Sustainable Dynamic Wrinkle Efficacy: Non-Invasive Peptides as the Future of Botox Alternatives
Abstract
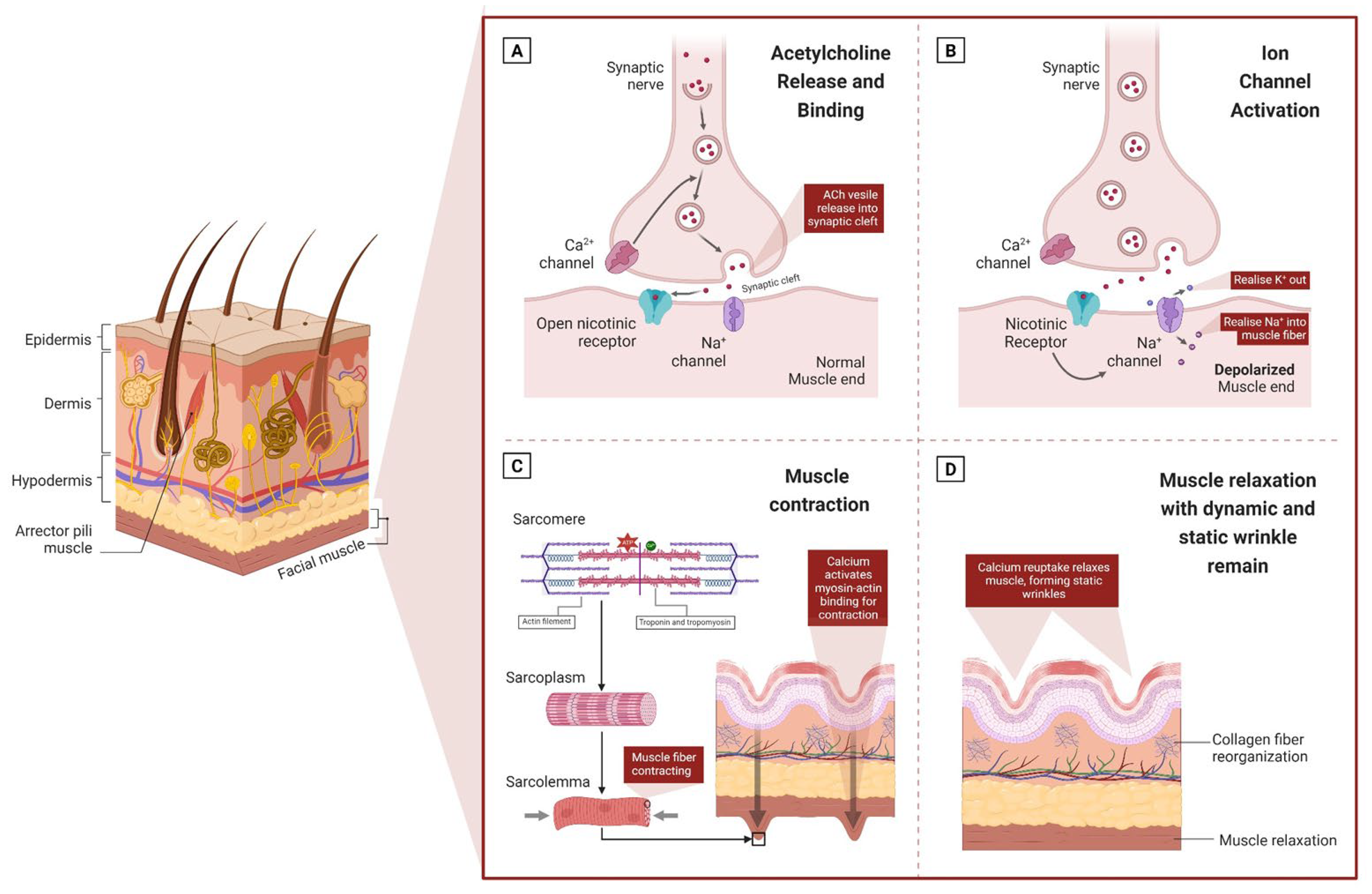
:1. Introduction
2. The Pathophysiology of Dynamic Wrinkles
2.1. Mechanisms of Wrinkle Formation
2.1.1. Acetylcholine Release and Neuromuscular Activation
2.1.2. Calcium Ion Release and Muscle Preparation
2.1.3. Muscle Contraction and Initial Skin Folding
2.1.4. Development of Dynamic and Static Wrinkles
2.2. Current Treatment of Dynamic Wrinkles
3. Current Standard Care: Botulinum Toxin
3.1. Botulinum Toxin Injection
3.2. Botulinum Toxin Topical Gel
- Nanoparticle-based formulations: Demonstrated a 25% reduction in wrinkle depth after four weeks of daily application, significantly higher than the 5% reduction observed in the placebo group [55].
