1. Introduction
Hair fibers comprise a fully formed dehydrated cuticle, a cortex, and sometimes a medulla [
1]. The cuticle is a chemically resistant area surrounding the cortex and typically consists of 6–8 layers. The cortex consists of cells and cell membrane complexes (CMCs). Generally, thicker hairs contain more than one loosely packed porous region, called the medulla, located near the center of the hair [
1]. These multiple porous regions appear spherical and hollow inside a series of vacuoles along the fiber axis. Medullation exhibits a positive relationship with hair diameter but not age [
2]. The medulla appears more frequently in the thick hair of Asians than in Caucasians and more often in beards than in scalp hair.
The CMC is found at the interface of the cuticle and cuticle, cuticle and cortex, and between the cortex and medulla [
3]. In addition, medullary cells bind to each other via CMC-type substances within the medulla. The fact that the CMC is sufficiently damaged by oxidation and the medulla is damaged by stimuli such as oxidation indicates that the composition of the medulla is largely related to the CMC [
4]. The CMC comprises two five-nanometer layers called beta layers with a 15 nm delta layer between them. The composition and bonding state of the beta layer are not yet clear; however, lipids and amino acids are known to be present [
1,
4]. Because it is difficult to separate the delta layer in the CMC, the composition of the delta layer is still unclear, but it is known to contain protein [
1].
Cuticle lifting is caused by reducing or oxidizing agents, such as permanent wave or bleaching treatments [
5,
6]. UV irradiation also causes cuticle lifting [
7]. When combing or brushing, the cuticles bend and stretch, causing them to lift or clump. Cuticle lifting is mainly caused by the formation of a hydrophobic surfactant layer owing to surfactant penetration into the cuticle and CMC [
8]. This cuticle lifting phenomenon leads to the adhesion failure of the CMC [
1].
Defects in the medulla have been discovered in studies through use of light microscopy imaging, whereby damage to the medulla indicates the presence of internal pores [
3,
9,
10,
11,
12]. In SEM images, the medulla of broken hair appears as hollow spheres rather than simple pores [
13]. Because the medulla has lower hydroxyl amino acid and high carboxylic acid contents compared to other regions, it is likely to have a high affinity for metals, acids, and alkalis. The medulla is more susceptible to ion exchange reactions, such as those with anionic and cationic surfactants [
4,
14]. Theoretically, the medulla is susceptible to surfactants; however, the actual effects have not yet been experimentally demonstrated.
Pores form inside hair due to oxidative damage or heat, and as the damage becomes more severe, the volume and size of the pores increase [
15]. These internal pores are also observed in SEM images [
9,
16] and Raman spectral images [
17]. Meanwhile, in the case of optical microscopy, pores inside hair can be easily discovered using a simple method because light is scattered due to changes in the refractive index of the medium. When light is shone on the hair under a microscope in reflection mode, the pores inside the hair appear bright due to scattering [
18,
19], and when light is shone through the microscope in transmission mode, the pores inside the hair appear dark [
11,
20]. It is interpreted that when the vacuoles that form the medulla are damaged, the internal pores form a porous-like structure [
11].
Previously, we found that surfactants cause the loss of lipids in hair [
21]. Additionally, hair protein loss occurs when hair is washed with surfactants at room temperature, and to a lesser extent, protein loss also occurs when hair is washed with water [
22]. Until now, it was thought that pores occur due to oxidative damage, heat, disease, etc. [
11], but this study revealed for the first time that pores occur when hair is washed with a surfactant.
This study focused on observing cuticle lifting and changes in medullary cells through the effects of surfactants on structural changes in hair. By observing the pores of the medulla, we studied the extent to which the surfactant damaged the hair, depending on the degree of cuticle lifting. In addition, surfactant-induced pore formation can be prevented by cuticle sealing, which reduces cuticle lifting.
