Piperine Extraction and Encapsulation in Polycaprolactone Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Piperine Extraction
2.3. Extract Characterization
2.3.1. Piperine Concentration
2.3.2. Antioxidant Activity
2.3.3. Tannin, Flavonoid and Phenol Concentration
2.4. Extract-Loaded PCL Nanoparticles Formulation
2.5. Nanoparticles Characterization
2.5.1. Nanoparticle’s Size Distribution and Zeta Potential
2.5.2. Nanoparticle’s Morphology
2.5.3. Piperine Encapsulation Efficiency
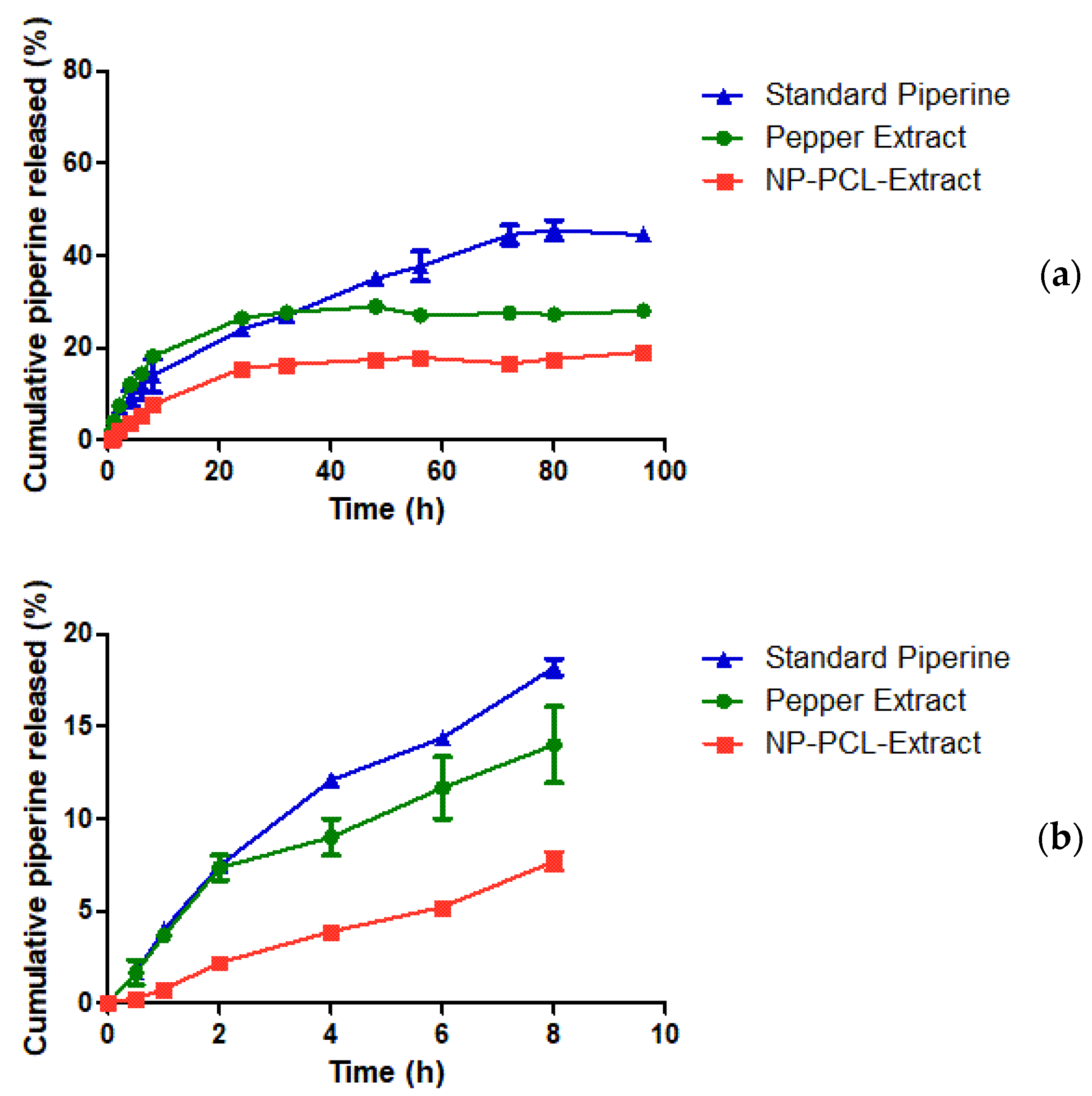
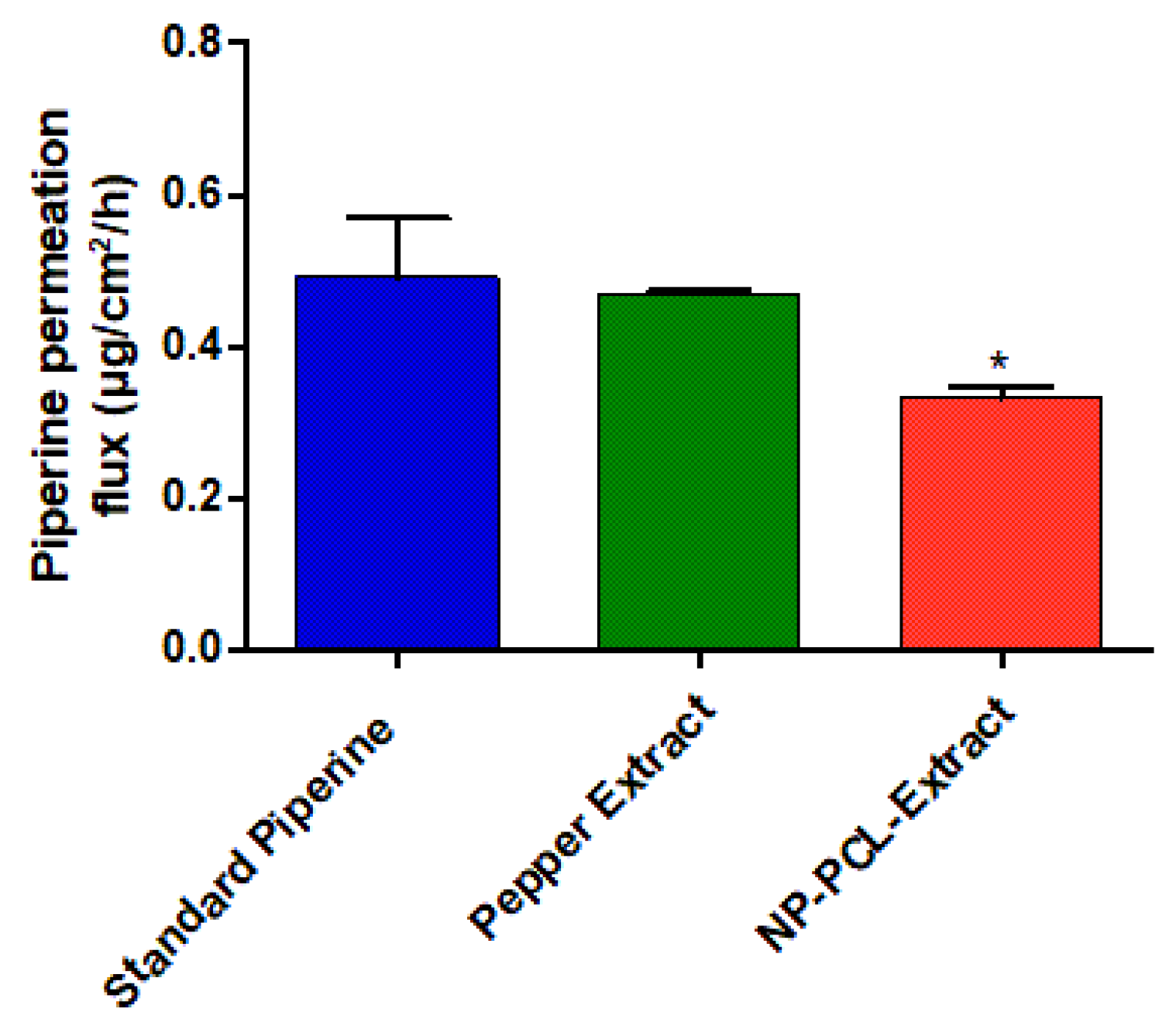
2.5.4. Piperine In Vitro Release
2.5.5. Nanoparticles Stability
2.6. Statistical Analysis
3. Results
3.1. Piperine Concentration
3.2. Antioxidant Activity
3.3. Tannin, Flavonoid and Phenol Concentration
3.4. Extract-Loaded PCL Nanoparticles Formulation
3.5. Piperine In Vitro Release
3.6. Nanoparticles Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia, J.; Kamada, T.; Jacobson, T.; Curado, M.; Oliveira, S. Super ação de dormência em sementes de pimenta-do-reino (Piper nigrum L.). Pesqui. Agropecuária Trop. 2007, 30, 51–54. [Google Scholar] [CrossRef]
- Pissinate, K. Atividade Citotóxica de Piper Nigrum e Struthanthusmarginatus. Estudo Preliminar da Correlação Entre a Citotoxicidade e Hidrofobicidade da Piperina e Derivados Sintéticos; Universidade Federal Rural do Rio de Janeiro: Rio de Janeiro, Brazil, 2006. [Google Scholar]
- Mailoo, V. The Ayurvedic Model of Human Occupation. Asian J. Occup. Ther. 2007, 6, 1–13. [Google Scholar] [CrossRef]
- Singletary, K. Black Pepper: Overview of Health Benefits. Nutr. Today 2010, 45, 43–47. [Google Scholar] [CrossRef]
- Houcine, B.; Romdhane, M.; Souchard, J.-P.; Cazaux, S.; Bouajila, J. Chemical Composition and Anticancer and Antioxidant Activities of Schinus Molle, L. and Schinus Terebinthifolius Raddi Berries Essential Oils. J. Food Sci. 2010, 75, C466–C472. [Google Scholar] [CrossRef]
- Stępniowska, A.; Cieplińska, P.; Fac, W.; Górska, J. Selected Alkaloids Used in the Cosmetics Industry. J. Cosmet. Sci. 2021, 72, 229–245. [Google Scholar]
- Abbasi, B.; Ahmad, N.; Fazal, H.; Mahmood, T. Conventional and modern propagation techniques in Piper nigrum. J. Med. Plants Res. 2010, 4, 7–12. [Google Scholar]