- Liposomal delivery systems: Reported a 30% improvement in wrinkle severity over an eight-week period [57].
- Peptide-based carriers: Achieved a 20% reduction in periorbital wrinkles after six weeks of treatment [60].
4. Emerging Peptide Topical Alternatives
4.1. Synthetic Peptide
4.1.1. Argireline (Acetyl Hexapeptide-8)
4.1.2. Snap-8 (Acetyl Octapeptide-3)
4.1.3. Leuphasyl (Pentapeptide-18)
4.1.4. Vialox (Pentapeptide-3)
4.2. Animal-Devired Synthesis Peptide
4.2.1. XEP-30 and XEP-018 (μ-Conotoxin CnIIIC)
4.2.2. Syn-Ake (Dipeptide Diaminobutyroyl Benzylamide Diacetate)
4.3. Plant-Based Extract
5. Market Insights and Consumer Trends
6. Challenges and Future Perspectives
7. Conclusions
- Effective, safer solutions: These ingredients meet the growing consumer demand for ‘clean beauty’ products, aligning with preferences for non-toxic and sustainable skincare.
- Technological advancements: Innovations in delivery technologies are overcoming challenges like skin penetration, ensuring these ingredients are not only effective but also reliable.
- Market expansion: The booming market, fueled by consumers seeking seamless and risk-free skincare routines, highlights the transformative potential of these alternatives.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chao, Y.Y.Y. The Aesthetic Standard for Contouring and Facial Dynamics. In Optimizing Aesthetic Toxin Results; CRC Press: London, UK, 2022; pp. 37–44. ISBN 978-1-00-300813-2. [Google Scholar]
- Kurosumi, M.; Mizukoshi, K.; Hongo, M.; Kamachi, M.G. Does Age-Dynamic Movement Accelerate Facial Age Impression? Perception of Age from Facial Movement: Studies of Japanese Women. PLoS ONE 2021, 16, e0255570. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, M.; He, Y.; Meng, H.; Meng, Q.; Shi, Q.; Yi, F. Facial Skin Aging Stages in Chinese Females. Front. Med. 2022, 9, 870926. [Google Scholar] [CrossRef]
- Fujimura, T.; Hotta, M. The Preliminary Study of the Relationship between Facial Movements and Wrinkle Formation. Skin Res. Technol. 2012, 18, 219–224. [Google Scholar] [CrossRef]
- Walker, H.M.; Chauhan, P.R. Anatomy, Head and Neck: Glabella. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Rahman, E.; Mosahebi, A.; Carruthers, J.D.A.; Carruthers, A. The Efficacy and Duration of Onabotulinum Toxin A in Improving Upper Facial Expression Lines with 64-Unit Dose Optimization: A Systematic Review and Meta-Analysis with Trial Sequential Analysis of the Randomized Controlled Trials. Aesthet. Surg. J. 2023, 43, 215–229. [Google Scholar] [CrossRef]
- Demchenko, I.; Swiderski, A.; Liu, H.; Jung, H.; Lou, W.; Bhat, V. Botulinum Toxin Injections for Psychiatric Disorders: A Systematic Review of the Clinical Trial Landscape. Toxins 2024, 16, 191. [Google Scholar] [CrossRef]
- de Jongh, F.W.; Wolf, O.; Wong, Z.Y.; Ingels, K.J.A.O.; Pouwels, S. Botulinum Toxin Treatment of the Buccinator Muscle Facial Synkinesis: A Systematic Review. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2023, 86, 88–93. [Google Scholar] [CrossRef]
- Ascher, B.; Rzany, B.-J.; Kestemont, P.; Redaelli, A.; Hendrickx, B.; Iozzo, I.; Martschin, C.; Milotich, A.; Molina, B.; Cartier, H.; et al. International Consensus Recommendations on the Aesthetic Usage of Ready-to-Use AbobotulinumtoxinA (Alluzience). Aesthet. Surg. J. 2024, 44, 192–202. [Google Scholar] [CrossRef]
