Exploring the In Vitro Antioxidant, Anti-Aging, and Cytotoxic Properties of Kaempferia galanga Linn. Rhizome Extracts for Cosmeceutical Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Kaempferia galanga Linn. Preparation and Extraction
2.3. Preliminary Phytochemical Screening of Kaempferia galanga Linn. Extracts
2.3.1. Determination of Flavonoid Compounds
2.3.2. Determination of Phenolic Compounds
2.3.3. Determination of Terpenoid Compounds
2.4. Determination of Total Phenolic Contents
2.5. Determination of Total Flavonoid Contents
2.6. Antioxidant Activities of Kaempferia galanga Linn. Extracts
2.6.1. DPPH• Radical Scavenging Activity
2.6.2. ABTS+• Radical Scavenging Activity
2.6.3. Ferric Reducing Antioxidant Power (FRAP)
2.7. Anti-Aging Activities
2.7.1. Collagenase Inhibitory Activity
2.7.2. Elastase Inhibitory Activity
2.8. Cytotoxicity of Kaempferia galanga Linn. Extracts
2.8.1. Cell Culture and Conditions
2.8.2. Determination of Cell Viability
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of Solvent Polarity on Kaempferia galanga Linn. Extraction Yield
3.2. Effect of Solvent Polarity on Phytochemical Screening, Total Phenolic Content, and Total Flavonoids Content
3.3. Effect of Solvent Polarity on Antioxidant Activities of Kaempferia galanga Linn. Extracts
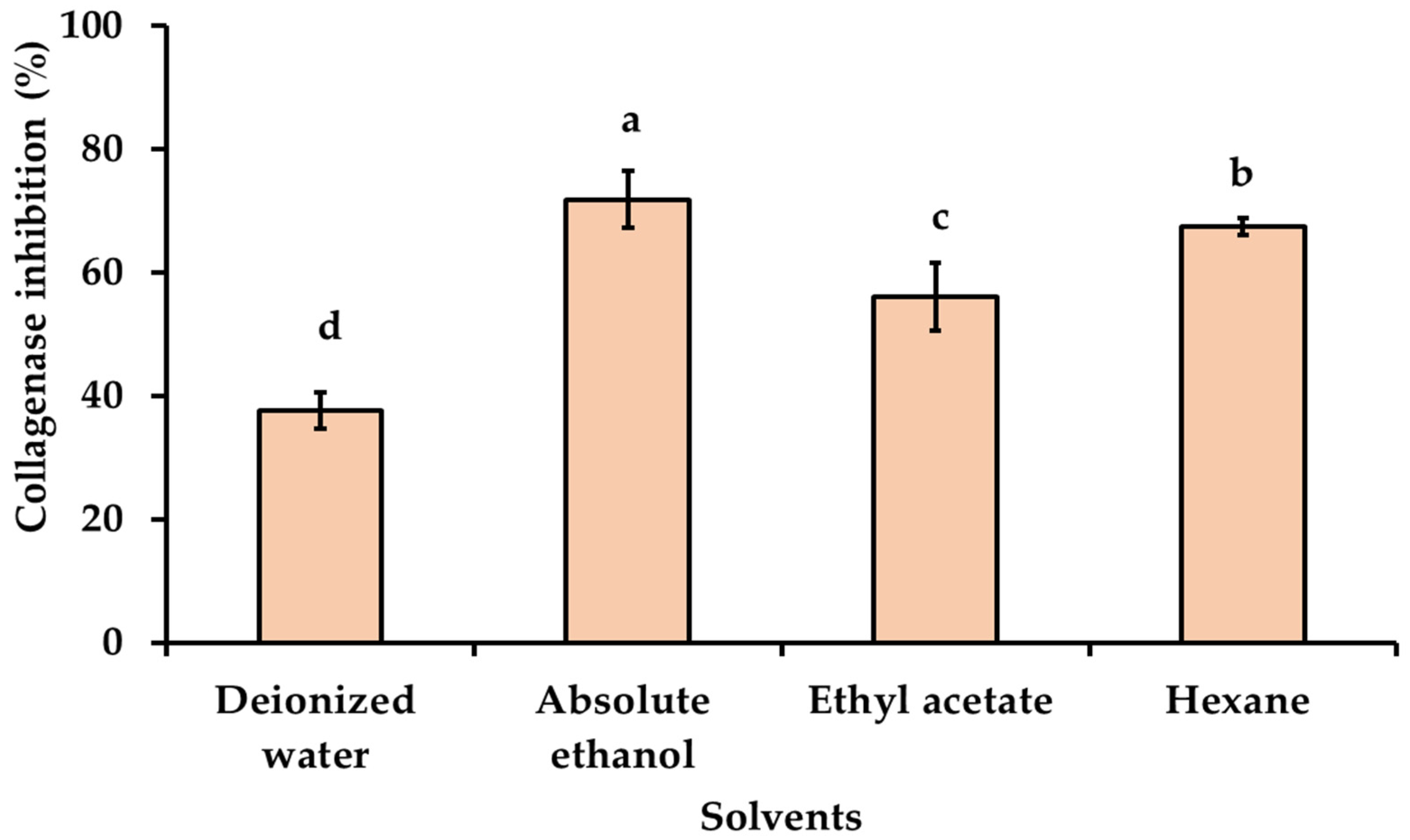
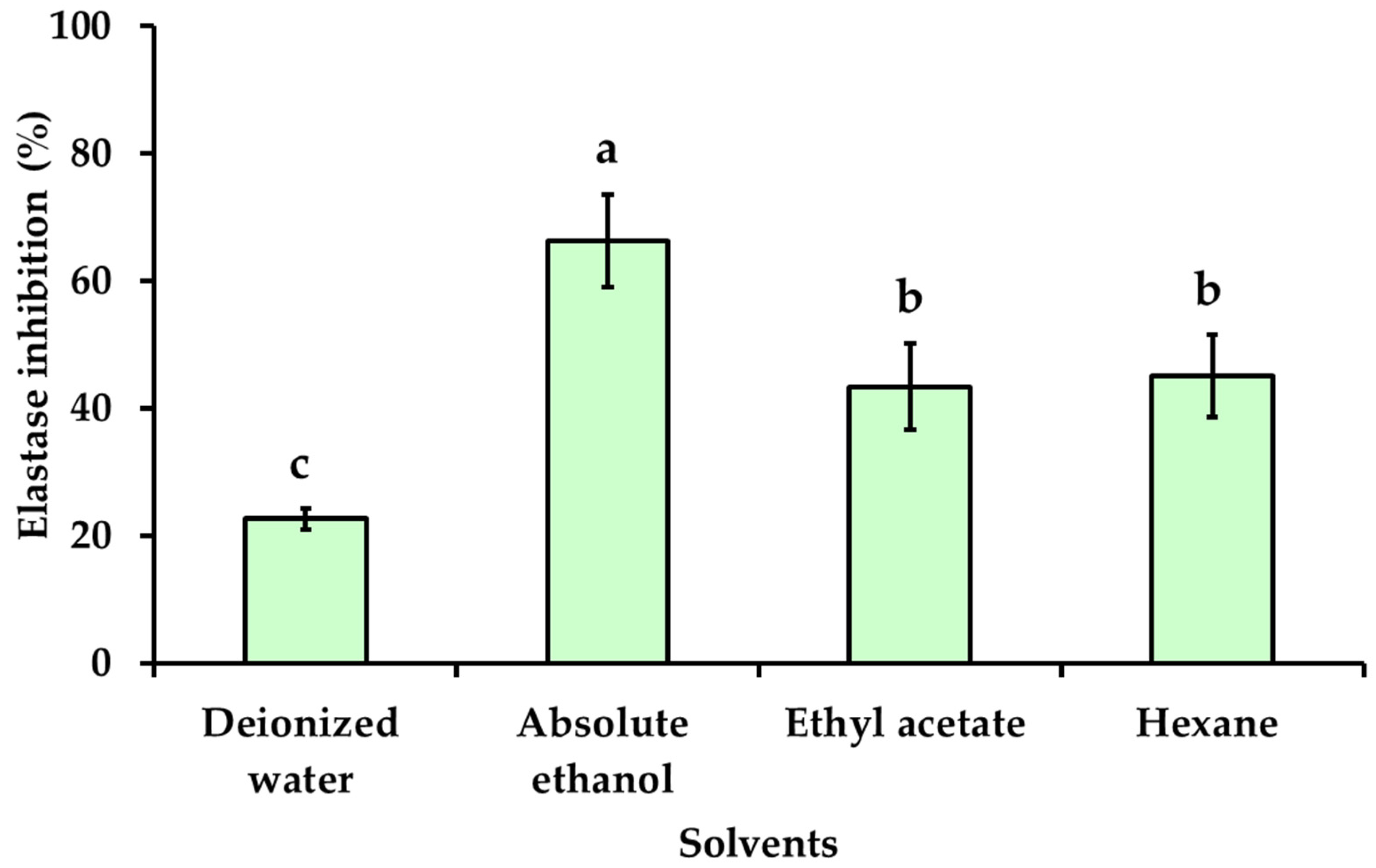
3.4. Effect of Solvent Polarity on Anti-Aging Activities of Kaempferia galanga Linn. Extracts
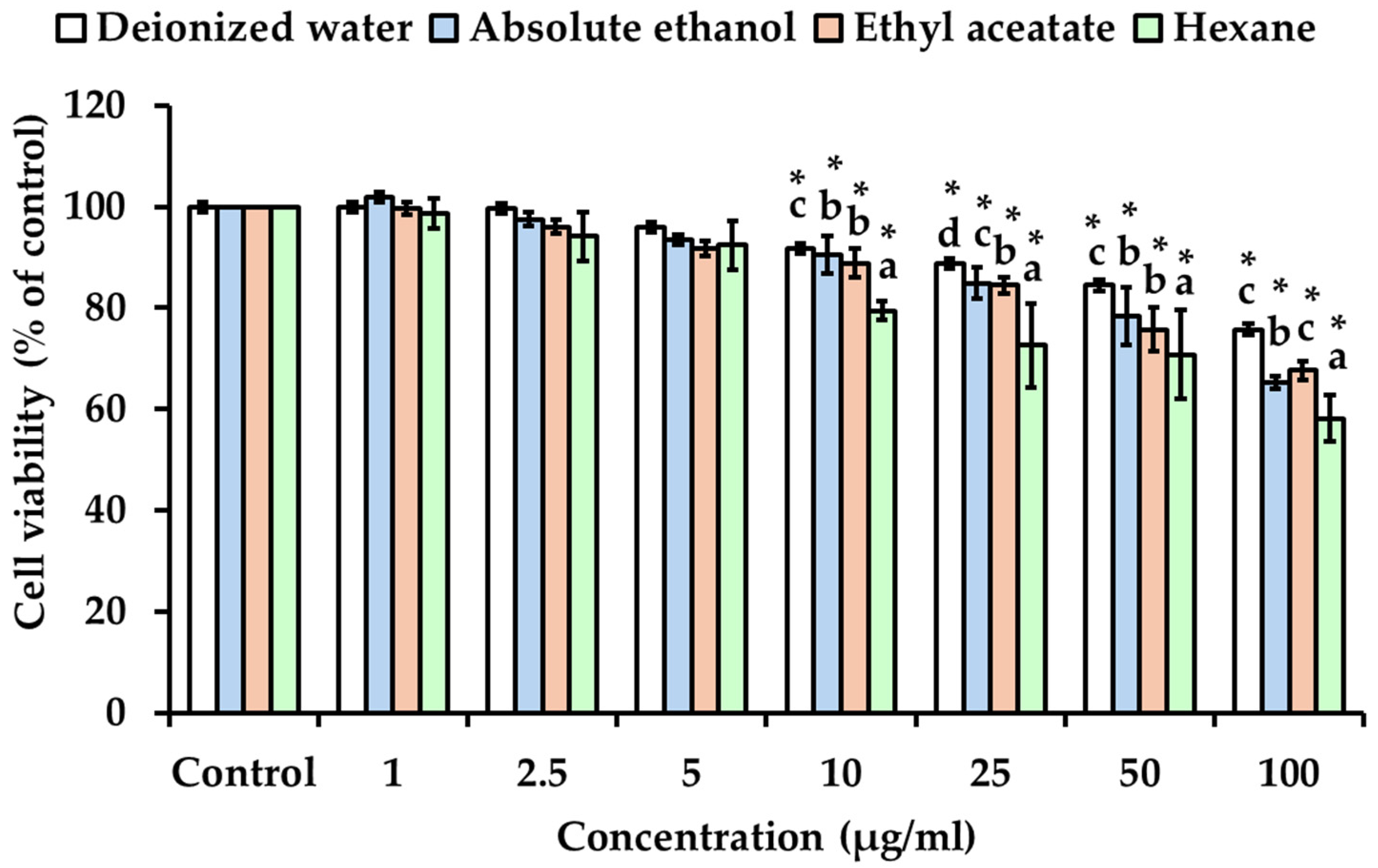
3.5. Effect of Solvent Polarity on the Cytotoxicity of Kaempferia galanga Linn. Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohiuddin, A.K. Skin Aging & Modern Age Anti-aging Strategies. Glob. J. Med. Res. B 2019, 19, 209–240. [Google Scholar]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; Camp, J.V.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef] [PubMed]