2. Materials and Methods
2.1. Materials
The Chinese hair swatches used in this study were commercial samples purchased from Bulex (Happy Call, Seoul, Republic of Korea). Glycine, arginine, alanine, methionine, phenylalanine, and tyrosine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium laureth sulfate (SLES) was obtained from LG H&H. Hydrogenated castor oil/sebacic acid (CSA) copolymer was purchased from Croda (Princeton, NJ, USA). Acrylamidopropyl trimonium chloride/acrylamide copolymer (ATC) was commercially obtained from Ashland (Wilmington, NC, USA) as n-durHance AA-2000. Polyethyleneimine was obtained from Nippon Shokubai (Osaka, Japan). Basic Brown 16 (BB 16), Basic Brown 99 (BB 99), Basic Red 51 (BR 51), Basic Yellow 87 (BY 87), Basic Yellow, and Acid Violet 43 (AV43) dyes were purchased from Sensient Beauty (Saint Ouen L’Aumone, France).
2.2. Analysis of the Internal Density of Hair
Images of the hair pores were obtained using an Olympus BX53M polarizing microscope. When calculating the black area, the black area at the edge of the hair was not counted because it is an area where light does not reach the detector. The images were imported into ImageJ as 8-bit grayscale stacks. Porosity analyses of the images were performed at equal brightness. To ensure that the focus of the hair images captured under the microscope was uniform, we created and tested a sample holder that could capture images while the hair was pulled tightly. Using the sample holder, images were captured at the same location, even after washing, and the location was rechecked based on the original porous point to secure the same location.
2.3. Treatment of Hair and Surface Modification
The hair swatch was prewashed with 10% SLES solution. To determine the effect of the surfactants on the pores of the hair caused by washing, the hair was threaded into the cylinder at 3-cm intervals and fixed. The hair was immersed in 10% SLES aqueous solution for 10 s and then rinsed in running water for 30 s (n = 9).
To check amino acid penetration, the hair was soaked in 1% amino acid solution for 15 min. Afterwards, the hair was lightly wiped with a paper towel and dried naturally. When sectioning the hair, the hair was cut with a medical knife (Microtome Blade S35, Feather, Osaka, Japan) without embedding.
During one experiment to classify or control cuticle lifting, 12 hair fibers were prepared and tested. Twelve images of different parts of one hair were obtained, and a total of three experiments were performed. To coat the hair with CSA, ATC, or polyethyleneimine, the hair was left in 5% targeting polymer solution for 15 min, lightly rinsed with water, and dried naturally (n = 36).
2.4. Hair Gloss Measurement
The SAMBA Hair System (Bossa Nova, Los Angeles, CA, USA) was used to measure hair gloss. For gloss measurement, 4 g hair samples were used, and the measured image was transferred to SAMBA software without additional editing to calculate the gloss value. The
LBNT representing the gloss was obtained through Equation (1).
where
W is the average width of the specular band and
S and
D are the integral values from the specular and diffused profiles, respectively [
23].
2.5. SEM
The extent of cuticle lifting was confirmed using scanning electron microscopy (SEM). The SEM images of the hair were obtained using a Hitachi S 4800 instrument (Hitachi, Tokyo, Japan). Based on the SEM images, the hair was classified based on the degree of cuticle lifting. To classify the hair using SEM, numerous hair strands were randomly selected from a 4 g sample of hair and many photographs were taken.
2.6. AFM
To confirm cuticle sealing, hair topography was evaluated using atomic force microscopy (AFM, XE-100, Park Systems, Suwon, Republic of Korea) in contact mode using an NSC36C cantilever (San Jose, CA, USA). The cantilever had a typical spring constant of 0.6 N·m−1 and resonant frequency of 65 kHz. The cuticle edge portion of a total of three hairs was scanned in contact mode.
2.7. Contact Angle Measurement
Static contact angle measurements were recorded at room temperature using a Sanyo camera with an FTA 32 (version 2.0). Ten strands of hair fibers were attached in parallel using adhesive tape and photographed using a DSA-100 (Krüss, Hamburg, Germany). The contact angle was measured after 10 s of water loading onto the hair fibers. For the contact angle experiment, the value was recorded 10 s after applying the water drop.
2.8. Color Measurement
Hair color can be expressed using the Commission Internationale de l’Éclairage (CIE) Lab system, in which any color can be described using three values: L refers to lightness, a refers to the red/green ratio, and b refers to the yellow/blue ratio. The CIE Lab value of hair color change was measured using a spectrophotometer (Labscan XE, HunterLab, Reston, Virginia VA, USA). All experiments were averaged over three repetitive cycles. Each test was repeated using three separate hair swatches. The color difference between the original hair and treated hair was calculated based on the CIE total color difference formula, ∆E = [(∆L)2 + (∆a)2 + (∆b)2]1/2.