- Park, J.; Jung, H.; Jang, B.; Song, H.-K.; Han, I.-O.; Oh, E.-S. D-Tyrosine Adds an Anti-Melanogenic Effect to Cosmetic Peptides. Sci. Rep. 2020, 10, 262. [Google Scholar] [CrossRef]
- Kluczyk, A.; Ludwiczak, J.; Modzel, M.; Kuczer, M.; Cebrat, M.; Biernat, M.; Bąchor, R. Argireline: Needle-Free Botox as Analytical Challenge. Chem. Biodivers. 2021, 18, e2000992. [Google Scholar] [CrossRef]
- Avcil, M.; Akman, G.; Klokkers, J.; Jeong, D.; Çelik, A. Efficacy of Bioactive Peptides Loaded on Hyaluronic Acid Microneedle Patches: A Monocentric Clinical Study. J. Cosmet. Dermatol. 2020, 19, 328–337. [Google Scholar] [CrossRef]
- Renzi, A.; Brillantino, A.; Di Sarno, G.; D’Aniello, F.; Ziccardi, S.; Paladino, F.; Iacobellis, F. Myoxinol (Hydrolyzed Hibiscus Esculentus Extract) in the Cure of Chronic Anal Fissure: Early Clinical and Functional Outcomes. Gastroenterol. Res. Pract. 2015, 2015, 567920. [Google Scholar] [CrossRef]
- Rubin, C.B.; Brod, B. Natural Does Not Mean Safe—The Dirt on Clean Beauty Products. JAMA Dermatol. 2019, 155, 1344. [Google Scholar] [CrossRef]
- Kuo, I.Y.; Ehrlich, B.E. Signaling in Muscle Contraction. Cold Spring Harb. Perspect. Biol. 2015, 7, a006023. [Google Scholar] [CrossRef]
- Dent, J.A. The Evolution of Pentameric Ligand-Gated Ion Channels. In Insect Nicotinic Acetylcholine Receptors; Thany, S.H., Ed.; Springer: New York, NY, USA, 2010; pp. 11–23. ISBN 978-1-4419-6445-8. [Google Scholar]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a Neuromodulator: Cholinergic Signaling Shapes Nervous System Function and Behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin Anti-Aging Strategies. Dermatoendocrinology 2012, 4, 308–319. [Google Scholar] [CrossRef]
- Catterall, W.A. Voltage Gated Sodium and Calcium Channels: Discovery, Structure, Function, and Pharmacology. Channels 2023, 17, 2281714. [Google Scholar] [CrossRef]
- Gharpure, A.; Noviello, C.M.; Hibbs, R.E. Progress in Nicotinic Receptor Structural Biology. Neuropharmacology 2020, 171, 108086. [Google Scholar] [CrossRef]
- Hołyńska-Iwan, I.; Szewczyk-Golec, K. Analysis of Changes in Sodium and Chloride Ion Transport in the Skin. Sci. Rep. 2020, 10, 18094. [Google Scholar] [CrossRef]
- Garbincius, J.F.; Elrod, J.W. Mitochondrial Calcium Exchange in Physiology and Disease. Physiol. Rev. 2022, 102, 893–992. [Google Scholar] [CrossRef]
- Rossi, D.; Pierantozzi, E.; Amadsun, D.O.; Buonocore, S.; Rubino, E.M.; Sorrentino, V. The Sarcoplasmic Reticulum of Skeletal Muscle Cells: A Labyrinth of Membrane Contact Sites. Biomolecules 2022, 12, 488. [Google Scholar] [CrossRef]
- Clausen, T. Na+-K+ Pump Regulation and Skeletal Muscle Contractility. Physiol. Rev. 2003, 83, 1269–1324. [Google Scholar] [CrossRef]
- Squire, J. Special Issue: The Actin-Myosin Interaction in Muscle: Background and Overview. Int. J. Mol. Sci. 2019, 20, 5715. [Google Scholar] [CrossRef]
- Campos, L.D.; Santos Junior, V.D.A.; Pimentel, J.D.; Carregã, G.L.F.; Cazarin, C.B.B. Collagen Supplementation in Skin and Orthopedic Diseases: A Review of the Literature. Heliyon 2023, 9, e14961. [Google Scholar] [CrossRef]