- Gupta, S.K.; Bansal, P.; Bhardwaj, R.K.; Velpandian, T. Comparative anti-nociceptive, anti-inflammatory and toxicity profile of nimesulide vs nimesulide and piperine combination. Pharmacol. Res. 2000, 41, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Masood Ahmed Chaudhry, N.; Tariq, P. Bactericidal activity of black pepper, bay leaf, aniseed and coriander against oral isolates. Pak. J. Pharm. Sci. 2006, 19, 214–218. [Google Scholar]
- Mittal, R.; Gupta, R. In vitro antioxidant activity of piperine. Methods Find. Exp. Clin. Pharmacol. 2000, 22, 271–274. [Google Scholar] [CrossRef]
- Parmar, V.; Jain, S.; Bisht, K.; Jain, R.; Taneja, P.; Jha, A.; Tyagi, O.; Prasad, A.; Wengel, J.; Olsen, C.; et al. Phytochemistry of genus Piper. Phytochemistry 1997, 46, 597–673. [Google Scholar] [CrossRef]
- Verma, A.; Kushwaha, H.N.; Srivastava, A.K.; Srivastava, S.; Jamal, N.; Srivastava, K.; Ray, R.S. Piperine attenuates UV-R induced cell damage in human keratinocytes via NF-kB, Bax/Bcl-2 pathway: An application for photoprotection. J. Photochem. Photobiol. B Biol. 2017, 172, 139–148. [Google Scholar] [CrossRef]
- Faas, L.; Venkatasamy, R.; Hider, R.C.; Young, A.R.; Soumyanath, A. In vivo evaluation of piperine and synthetic analogues as potential treatments for vitiligo using a sparsely pigmented mouse model. Br. J. Dermatol. 2008, 158, 941–950. [Google Scholar] [CrossRef]
- Mao, Y.; Xu, Z.; Song, J.; Xie, Y.; Mei, X.; Shi, W. Efficacy of a mixed preparation containing piperine, capsaicin and curcumin in the treatment of alopecia areata. J. Cosmet. Dermatol. 2022, 21, 4510–4514. [Google Scholar] [CrossRef]
- Ye, Y.-J.; Wang, Y.; Lou, K.; Chen, Y.-Z.; Chen, R. The preparation, characterization, and pharmacokinetic studies of chitosan nanoparticles loaded with paclitaxel/dimethyl-β-cyclodextrin inclusion complexes. Int. J. Nanomed. 2015, 10, 4309–4319. [Google Scholar] [CrossRef]
- Ataide, J.A.; Coco, J.C.; dos Santos, É.M.; Beraldo-Araujo, V.; Silva, J.R.A.; de Castro, K.C.; Lopes, A.M.; Filipczak, N.; Yalamarty, S.S.K.; Torchilin, V.P.; et al. Co-Encapsulation of Drugs for Topical Application-A Review. Molecules 2023, 28, 1449. [Google Scholar] [CrossRef] [PubMed]