2.9. The Degree of Cuticle Lifting
To quantify the degree of cuticle lifting, a single hair analyzer for damage assessment (IKLab Inc., Siheung, Republic of Korea) was used. This is a custom-made commercial device that can determine the degree of cuticle lifting based on the degree of light reflection, and detailed information is provided elsewhere [
24]. This is a device that fixes hair and shines light on the hair to determine which part of the hair shines brighter than the surrounding area through numerical analysis.
3. Results
3.1. Surfactant Washing Decreases the Internal Density inside the Hair
To determine whether surfactants cause structural changes in the hair, internal images of the hair that changed depending on the number of washes were observed using an optical microscope. The hair was measured under a microscope using a sample holder that could be held in the same position before and after washing the hair.
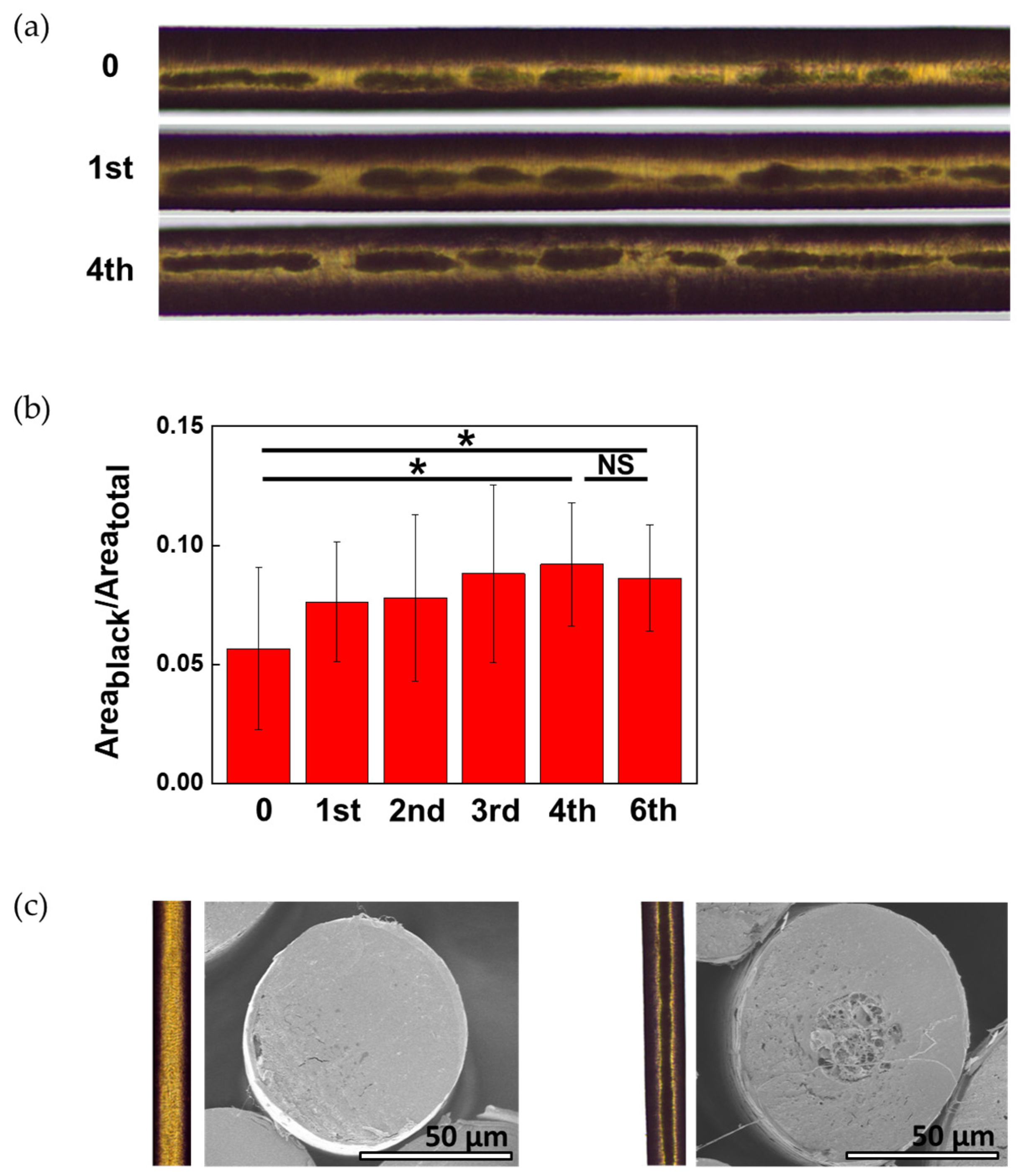
Figure 1a shows transmission images of the inside of the hair taken by focusing the light on the inside of the hair in transmission mode. Because hair is circular, the transmitted light at the sides of the hair is refracted and appears black (the light does not reach the senses). The black area in the middle is believed to be a porous structure formed when the vacuoles inside the hair are damaged [
11]. There were cases where new black areas were created in parts that did not initially have black areas after washing. In the areas where black areas existed from the beginning, the black areas became thicker as the number of washes increased.
