOP Conf. Ser. Earth Environ. Sci. 2019, 292, 12–64. [Google Scholar] [CrossRef]
- Kobori, M.; Takahashi, Y.; Akimoto, Y.; Sakurai, M.; Matsunaga, I.; Nishimuro, H.; Ippoushi, K.; Oike, H.; Ohnishi-Kameyama, M. Chronic high intake of quercetin reduces oxidative stress and induces expression of the antioxidant enzymes in the liver and visceral adipose tissues in mice. J. Funct. Foods 2015, 15, 551–560. [Google Scholar] [CrossRef]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: Involvement of p38. Chem. Biol. Interact. 2012, 195, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health benefits of quercetin in age-related diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef] [PubMed]
- García-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar] [CrossRef]
- Ilyasov, I.; Beloborodov, V.; Antonov, D.; Dubrovskaya, A.; Terekhov, R.; Zhevlakova, A.; Saydasheva, A.; Evteev, V.; Selivanova, I. Flavonoids with glutathione antioxidant synergy: Influence of free radicals inflow. Antioxidants 2020, 9, 695. [Google Scholar] [CrossRef]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kürbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef]
- Han, M.K.; Barreto, T.A.; Martinez, F.J.; Comstock, A.T.; Sajjan, U.S. Randomised clinical trial to determine the safety of quercetin supplementation in patients with chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2020, 7, e000392. [Google Scholar] [CrossRef]