- Politi, F.A.S.; Carvalho, S.G.; Rodero, C.F.; dos Santos, K.P.; Meneguin, A.B.; Sorrechia, R.; Chiavacci, L.A.; Chorilli, M. Piperine-loaded nanoparticles incorporated into hyaluronic acid/sodium alginate-based membranes for the treatment of inflammatory skin diseases. Int. J. Biol. Macromol. 2023, 227, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Budama-Kilinc, Y. Piperine Nanoparticles for Topical Application: Preparation, Characterization, In vitro and In silico Evaluation. ChemistrySelect 2019, 4, 11693–11700. [Google Scholar] [CrossRef]
- Ozkan, B.; Altuntas, E.; Cakir Koc, R.; Budama-Kilinc, Y. Development of piperine nanoemulsions: An alternative topical application for hypopigmentation. Drug Dev. Ind. Pharm. 2022, 48, 117–127. [Google Scholar] [CrossRef]
- Alshehri, S.; Bukhari, S.I.; Imam, S.S.; Hussain, A.; Alghaith, A.F.; Altamimi, M.A.; AlAbdulkarim, A.S.; Almurshedi, A. Formulation of Piperine-Loaded Nanoemulsion: In Vitro Characterization, Ex Vivo Evaluation, and Cell Viability Assessment. ACS Omega 2023, 8, 22406–22413. [Google Scholar] [CrossRef]
- Kaur, J.; Singh, R.R.; Khan, E.; Kumar, A.; Joshi, A. Piperine-Loaded PLGA Nanoparticles as Cancer Drug Carriers. ACS Appl. Nano Mater. 2021, 4, 14197–14207. [Google Scholar] [CrossRef]
- Kazmi, I.; Al-Abbasi, F.A.; Imam, S.S.; Afzal, M.; Nadeem, M.S.; Altayb, H.N.; Alshehri, S. Formulation of Piperine Nanoparticles: In Vitro Breast Cancer Cell Line and In Vivo Evaluation. Polymers 2022, 14, 1349. [Google Scholar] [CrossRef]
- Alvarez-Román, R.; Naik, A.; Kalia, Y.N.; Guy, R.H.; Fessi, H. Skin penetration and distribution of polymeric nanoparticles. J. Control. Release 2004, 99, 53–62. [Google Scholar] [CrossRef]
- Leroueil-Le Verger, M.; Fluckiger, L.; Kim, Y.-I.; Hoffman, M.; Maincent, P. Preparation and characterization of nanoparticles containing an antihypertensive agent. Eur. J. Pharm. Biopharm. 1998, 46, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ϵ-caprolactone microspheres and nanospheres: An overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.; Laurencin, C. Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Tundisi, L.L.; Ataide, J.A.; da Fonseca, J.H.L.; Silvério, L.A.L.; Lancellotti, M.; Paiva-Santos, A.C.; d’Ávila, M.A.; Kohane, D.S.; Mazzola, P.G. Terbinafine Nanohybrid: Proposing a Hydrogel Carrying Nanoparticles for Topical Release. Pharmaceutics 2023, 15, 841. [Google Scholar] [CrossRef]