- Gillies, A.R.; Lieber, R.L. Structure and Function of the Skeletal Muscle Extracellular Matrix. Muscle Nerve 2011, 44, 318–331. [Google Scholar] [CrossRef]
- Dierckx, S.; Patrizi, M.; Merino, M.; González, S.; Mullor, J.L.; Nergiz-Unal, R. Collagen Peptides Affect Collagen Synthesis and the Expression of Collagen, Elastin, and Versican Genes in Cultured Human Dermal Fibroblasts. Front. Med. 2024, 11, 1397517. [Google Scholar] [CrossRef]
- Burns, E.; Ahmed, H.; Isedeh, P.; Kohli, I.; Van der Pol, W.; Shaheen, A.; Muzaffar, A.; Al-Sadek, C.; Foy, T.; Abdelgawwad, M.; et al. Ultraviolet Radiation, Both UVA and UVB, Influences the Composition of the Skin Microbiome. Exp. Dermatol. 2019, 28, 136–141. [Google Scholar] [CrossRef]
- Sparavigna, A.; Tenconi, B.; Giori, A.M.; Bellia, G.; La Penna, L. Evaluation of the Efficacy of a New Hyaluronic Acid Gel on Dynamic and Static Wrinkles in Volunteers with Moderate Aging/Photoaging. Clin. Cosmet. Investig. Dermatol. 2019, 12, 81–90. [Google Scholar] [CrossRef]
- Susmita, A. An Evaluation of Use of Botulinum Toxin Type A in the Management of Dynamic Forehead Wrinkles—A Clinical Study. J. Clin. Diagn. Res. 2016, 10, ZC127. [Google Scholar] [CrossRef]
- Wright, G.; Lax, A.; Mehta, S.B. A Review of the Longevity of Effect of Botulinum Toxin in Wrinkle Treatments. Br. Dent. J. 2018, 224, 255–260. [Google Scholar] [CrossRef]
- Piewngam, P.; Khongthong, S.; Roekngam, N.; Theapparat, Y.; Sunpaweravong, S.; Faroongsarng, D.; Otto, M. Probiotic for Pathogen-Specific Staphylococcus Aureus Decolonisation in Thailand: A Phase 2, Double-Blind, Randomised, Placebo-Controlled Trial. Lancet Microbe 2023, 4, e75–e83. [Google Scholar] [CrossRef]
- Cavallini, M.; Dell’Avanzato, R.; Fundarò, S.P.; Urdiales-Gálvez, F.; Papagni, M.; Trocchi, G.; Raichi, M.; Zazzaron, M. Treating Glabellar Lines With Botulinum Toxin: Does Your Patient Need to Frown Steadily? Aesthet. Surg. J. 2024, 44, 421–427. [Google Scholar] [CrossRef]
- El-Garem, Y.F.; Eid, A.A.; Leheta, T.M. Locking the Line of Convergence by Botulinum Toxin Type A for the Treatment of Dynamic Forehead Wrinkles. J. Cosmet. Dermatol. 2023, 22, 186–192. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. BOTOX (onabotulinumtoxinA) Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/103000s5236lbl.pdf (accessed on 1 June 2024).
- Food and Drug Administration. BOTOX Cosmetic (onabotulinumtoxinA) for Injection, for Intramuscular Use 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/103000s5303lbl.pdf (accessed on 1 June 2024).
- Erbguth, F.J. Historical Notes on Botulism, Clostridium Botulinum, Botulinum Toxin, and the Idea of the Therapeutic Use of the Toxin. Mov. Disord. 2004, 19, S2–S6. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. DYSPORT® (abobotulinumtoxinA) for Injection. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125274s107lbl.pdf (accessed on 1 June 2024).
- U.S. Food and Drug Administration. XEOMIN (incobotulinumtoxinA) for Injection, for Intramuscular or Intraglandular Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125360s078lbl.pdf (accessed on 1 June 2024).