- Di Pierro, F.; Khan, A.; Iqtadar, S.; Mumtaz, S.U.; Chaudhry, M.N.A.; Bertuccioli, A.; Derosa, G.; Maffioli, P.; Togni, S.; Riva, A.; et al. Quercetin as a possible complementary agent for early-stage COVID-19: Concluding results of a randomized clinical trial. Front. Pharmacol. 2022, 13, 1096853. [Google Scholar] [CrossRef]
- Pawar, A.; Russo, M.; Rani, I.; Goswami, K.; Russo, G.L.; Pal, A. A critical evaluation of risk to reward ratio of quercetin supplementation for COVID-19 and associated comorbid conditions. Phytother. Res. 2022, 36, 2394–2415. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, J.; Chazarra, S.; Garcia-Carmona, F. Kojic acid, a cosmetic skin whitening agent, is a slow-binding inhibitor of catecholase activity of tyrosinase. J. Pharm. Pharmacol. 1994, 46, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]


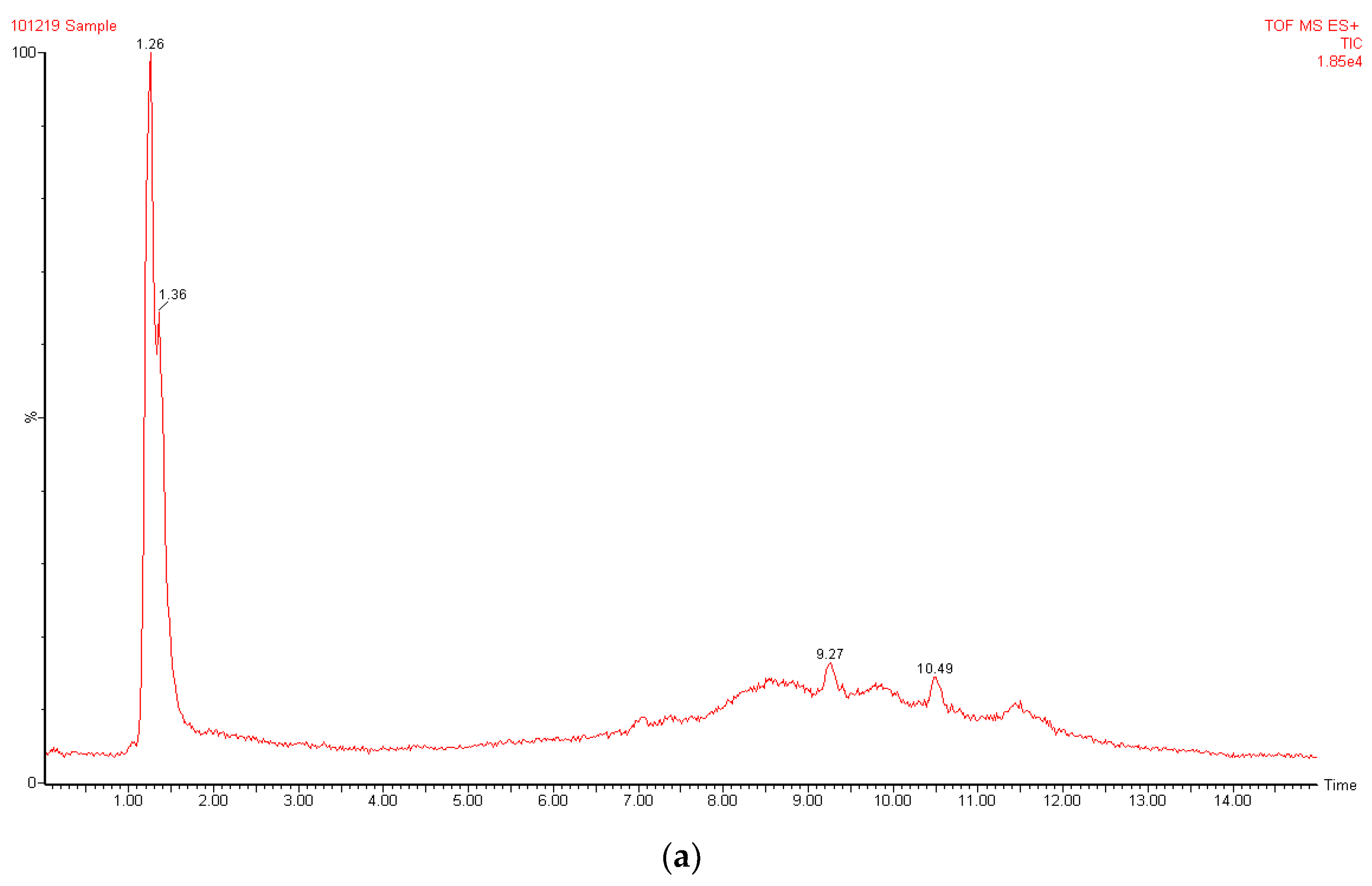
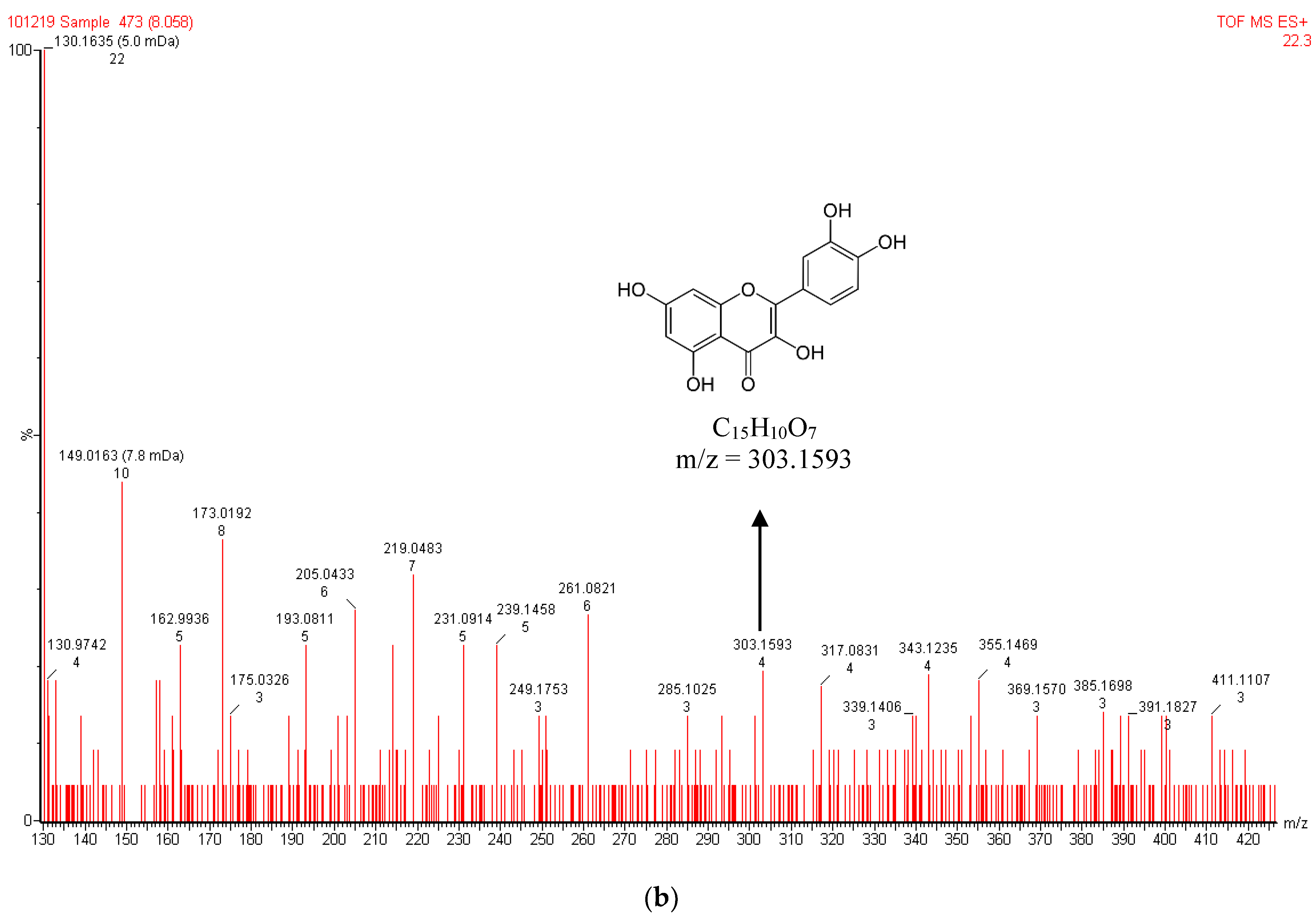
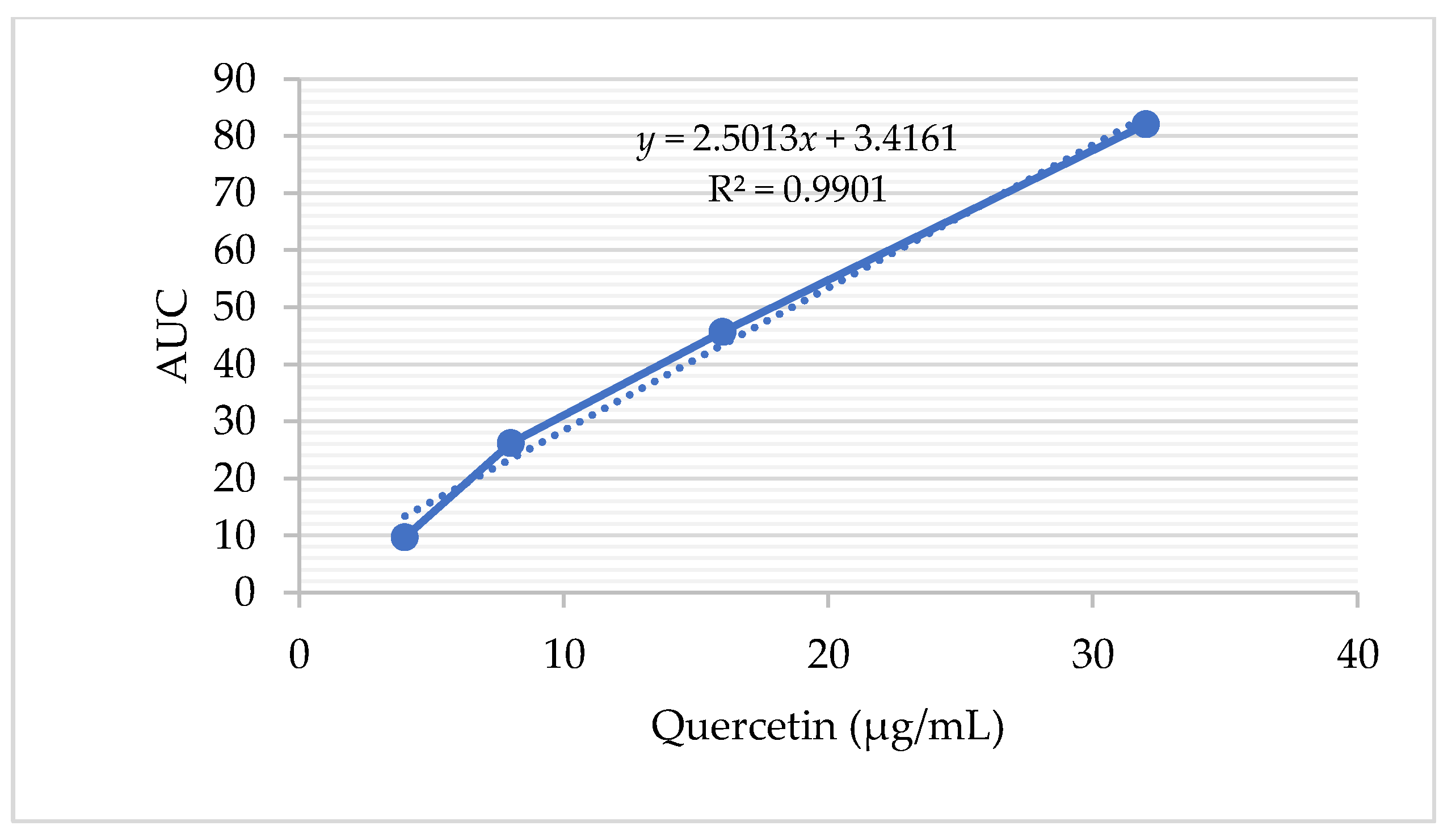


| Tested Plant | Total Phenolics (Equivalent to mg GAE/g Extract) Using Folin–Ciocâlteu (Linear Regression Equation Obtained from the Calibration Graph) | DPPH Radical Scavenging Capacity Presented as an IC50 Value in μg/mL |
|---|---|---|
| H. coronarium rhizome (ethanol extract) | 31.03 (y = 0.0114x + 1.4152 R2 = 0.9933) | 243.40 (weak) |
| C. zedoaria rhizome (ethanol extract) | 17.92 (y = 0.0556x + 0.1594 R2 = 0.9773) | 251.70 (weak) |
| C. zedoaria rhizome (ethyl acetate fraction) | 22.54 (y = 0.0343x + 0.1237 R2 = 0.9936) | 171.86 (weak) |