- Shin, R.; Berg, R.H.; Schachtman, D.P. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol. 2005, 46, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, K.; Akamatsu, H.; Okano, Y.; Matsunaga, K.; Masaki, H. Exogenous nitric oxide enhances the synthesis of type I collagen and heat shock protein 47 by normal human dermal fibroblasts. J. Dermatol. Sci. 2006, 41, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zheng, N.; Niu, N.; Tan, Y.; Li, Y.; Tian, H. Potent anti-angiogenic component in Kaempferia galanga L. and its mechanism of action. J. Ethnopharmacol. 2024, 324, 117811. [Google Scholar] [CrossRef] [PubMed]
- Wahyuni, I.S.; Sufiawati, I.; Shafuria, A.; Nittayananta, W.; Levita, J. Formulation and Evaluation of Mucoadhesive Oral Care Gel Containing Kaempferia galanga Extract. Pharmaceutics 2024, 16, 421. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, R.; Hu, H.; Zhao, X.; Yin, Z.; Zou, Y.; Li, L.; Jia, R.; Zhang, Y.; Song, X. Antiviral effect of an extract from Kaempferia galanga L. rhizome in mice infected with pseudorabies virus. J. Virol. Methods. 2022, 307, 114573. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. Phytochemistry, pharmacological activities and uses of traditional medicinal plant Kaempferia galanga L.—An overview. J. Ethnopharmacol. 2020, 253, 112667. [Google Scholar] [CrossRef] [PubMed]
- Lallo, S.; Hardianti, B.; Sartini, S.; Ismail, I.; Laela, D.; Hayakawa, Y. Ethyl P-Methoxycinnamate: An Active Anti-Metastasis Agent and Chemosensitizer Targeting NFκB from Kaempferia galanga for Melanoma Cells. Life 2022, 12, 337. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.I.; Asmawi, M.Z.; Sadikun, A.; Atangwho, I.J.; Yam, M.F.; Altaf, R.; Ahmed, A. Bioactivity-guided isolation of ethyl-p-methoxycinnamate, an anti-inflammatory constituent, from Kaempferia galanga L. extracts. Molecules 2012, 17, 8720–8734. [Google Scholar] [CrossRef] [PubMed]
- Abioye, E.O.; Akinpelu, D.A.; Aiyegoro, O.A.; Adegboye, M.F.; Oni, M.O.; Okoh, A.I. Preliminary Phytochemical Screening and Antibacterial Properties of Crude Stem Bark Extracts and Fractions of Parkia biglobosa (Jacq.). Molecules 2013, 18, 8485–8499. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Phytochemical Methods A Guide to Modern Techniques of Plant Analysis, 3rd ed.; Chapman & Hall: London, UK, 1998. [Google Scholar]