In
Figure 1a, the boundary of the black area is unclear. As the number of washings increased, the boundary of the black area became clearer.
Figure 1b shows the digitized area obtained by ImageJ. The black areas significantly increased after up to four washes. After washing the hair four times, there was no significant difference in the black area even with additional washing.
When the experiment was conducted using only water without a surfactant, black areas were not generated; however, the number of black areas was reduced. Therefore, the black areas shown in
Figure 1 were caused by surfactants.
Images were taken with SEM to establish what the black areas inside real hair look like. Due to limitations in cross-sectional technology, cross-sections of hair without black areas and hair with a black area in the middle of the hair were photographed using SEM. Unlike the cross-section of hair without a black area in the center of the hair, pores were found in the cross-section of hair with a black area.
We determined whether the black areas disappeared when the interior was filled, as shown in
Figure 2. When hair is filled with a material, determining whether it is actually filled is very difficult using a mass spectrometer because the amount of filling is small compared to the total amount of hair. The degree of hair filling affects hair glossiness [
25]. To confirm whether the hair interior was filled, amino acids with different refractive indices were used. When the refractive index changes owing to the material being filled inside, the gloss of the hair also changes.
The correlation between the black area and hair gloss was investigated to confirm whether the black area inside the hair was related to material defects inside the hair. The refractive index of hair is 1.55 [
26], and amino acids with higher refractive indices than hair, such as glycine, arginine, alanine, methionine, phenylalanine, and tyrosine, were used in the experiment [
27]. The glossiness values with the refractive index are shown in
Figure 2, along with the molecular weight.
As shown in
Figure 2, the gloss of hair soaked in glycine, which has a high refractive index, was evaluated as being the highest. However, in the case of amino acids with a high refractive index but high molecular weight, no change in the black area was observed. Phenylalanine and methionine did not reduce black areas and increase the shine content. These amino acids have high molecular weights, making it difficult for them to penetrate hair. In the case of glycine, it was surprising that the black areas disappeared after immersion (
Figure 3b).
These results suggest that the black areas form as the components inside the hair escape. Therefore, these black areas result from a decrease in the internal density of the hair. These appear to represent pores inside the hair.
3.2. Loss of Internal Hair Components through the CMC
The absorbed molecules penetrate the hair through the CMC at the entrance of the cuticle [
28,
29]. Assuming that the release of internal components due to surfactant washing also occurs through the CMC, the degree to which pores form after surfactant washing may vary depending on the state of the CMC. Because the CMC is located between the cuticle and cuticle and between the cuticle and cortex, the condition of the CMC is inevitably affected by cuticle lifting.
Using SEM, the hairs were divided into four groups according to their cuticle lifting status (
Figure 3a): hair with no raised cuticles (group I), hair with slightly raised cuticles (groups II and III), and hair with highly lifted cuticles (group IV).
To capture images of the same location before and after washing, the hair tip was measured to facilitate identification. There was a part that originally had no black areas, but after washing with surfactant, black areas appeared. The degree of formation of black areas differed for each hair group, as shown in
Figure 3b. More black areas were created in the hair where the cuticle was lifted the most. The black areas of the four groups were calculated, and the changes in area are plotted in
Figure 3c. It was observed that most changes occurred in the hair of group D owing to the surfactant.