- Babadi, D.; Dadashzadeh, S.; Shahsavari, Z.; Shahhosseini, S.; ten Hagen, T.L.M.; Haeri, A. Piperine-loaded electrospun nanofibers, an implantable anticancer controlled delivery system for postsurgical breast cancer treatment. Int. J. Pharm. 2022, 624, 121990. [Google Scholar] [CrossRef]
- Jain, S.; Meka, S.R.K.; Chatterjee, K. Engineering a Piperine Eluting Nanofibrous Patch for Cancer Treatment. ACS Biomater. Sci. Eng. 2016, 2, 1376–1385. [Google Scholar] [CrossRef]
- Alvarez-Román, R.; Barré, G.; Guy, R.H.; Fessi, H. Biodegradable polymer nanocapsules containing a sunscreen agent: Preparation and photoprotection. Eur. J. Pharm. Biopharm. 2001, 52, 191–195. [Google Scholar] [CrossRef]
- Javaid, S.; Ahmad, N.M.; Mahmood, A.; Nasir, H.; Iqbal, M.; Ahmad, N.; Irshad, S. Cefotaxime Loaded Polycaprolactone Based Polymeric Nanoparticles with Antifouling Properties for In-Vitro Drug Release Applications. Polymers 2021, 13, 2180. [Google Scholar] [CrossRef] [PubMed]
- Kondiah, P.P.D.; Rants’o, T.A.; Mdanda, S.; Mohlomi, L.M.; Choonara, Y.E. A Poly(Caprolactone)-Cellulose Nanocomposite Hydrogel for Transdermal Delivery of Hydrocortisone in Treating Psoriasis Vulgaris. Polymers 2022, 14, 2633. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Mikołajczyk, A.; Błasiak, E.; Fic, E.; Dziedzicka-Wasylewska, M. Polycaprolactone Nanoparticles as Promising Candidates for Nanocarriers in Novel Nanomedicines. Pharmaceutics 2021, 13, 191. [Google Scholar] [CrossRef]
- El Yousfi, R.; Brahmi, M.; Dalli, M.; Achalhi, N.; Azougagh, O.; Tahani, A.; Touzani, R.; El Idrissi, A. Recent Advances in Nanoparticle Development for Drug Delivery: A Comprehensive Review of Polycaprolactone-Based Multi-Arm Architectures. Polymers 2023, 15, 1835. [Google Scholar] [CrossRef] [PubMed]
- Pawar, R.; Pathan, A.; Nagaraj, S.; Kapare, H.; Giram, P.; Wavhale, R. Polycaprolactone and its derivatives for drug delivery. Polym. Adv. Technol. 2023, 34, 3296–3316. [Google Scholar] [CrossRef]