- Eun, C.-H.; Kang, M.-S.; Kim, I.-J. Elastase/Collagenase Inhibition Compositions of Citrus unshiu and Its Association with Phenolic Content and Anti-Oxidant Activity. Appl. Sci. 2020, 10, 4838. [Google Scholar] [CrossRef]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Vistica, D.T.; Skehan, P.; Scudiero, D.; Monks, A.; Pittman, A.; Boyd, M.R. Tetrazolium-based assays for cellular viability: A critical examination of selected parameters affecting formazan production. Cancer Res. 1991, 51, 2515–2520. [Google Scholar] [PubMed]
- Abarca-Vargas, R.; Pena Malacara, C.F.; Petricevich, V.L. Characterization of Chemical Compounds with Antioxidant and Cytotoxic Activities in Bougainvillea x buttiana Holttum and Standl, (var. Rose) Extracts. Antioxidants 2016, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Ganjloo, A.; Salleh, L.M.; Selamat, J.; Hamid, A.; Zaidul, I.S.M. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod. Process. 2011, 89, 67–72. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.A.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz. J. Pharm. Sci. 2020, 56, e17129. [Google Scholar] [CrossRef]
- Sani, S.A.; Faik, A.A.M.; Abdulla, R.; Kunasekaran, S. Phytochemical, antioxidant and antibacterial activities of two kinds of Sabah Zingberaceae. J. Phys. Conf. Ser. 2019, 1358, 012012. [Google Scholar] [CrossRef]
- Uba, G.; Dauda, H.; Aujara, K.M.; Ali, U. Solvent Extraction and its Effects on the Phytochemical Yield and Antioxidant Capacity of Commiphora africana (Burseraceae). Bioremediation Sci. Technol. Res. 2020, 8, 8–11. [Google Scholar] [CrossRef]
- Snyder, L.R. Classification of the solvent properties of common liquids. J. Chromatogr. A 1974, 92, 223–230. [Google Scholar] [CrossRef]
- Rao, A.; Pandey, V.N. Phytochemical Screening of Tubers and Leaf extracts of Sagittaria sagittifolia L.: Newsa (Arrowhead). Int. J. Sci. Res. Publ. 2017, 7, 431–437. [Google Scholar]