3.3. Prevention of Pore Formation through Sealing
Hair with more cuticle lifting will have more black areas due to surfactants. The penetration of surfactants into hair and the loss of internal components within the hair may occur through the CMCs between the cuticles [
21]. We changed the hair surface properties in three ways and tested the properties that effectively prevented the loss of internal components owing to the anionic surfactants. In the first sample, the cuticle surface was sealed with a polymer oil, CSA; in the second sample, it was treated with a hydrophobic coating by an acrylamide copolymer, ATC; and in the third sample, it was cationized with polyethyleneimine. These coatings were applied to the hair of the class III group, as shown in
Figure 2.
Using different methods, we checked whether each material was properly coated on the hair. AFM confirmed that the hair was coated with CSA. Because the thickness of the coating is on the nanometer scale, it is difficult to determine whether it is coated or not; therefore, the thickness before and after the coating was scanned at the same location.
Figure 4a shows that hair cuticle height was reduced by up to 110 nm after sealing.
The change in contact angle was measured to confirm that the hydrophobic coating on the hair surface was properly applied. When a drop of water was dropped onto the hair, the contact angle decreased as the water was absorbed by the hair; therefore, the contact angle was measured after 10 s. The water drop disappeared in the case of the control hair, and the contact angle (
Figure 4b) could not be measured after 10 s. However, in the hydrophobically treated hair, the value was 28–51°.
Zeta potential is used to check the degree of cationization of the surface [
21]; however, because the measurement must be performed in water, the water-soluble coatings may be damaged. To ensure that the hair surface was cationic with polyethyleneimine, anionic acid dyes were adsorbed. As can be seen in
Figure 4c, the hair treated with 1% polyethyleneimine aqueous solution showed high color change (ΔE) regardless of the molecular weight of the dye. Therefore, it can be concluded that the hair treated with polyethyleneimine was cationic.
After washing the hair with the surfactant, changes in the black areas were observed. As shown in
Figure 4a, changes in cuticle lifting owing to the hair surface coating can be as small as 100 nm, making it difficult to observe these changes with the naked eye. Therefore, we used single hair analysis for damage assessment, which could numerically check the degree of cuticle lifting [
24].
The statistical distribution of spatial properties was determined using the Delaunay triangle (DT) [
30]. The scattered light emitted from the cuticle of the surface-coated hair was analyzed using the DT. If the points from the scattered light are evenly distributed, the average angle of the Delaunay triangle (DT) created by connecting the points is small; if the surface is rough, the value of the largest angle forming the DT is large. In addition, after extracting the points from the scattered light, the ratio of the area containing the points in the entire area was calculated as histogram density (HD). Based on a study that calculated the degree of cuticle lifting by calculating DT and HD using reflected light, cuticle lifting was detected.
In
Figure 5a, the left image was acquired from the hair, and the right image is a scattered-light image measured from the same hair. Areas with severe cuticle lifting had a higher intensity of reflected light than the surrounding areas; these areas are represented by red spots. The degree of cuticle lifting was calculated for cases where the hair surface was sealed, hydrophobized, or cationized.
The greater lifting value shown in
Figure 5b indicates that more cuticle lifting had occurred. The average value for the hydrophobic surface treated with ATC was 0.9213, whereas for the cationic treatment with polyethyleneimine, the average was 1.0789. When the cuticle was sealed with a polymer oil, CSA, the degree of hair cuticle formation was 0.3407. Cuticle-sealing treatment flattened the cuticle.
When these hair samples were washed with a surfactant, a change in the dark area was observed. For comparison, under the same conditions, an area without a dark domain was selected and observed before washing with the surfactant. Areas without a dark domain have the advantage of allowing an easy comparison of changes in the pores.
When cuticle sealing was performed, the change in the dark area was 5.11%. In the case of the hydrophobic coating, it was 12.14%, and in the case of the cationic coating, it was slightly lower at 11.45%. In the case of untreated hair, the change in porosity was 15.5%, indicating that the hydrophobic and cationic treatments reduced the porosity.
The changes in the dark areas evaluated after washing the hair are shown in
Figure 5b, along with the cuticle lifting values for each hair. A lower degree of cuticle lifting reduces the incidence of dark areas after washing.