- Bhairy, S.; Shaikh, A.; Nalawade, V.; Hirlekar, R. Development and validation of bivariate UV-visible spectroscopic method for simultaneous estimation of curcumin and piperine in their combined nanoparticulate system. J. Appl. Pharm. Sci. 2021, 11, 064–070. [Google Scholar]
- Pires, J.; Torres, P.B.; Santos, D.Y.A.C.d.; Chow, F. Ensaio em microplaca do potencial antioxidante através do método de sequestro do radical livre DPPH para extratos de algas. Inst. Biociências Univ. São Paulo 2017, 12, 1–6. [Google Scholar]
- Urrea-Victoria, V.; Pires, J.; Torres, P.B.; Alves, D.Y.; Santos, C.d.; Chow, F. Ensaio Antioxidante em Microplaca do Poder de Redução do Ferro (FRAP) Para Extratos de Algas; Instituto de Biociências, Universidade de São Paulo: São Paulo, Brazil, 2016; pp. 1–6. ISBN 978-85-85658-62-5. [Google Scholar]
- Shad, M.; Nawaz, H.; Rehman, T.; Badar, H.; Hussain, M. Optimization of extraction efficiency of tannins from Cichorium intybus L.: Application of response surface methodology. J. Med. Plant Res. 2012, 6, 4467–4474. [Google Scholar]
- Alves, E.; Kubota, E.H. Conteúdo de fenólicos, flavonoides totais e atividade antioxidante de amostras de própolis comerciais. Rev. Ciências Farm. Básica E Apl. 2013, 34, 37–41. [Google Scholar]
- Caetano, K.; Ceotto, J.; Ribeiro, A.; de Morais, F.; Ferrari, R.; Pacheco, M.; Capitani, C.; Caetano, K.A.; Ceotto, J.M.; Badan Ribeiro, A.P.; et al. Effect of baru (Dipteryx alata Vog.) addition on the composition and nutritional quality of cookies. Food Sci. Technol. 2017, 37, 239–245. [Google Scholar] [CrossRef]
- Massella, D.; Leone, F.; Peila, R.; Barresi, A.A.; Ferri, A. Functionalization of Cotton Fabrics with Polycaprolactone Nanoparticles for Transdermal Release of Melatonin. J. Funct. Biomater. 2018, 9, 1. [Google Scholar] [CrossRef]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Sequential Microwave-Ultrasound-Assisted Extraction for Isolation of Piperine from Black Pepper (Piper nigrum L.). Food Bioprocess Technol. 2017, 10, 2199–2207. [Google Scholar] [CrossRef]