- Scott, A.B.; Honeychurch, D.; Brin, M.F. Early Development History of Botox (onabotulinumtoxinA). Medicine 2023, 102, e32371. [Google Scholar] [CrossRef]
- Global Opportunity Analysis and Industry Forecast Botulinum Toxin Market by Product Type (Toxin Type A and Toxin Type B), by Application (Therapeutic and Aesthetic), by Gender (Male and Female), by Age Group (13–19, 20–29, 30–39, 40–54, and above), by End User (Hospitals, Dermatology Clinics, Spas & Cosmetic Centers)—Global Opportunity Analysis and Industry Forecast, 2024–2030. Available online: https://www.nextmsc.com/report/botulinum-toxin-market (accessed on 1 June 2024).
- Food and Drug Administration. Product Approval Information—Licensing Action. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2002/botuall041202L.htm (accessed on 1 June 2024).
- Joseph, J.H.; Eaton, L.L.; Robinson, J.; Pontius, A.; Williams, E.F. Does Increasing the Dose of Abobotulinumtoxina Impact the Duration of Effectiveness for the Treatment of Moderate to Severe Glabellar Lines? J. Drugs Dermatol. JDD 2016, 15, 1544–1549. [Google Scholar]
- Food and Drug Administration. BOTOX COSMETIC (onabotulinumtoxinA) for Injection, for Intramuscular Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103000s5260lbl.pdf (accessed on 1 June 2024).
- Karsai, S.; Adrian, R.; Hammes, S.; Thimm, J.; Raulin, C. A Randomized Double-Blind Study of the Effect of Botox and Dysport/Reloxin on Forehead Wrinkles and Electromyographic Activity. Arch. Dermatol. 2007, 143, 1447–1462. [Google Scholar] [CrossRef]
- Yeilding, R.H.; Fezza, J.P. A Prospective, Split-Face, Randomized, Double-Blind Study Comparing OnabotulinumtoxinA to IncobotulinumtoxinA for Upper Face Wrinkles. Plast. Reconstr. Surg. 2015, 135, 1328–1335. [Google Scholar] [CrossRef]
- Prager, W.; Bee, E.K.; Havermann, I.; Zschocke, I. IncobotulinumtoxinA for the Treatment of Platysmal Bands: A Single-Arm, Prospective Proof-of-Concept Clinical Study. Dermatol. Surg. 2015, 41, S88–S92. [Google Scholar] [CrossRef]
- Brin, M.F.; Burstein, R. Botox (onabotulinumtoxinA) Mechanism of Action. Medicine 2023, 102, e32372. [Google Scholar] [CrossRef] [PubMed]
- Blasi, J.; Chapman, E.R.; Link, E.; Binz, T.; Yamasaki, S.; De Camilli, P.; Südhof, T.C.; Niemann, H.; Jahn, R. Botulinum Neurotoxin A Selectively Cleaves the Synaptic Protein SNAP-25. Nature 1993, 365, 160–163. [Google Scholar] [CrossRef]
- Schiavo, G.; Santucci, A.; Dasgupta, B.R.; Mehta, P.P.; Jontes, J.; Benfenati, F.; Wilson, M.C.; Montecucco, C. Botulinum Neurotoxins Serotypes A and E Cleave SNAP-25 at Distinct COOH-Terminal Peptide Bonds. FEBS Lett. 1993, 335, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Drugs.com. OnabotulinumtoxinA (Botox/Botox Cosmetic). Available online: https://www.drugs.com/onabotulinumtoxina.html (accessed on 1 June 2024).
- Mordor Intelligence BOTULINUM TOXIN COMPANIES (2024–2029). Available online: https://www.mordorintelligence.com/industry-reports/global-botulinum-toxin-market/companies (accessed on 1 June 2024).
- aedit.com. How Much Does Botox Cost? Available online: https://aedit.com/procedure/botox/cost (accessed on 1 June 2024).