- Ncube, B.; Finnie, J.F.; Van Staden, J. Quality from the field: The impact of environmental factors as quality determinants in medicinal plants. S. Afr. J. Bot. 2012, 82, 11–20. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Raudone, L. Phenological and Geographical Effects on Phenolic and Triterpenoid Content in Vaccinium vitis-idaea L. Leaves. Plants 2021, 10, 1986. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Huang, Y.; Wang, Y.; He, X. Anti-inflammatory diarylheptanoids and phenolics from the rhizomes of kencur (Kaempferia galanga L.). Ind. Crops Prod. 2018, 125, 454–461. [Google Scholar] [CrossRef]
- Swapana, N.; Tominaga, T.; Elshamy, A.I.; Ibrahim, M.A.A.; Hegazy, M.-E.F.; Brajakishor Singh, C.; Suenaga, M.; Imagawa, H.; Noji, M.; Umeyama, A. Kaemgalangol A: Unusual seco-isopimarane diterpenoid from aromatic ginger Kaempferia galanga. Fitoterapia 2018, 129, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, P.C.; Latha, K.P.; Mudgal, J.; Nampurath, G.K. Extraction, characterization and evaluation of Kaempferia galanga L. (Zingiberaceae) rhizome extracts against acute and chronic inflammation in rats. J. Ethnopharmacol. 2016, 194, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Iloki-Assanga, S.B.; Lewis-Lujan, L.M.; Lara-Espinoza, C.L.; Gil-Salido, A.A.; Fernandez-Angulo, D.; Rubio-Pino, J.L.; Haines, D.D. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Res. Notes 2015, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Hirano, R.; Sasamoto, W.; Matsumoto, A.; Itakura, H.; Igarashi, O.; Kondo, K. Antioxidant Ability of Various Flavonoids against DPPH Radicals and LDL Oxidation. J. Nutr. Sci. Vitaminol. 2001, 47, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Zdunska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Begum, T.; Gogoi, R.; Sarma, N.; Pandey, S.K.; Lal, M. Novel ethyl p-methoxy cinnamate rich Kaempferia galanga (L.) essential oil and its pharmacological applications: Special emphasis on anticholinesterase, anti-tyrosinase, alpha-amylase inhibitory, and genotoxic efficiencies. PeerJ 2023, 11, e14606. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Kabir, M.T.; Islam, M.N.; Muqaddim, M.; Sharmin, S.; Ullah, M.S.; Uddin, M.S. Investigation of Antioxidant and Cytotoxic Activities of Kaempferia galanga L. Res. J. Pharm. Technol. 2019, 12, 6. [Google Scholar] [CrossRef]