- Yu, L.; Hu, X.; Xu, R.; Ba, Y.; Chen, X.; Wang, X.; Cao, B.; Wu, X. Amide alkaloids characterization and neuroprotective properties of Piper nigrum L.: A comparative study with fruits, pericarp, stalks and leaves. Food Chem. 2022, 368, 130832. [Google Scholar] [CrossRef] [PubMed]
- Narendra Babu, K.; Hemalatha, R.; Satyanarayana, U.; Shujauddin, M.; Himaja, N.; Bhaskarachary, K.; Dinesh Kumar, B. Phytochemicals, polyphenols, prebiotic effect of Ocimum sanctum, Zingiber officinale, Piper nigrum extracts. J. Herb. Med. 2018, 13, 42–51. [Google Scholar] [CrossRef]
- Grinevicius, V.M.A.S.; Andrade, K.S.; Ourique, F.; Micke, G.A.; Ferreira, S.R.S.; Pedrosa, R.C. Antitumor activity of conventional and supercritical extracts from Piper nigrum L. cultivar Bragantina through cell cycle arrest and apoptosis induction. J. Supercrit. Fluids 2017, 128, 94–101. [Google Scholar] [CrossRef]
- Subramanian, R.; Subbramaniyan, P.; Noorul Ameen, J.; Raj, V. Double bypasses soxhlet apparatus for extraction of piperine from Piper nigrum. Arab. J. Chem. 2016, 9, S537–S540. [Google Scholar] [CrossRef]
- Vieira, L.V.; Juvenato, M.M.E.; Krause, M.; Heringer, O.A.; Ribeiro, J.S.; Brandão, G.P.; Kuster, R.M.; Carneiro, M.T.W.D. The effects of drying methods and harvest season on piperine, essential oil composition, and multi-elemental composition of black pepper. Food Chem. 2022, 390, 133148. [Google Scholar] [CrossRef]
- Gülçin, İ. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int. J. Food Sci. Nutr. 2005, 56, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Onyesife, C.O.; Chukwuma, I.F.; Okagu, I.U.; Ndefo, J.C.; Amujiri, N.A.; Ogugua, V.N. Nephroprotective effects of Piper nigrum extracts against monosodium glutamate-induced renal toxicity in rats. Sci. Afr. 2023, 19, e01453. [Google Scholar] [CrossRef]
- Tedasen, A.; Khoka, A.; Madla, S.; Sriwiriyajan, S.; Graidist, P. Anticancer effects of piperine-free Piper nigrum extract on cholangiocarcinoma cell lines. Pharmacogn. Mag. 2020, 16, S28–S38. [Google Scholar]
- Saha, S.; Verma, R.J. In vitro and in silico study of Piper nigrum on cyclooxygenase-2, inducible nitric oxide synthase and antioxidant enzymes. J. Herb. Med. 2015, 5, 86–98. [Google Scholar] [CrossRef]