| C. heyneana rhizome (ethanol extract) | 83.69 (y = 0.0668x + 0.1789 R2 = 0.9945) | 320.00 (weak) |
| C. heyneana rhizome (ethyl acetate fraction) | 90.32 (y = 0.0388x + 0.1897 R2 = 0.9847) | 298.80 (weak) |
| A. galanga rhizome (ethanol extract) | 252.36 (y = 2.5013x + 3.4161 R2 = 0.9901) | 66.67 (moderate) |
| Ascorbic acid | 1.39 (strong) | |
| Quercetin | 0.59 (strong) | |
| Phenol Compound (Ligand) | Binding Affinity in Terms of Docking Score (kcal/mol) | Residues Involved in the Hydrogen Bond Interaction (Distance in Ȧ) | Residues Involved in the Hydrophobic Interaction (Distance in Ȧ) | Close Contact Residues in the Catalytic Site |
|---|---|---|---|---|
| Kaempferol | −7.6 | N/A | Pro201 (3.6–3.7 Å) | Glu158, Gly200, Pro201, His208, Arg209, Gly216, Val 218, Cu501, Cu502 |
| Galangin | −7.1 | Gly216 (1.754 Ȧ) | Asn205 (3.6 Å) His208 (3.9 Å) Gly216 (3.9 Å) Val218 (3.6–3.7 Å) | Asn205, His208, Gly216, Val218, Cu501 |
| Arbutin | −6.5 | Asn205 (2.075 Ȧ) | Pro201 (3.5–3.8 Å) Arg209 (3.6–3.9 Å) | Pro201, His204, Asn205, Arg209, Val218 |
| Ethyl p-methoxy cinnamate | −5.7 | N/A | Pro201 (3.6–3.9 Å) Asn205 (3.6–3.9 Å) Arg209 (3.7–3.9 Å) | Met184, Pro201, Asn205, Arg209 |
| Kojic Acid | −5.6 | N/A | His204 (3.7 Å) His208 (3.6–3.9 Å) Gly216 (3.8 Å) | His204, His208, Cu501, Cu502 |
| 6-Gingerol | −5.4 | N/A | Asn205 (3.6 Å) Arg209 (3.5–3.9 Å) Gly216 (3.7 Å) Val218 (3.6–3.7 Å) | His60, Asn205, His208, Arg209, Gly216, Val218, Cu501 |