- Yeap, Y.S.Y.; Kassim, N.K.; Ng, R.C.; Ee, G.C.L.; Saiful Yazan, L.; Musa, K.H. Antioxidant properties of ginger (Kaempferia angustifolia Rosc.) and its chemical markers. Int. J. Food Prop. 2017, 20, 1158–1172. [Google Scholar] [CrossRef]
- Kaczorová, D.; Karalija, E.; Dahija, S.; Bešta-Gajević, R.; Parić, A.; Ćavar Zeljković, S. Influence of Extraction Solvent on the Phenolic Profile and Bioactivity of Two Achillea Species. Molecules 2021, 26, 1601. [Google Scholar] [CrossRef] [PubMed]
- Utami, S.; Sachrowardi, Q.R.; Damayanti, N.A.; Wardhana, A.; Syarif, I.; Nafik, S.; Arrahman, B.C.; Kusuma, H.S.W.; Widowati, W. Antioxidants, anticollagenase and antielastase potentials of ethanolic extract of ripe sesoot (Garcinia picrorrhiza Miq.) fruit as antiaging. J. Herbmed Pharmacol. 2018, 7, 88–93. [Google Scholar] [CrossRef]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Zorina, A.; Zorin, V.; Kudlay, D.; Kopnin, P. Molecular Mechanisms of Changes in Homeostasis of the Dermal Extracellular Matrix: Both Involutional and Mediated by Ultraviolet Radiation. Int. J. Mol. Sci. 2022, 23, 6655. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Santoyo, S.; Jaime, L.; García-Blairsy Reina, G.; Herrero, M.; Señoráns, F.J.; Ibáñez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Starmans, D.A.J.; Nijhuis, H.H. Extraction of secondary metabolites from plant material: A review. Trends Food Sci. Technol. 1996, 7, 191–197. [Google Scholar] [CrossRef]


| Solvents | Phytochemical Screening | ||
|---|---|---|---|
| Flavonoid Compound | Phenolic Compound | Terpenoid Compound | |
| Deionized water | + | ++ | + |
| Absolute ethanol | ++ | +++ | + |
| Ethyl acetate | ++ | +++ | + |
| Hexane | + | ++ | + |
| Solvents | Total Flavonoid Contents (mg QE/g Extract) | Total Phenolic Contents (mg GAE/g Extract) |
|---|---|---|