4. Discussion
Because strands of hair stick together, it takes less time for the surfactants to reach each individual hair than the total time it takes for the surfactants to lather the entire hair. In this experiment, the hair had to be kept in the same position before and after washing, so one strand of hair was fixed to the sample holder and then washed using SLES. Therefore, the hair washing time was set to 10 s, which is shorter than the in vivo wash time.
Surfactants remove lipids from hair during washing [
21]. Surfactants penetrate directly into hair and remove more hydrophobic lipids, such as squalene and esters. Although the route by which surfactants penetrate hair is unknown, the CMC may be the most likely candidate because it has a noncovalent structure rather than a covalent lipid structure. Other hair components, such as the cuticle and cortex, are keratin fibrous proteins that render the passage of surfactants and lipids inefficient.
When lipids in the hair are lost and hydrogen bonds within the hair are broken, the internal structure of the hair is disturbed. This structural perturbation was observed in black areas, as shown in
Figure 1. The surfactants may have caused internal changes such as protein loss or lipid loss [
21,
22] or pores may have formed due to the porous-like structure in the vacuole [
11]. Pores, which are represented by a region of low internal density, formed immediately after washing up to four times, but no pores were formed after four washes. This indicated that the bonding strength in the area where the pores were created was weak. As shown in
Figure 2, the region of low internal density created this way was easily filled with other amino acids. Therefore, the portion lost by the surfactant can be replenished with lipid components supplied by the sebaceous glands of the scalp or with external nutrients supplied during shampooing. Nonetheless, the formation of internal pores is noteworthy because they can lead to certain changes inside the hair.
Owing to methodological limitations, the components lost in the pores are unknown. Based on previous gas chromatography–mass spectrometry (GC/MS) studies, the lipids were predicted to be squalene and wax ester [
21]. However, the fact that hair is also filled with glycine means that the pores inside the hair are spaces where not only lipids but also other substances can enter. Phenylalanine is more lipophilic than tyrosine, and its inability to fill the inside of the hair appears to be due to its inability to pass through the delta layer of the CMC, which is thought to be made of protein (its composition is unknown).
If hair cuticle lifting is severe, the incidence of pores increases, even after washing. As the CMC is located between the cuticles, cuticle lifting is closely related to the collapse of the CMC structure. We cannot be sure that cuticle lifting increases the size of the CMC, but it makes it easier for materials from the inside and outside of the hair to pass through. When the cuticle is lifted, the amount of surfactant penetrating the CMC increases, accelerating the loss of internal components.
Because the increase in the size of the CMC is due to the collapse of the structure formed by noncovalently bound lipids, restoring the collapsed structure is difficult. Ultimately, preventing surfactants from penetrating hair is the most efficient way to prevent pores from forming inside the hair.
In this study, three methods were used to prevent the reduction in the internal density of hair caused by surfactants. First, the cuticle was sealed to prevent direct contact between the surfactants and CMC. Second, we attempted to prevent access to the surfactants by applying a hydrophobic coating to the hair surface. The last method involved cationizing the hair surface to induce the adsorption of anionic surfactants on the hair surface and prevent them from penetrating the hair. As shown in
Figure 5, cuticle sealing was most effective in preventing pores from forming inside the hair. This result proves that pores inside hair could be created through the CMC.
5. Conclusions
In this study, the effect of surfactants on the interior region of hair was observed using an optical microscope. Areas presumed to be pores are created by surfactants inside the hair. It was confirmed that, in terms of the pores, the low internal density region inside the hair could be filled with low-molecular-weight amino acids. We focused on the CMC as an outlet for internal material loss. Three methods were used to prevent internal components from leaking into the CMC. The hydrophobic coating, which prevents surfactants from accessing the surface, and the cationic coating, which prevents the surface penetration of anionic surfactants, were not very effective in preventing the creation of internal pores. To prevent the loss of internal components through the CMC, we attempted to seal the cuticle, which was found to prevent the loss of pores more effectively than the other surface treatments. This implies that the ingredients inside the hair leak out through the CMC, and cuticle-sealing technology is important for hair damage repair. In the future, additional research will need to be conducted to identify the components released from inside hair.