| Hydroquinone | −5.4 | Met215 (1.885 Ȧ) | N/A | His60, Asn205, His208, Met215, Val217, Val218, Cu501 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutakin; Saptarini, N.M.; Amalia, R.; Sumiwi, S.A.; Megantara, S.; Saputri, F.A.; Levita, J. Molecular Docking Simulation of Phenolics towards Tyrosinase, Phenolic Content, and Radical Scavenging Activity of Some Zingiberaceae Plant Extracts. Cosmetics 2023, 10, 149. https://doi.org/10.3390/cosmetics10060149
Mutakin, Saptarini NM, Amalia R, Sumiwi SA, Megantara S, Saputri FA, Levita J. Molecular Docking Simulation of Phenolics towards Tyrosinase, Phenolic Content, and Radical Scavenging Activity of Some Zingiberaceae Plant Extracts. Cosmetics. 2023; 10(6):149. https://doi.org/10.3390/cosmetics10060149
Chicago/Turabian StyleMutakin, Nyi Mekar Saptarini, Riezki Amalia, Sri Adi Sumiwi, Sandra Megantara, Febrina Amelia Saputri, and Jutti Levita. 2023. "Molecular Docking Simulation of Phenolics towards Tyrosinase, Phenolic Content, and Radical Scavenging Activity of Some Zingiberaceae Plant Extracts" Cosmetics 10, no. 6: 149. https://doi.org/10.3390/cosmetics10060149
APA StyleMutakin, Saptarini, N. M., Amalia, R., Sumiwi, S. A., Megantara, S., Saputri, F. A., & Levita, J. (2023). Molecular Docking Simulation of Phenolics towards Tyrosinase, Phenolic Content, and Radical Scavenging Activity of Some Zingiberaceae Plant Extracts. Cosmetics, 10(6), 149. https://doi.org/10.3390/cosmetics10060149