- Cortés, H.; Hernández-Parra, H.; Bernal-Chávez, S.A.; Prado-Audelo, M.L.D.; Caballero-Florán, I.H.; Borbolla-Jiménez, F.V.; González-Torres, M.; Magaña, J.J.; Leyva-Gómez, G. Non-Ionic Surfactants for Stabilization of Polymeric Nanoparticles for Biomedical Uses. Materials 2021, 14, 3197. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.M.; Jäger, E.; Jäger, A.; Stepánek, P.; Giacomelli, F.C. Physicochemical aspects behind the size of biodegradable polymeric nanoparticles: A step forward. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 1092–1102. [Google Scholar] [CrossRef]
- Bell, N.C.; Minelli, C.; Tompkins, J.; Stevens, M.M.; Shard, A.G. Emerging Techniques for Submicrometer Particle Sizing Applied to Stöber Silica. Langmuir 2012, 28, 10860–10872. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Bolzinger, M.-A.; Briançon, S.; Chevalier, Y. Nanoparticles through the skin: Managing conflicting results of inorganic and organic particles in cosmetics and pharmaceutics. WIREs Nanomed. Nanobiotechnol. 2011, 3, 463–478. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Bogatyrov, V.; Ostolska, I.; Szewczuk-Karpisz, K.; Terpiłowski, K.; Nosal-Wiercińska, A. Impact of poly(vinyl alcohol) adsorption on the surface characteristics of mixed oxide MnxOy–SiO2. Adsorption 2016, 22, 417–423. [Google Scholar] [CrossRef]
- Baspinar, Y.; Üstündas, M.; Bayraktar, O.; Sezgin, C. Curcumin and piperine loaded zein-chitosan nanoparticles: Development and in-vitro characterisation. Saudi Pharm. J. 2018, 26, 323–334. [Google Scholar] [CrossRef]
- Li, S.; Guo, J.; Tian, Z.; Chen, J.; Gou, G.; Niu, Y.; Li, L.; Yang, J. Piperine-Loaded Glycyrrhizic Acid- and PLGA-Based Nanoparticles Modified with Transferrin for Antitumor. AAPS PharmSciTech 2021, 22, 239. [Google Scholar] [CrossRef]
- Hashemi, S.; Mortazavi, S.A.; Moghimi, H.R.; Darbasizadeh, B. Development and evaluation of a novel methotrexate-loaded electrospun patch to alleviate psoriasis plaques. Drug Dev. Ind. Pharm. 2022, 48, 355–366. [Google Scholar] [CrossRef]
- de Oliveira, J.G.; Pilz-Júnior, H.L.; de Lemos, A.B.; da Silva da Costa, F.A.; Fernandes, M.; Gonçalves, D.Z.; Variza, P.F.; de Moraes, F.M.; Morisso, F.D.P.; Magnago, R.F.; et al. Polymer-based nanostructures loaded with piperine as a platform to improve the larvicidal activity against Aedes aegypti. Acta Trop. 2022, 230, 106395. [Google Scholar] [CrossRef]