| Deionized water | 1.19 ± 0.09 d | 13.36 ± 0.48 c |
| Absolute ethanol | 12.63 ± 1.64 a | 29.70 ± 0.19 b |
| Ethyl acetate | 9.13 ± 1.04 b | 32.16 ± 0.62 a |
| Hexane | 2.80 ± 0.07 c | 12.96 ± 0.96 c |
| Solvents | Antioxidant Activity | ||
|---|---|---|---|
| DPPH• Assay (IC50 Value, mg/mL) | FRAP Assay (mmol Fe2+/g Extract) | ABTS+• Assay (mg TE/g Extract) | |
| Deionized water | 3.472 ± 0.040 e | 44.209 ± 1.822 c | 11.853 ± 0.451 a |
| Absolute ethanol | 0.612 ± 0.020 b | 62.791 ± 1.866 b | 9.214 ± 0.574 b |
| Ethyl acetate | 2.573 ± 0.016 c | 28.451 ± 8.005 d | 8.799 ± 0.169 c |
| Hexane | 3.004 ± 0.062 d | 25.129 ± 4.872 d | 3.342 ± 0.370 d |
| Trolox | 0.005 ± 0.001 a | 182.069 ± 6.604 a | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wichayapreechar, P.; Charoenjittichai, R.; Prasansuklab, A.; Vinardell, M.P.; Rungseevijitprapa, W. Exploring the In Vitro Antioxidant, Anti-Aging, and Cytotoxic Properties of Kaempferia galanga Linn. Rhizome Extracts for Cosmeceutical Formulations. Cosmetics 2024, 11, 97. https://doi.org/10.3390/cosmetics11030097
Wichayapreechar P, Charoenjittichai R, Prasansuklab A, Vinardell MP, Rungseevijitprapa W. Exploring the In Vitro Antioxidant, Anti-Aging, and Cytotoxic Properties of Kaempferia galanga Linn. Rhizome Extracts for Cosmeceutical Formulations. Cosmetics. 2024; 11(3):97. https://doi.org/10.3390/cosmetics11030097
Chicago/Turabian StyleWichayapreechar, Panikchar, Ranit Charoenjittichai, Anchalee Prasansuklab, Maria Pilar Vinardell, and Wandee Rungseevijitprapa. 2024. "Exploring the In Vitro Antioxidant, Anti-Aging, and Cytotoxic Properties of Kaempferia galanga Linn. Rhizome Extracts for Cosmeceutical Formulations" Cosmetics 11, no. 3: 97. https://doi.org/10.3390/cosmetics11030097
APA StyleWichayapreechar, P., Charoenjittichai, R., Prasansuklab, A., Vinardell, M. P., & Rungseevijitprapa, W. (2024). Exploring the In Vitro Antioxidant, Anti-Aging, and Cytotoxic Properties of Kaempferia galanga Linn. Rhizome Extracts for Cosmeceutical Formulations. Cosmetics, 11(3), 97. https://doi.org/10.3390/cosmetics11030097