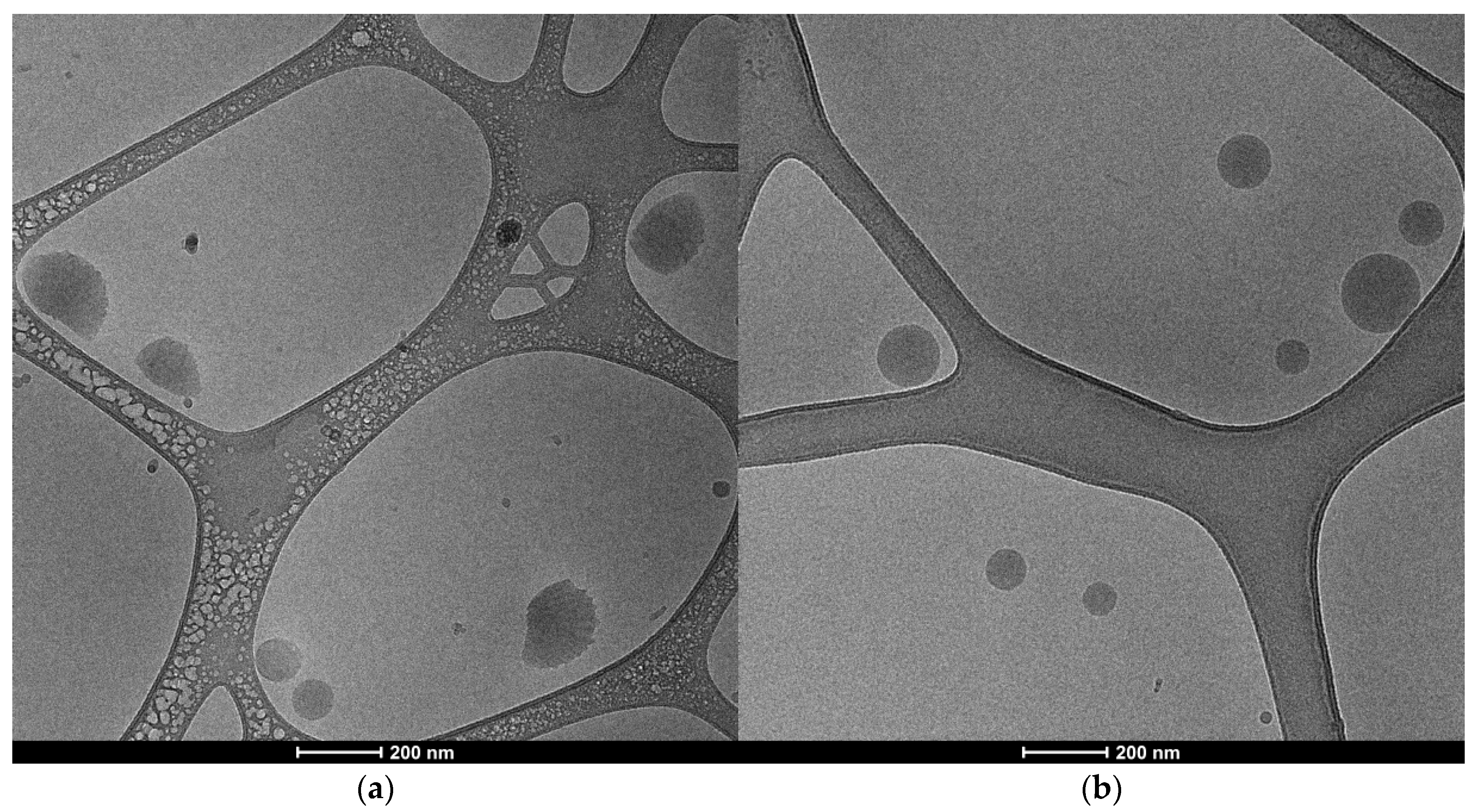


| Grain | Grain Powder | |
|---|---|---|
| Tannins (mg TAE/L) | 0.96 ± 0.28 | 20.57 ± 3.63 |
| Flavonoids (mg QE/g sample) | 313.18 ± 6.06 | 418.50 ± 24.83 |
| Total phenolics (mg GAE/g sample) | 0.36 ± 0.01 | 1.0 ± 0.2 |
| NP-PCL | NP-PCL-Extract | |
|---|---|---|
| Mean diameter (nm) * | 391.1 ± 6.4 | 374.2 ± 1.5 |
| Polydispersity index | 0.05 ± 0.04 | 0.07 ± 0.01 |
| D10 * | 285.0 ± 5.1 | 271.0 ± 1.2 |
| D50 | 407.0 ± 11.1 | 392.0 ± 1.2 |
| D90 | 583.0 ± 36.4 | 575.0 ± 5.29 |
| Zeta potential (mV) | −1.06 ± 0.02 | −1.17 ± 0.26 |
| NP-PCL | NP-PCL-Extract | |
|---|---|---|
| Mean diameter (nm) | 220.9 ± 10.6 | 221.0 ± 5.9 |
| D10 | 155.7 ± 18.8 | 171.4 ± 5.0 |
| D50 | 224.6 ± 8.2 | 215.1 ± 6.0 |
| D90 | 279.7 ± 8.2 | 270.6 ± 9.2 |
| Concentration (particles/mL) * | 9.5 × 1011 ± 7.1 × 1010 | 3.1 × 1011 ± 2.5 × 1010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coco, J.C.; Silvério, L.A.L.; Santos, É.M.d.; Sueiro, A.C.; Ataide, J.A.; Paiva-Santos, A.C.; Mazzola, P.G. Piperine Extraction and Encapsulation in Polycaprolactone Nanoparticles. Cosmetics 2023, 10, 152. https://doi.org/10.3390/cosmetics10060152
Coco JC, Silvério LAL, Santos ÉMd, Sueiro AC, Ataide JA, Paiva-Santos AC, Mazzola PG. Piperine Extraction and Encapsulation in Polycaprolactone Nanoparticles. Cosmetics. 2023; 10(6):152. https://doi.org/10.3390/cosmetics10060152
Chicago/Turabian StyleCoco, Julia Cedran, Luiza Aparecida Luna Silvério, Érica Mendes dos Santos, Ana Claudia Sueiro, Janaína Artem Ataide, Ana Cláudia Paiva-Santos, and Priscila Gava Mazzola. 2023. "Piperine Extraction and Encapsulation in Polycaprolactone Nanoparticles" Cosmetics 10, no. 6: 152. https://doi.org/10.3390/cosmetics10060152
APA StyleCoco, J. C., Silvério, L. A. L., Santos, É. M. d., Sueiro, A. C., Ataide, J. A., Paiva-Santos, A. C., & Mazzola, P. G. (2023). Piperine Extraction and Encapsulation in Polycaprolactone Nanoparticles. Cosmetics, 10(6), 152. https://doi.org/10.3390/cosmetics10060152