- Brandt, F.; O’Connell, C.; Cazzaniga, A.; Waugh, J.M. Efficacy and Safety Evaluation of a Novel Botulinum Toxin Topical Gel for the Treatment of Moderate to Severe Lateral Canthal Lines. Dermatol. Surg. 2010, 36, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Nasir, A. Topical Botulinum Toxin. J. Clin. Aesthetic Dermatol. 2010, 3, 35–39. [Google Scholar]
- Araco, A.; Francesco, A. Prospective Randomized Clinical Study of a New Topical Formulation for Face Wrinkle Reduction and Dermal Regeneration. J. Cosmet. Dermatol. 2021, 20, 2832–2840. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, B.M.; Zarnitsyn, V.; Prausnitz, M.R.; Anstey, A.; Gateley, C.; Birchall, J.C.; Coulman, S.A. Pocketed Microneedles for Rapid Delivery of a Liquid-State Botulinum Toxin A Formulation into Human Skin. J. Control. Release 2013, 165, 146–152. [Google Scholar] [CrossRef]
- Giordano, C.N.; Matarasso, S.L.; Ozog, D.M. Injectable and Topical Neurotoxins in Dermatology. J. Am. Acad. Dermatol. 2017, 76, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Beer, K.R. Comparative Evaluation of the Safety and Efficacy of Botulinum Toxin Type A and Topical Creams for Treating Moderate-to-Severe Glabellar Rhytids. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. Al 2006, 32, 184–197. [Google Scholar] [CrossRef]
- Blanes-Mira, C.; Clemente, J.; Jodas, G.; Gil, A.; Fernández-Ballester, G.; Ponsati, B.; Gutierrez, L.; Pérez-Payá, E.; Ferrer-Montiel, A. A Synthetic Hexapeptide (Argireline) with Antiwrinkle Activity. Int. J. Cosmet. Sci. 2002, 24, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, M.; Xiao, S.; Pan, P.; Li, P.; Huo, J. The Anti-Wrinkle Efficacy of Argireline, a Synthetic Hexapeptide, in Chinese Subjects: A Randomized, Placebo-Controlled Study. Am. J. Clin. Dermatol. 2013, 14, 147–153. [Google Scholar] [CrossRef]
- Grosicki, M.; Latacz, G.; Szopa, A.; Cukier, A.; Kieć-Kononowicz, K. The Study of Cellular Cytotoxicity of Argireline—An Anti-Aging Peptide. Acta Biochim. Pol. 2014, 61, 29–32. [Google Scholar] [CrossRef]
- Henseler, H. Investigating the Effects of Argireline in a Skin Serum Containing Hyaluronic Acids on Skin Surface Wrinkles Using the Visia® Complexion Analysis Camera System for Objective Skin Analysis. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2023, 12, Doc09. [Google Scholar] [CrossRef]
- Shin, J.Y.; Han, D.; Yoon, K.Y.; Jeong, D.H.; Park, Y.I. Clinical Safety and Efficacy Evaluation of a Dissolving Microneedle Patch Having Dual Anti-Wrinkle Effects With Safe and Long-Term Activities. Ann. Dermatol. 2024, 36, e37. [Google Scholar] [CrossRef]
- Ji, M.; Lee, H.-S.; Kim, Y.; Seo, C.; Choi, S.; Oh, S.; Min, J.; Park, H.-J.; Kim, J.D.; Jeong, D.H.; et al. Method Development for Acetyl Octapeptide-3 Analysis by Liquid Chromatography-Tandem Mass Spectrometry. J. Anal. Sci. Technol. 2020, 11, 34. [Google Scholar] [CrossRef]
- Dragomirescu, A.; Andoni, M.; Ionescu, D.; Andrei, F. The Efficiency and Safety of Leuphasyl—A Botox-Like Peptide. Cosmetics 2014, 1, 75–81. [Google Scholar] [CrossRef]
- Rossello, C. Evaluation of effectiveness of viper serum for topical use as facial anti-aging. Capsul. Eburnea 2009, 4, 1–6. [Google Scholar] [CrossRef]
- Reddy, B.Y.; Jow, T.; Hantash, B.M. Bioactive Oligopeptides in Dermatology: Part II. Exp. Dermatol. 2012, 21, 569–575. [Google Scholar] [CrossRef]
- Gorouhi, F.; Maibach, H.I. Role of Topical Peptides in Preventing or Treating Aged Skin. Int. J. Cosmet. Sci. 2009, 31, 327–345. [Google Scholar] [CrossRef]
- Del Río-Sancho, S.; Cros, C.; Coutaz, B.; Cuendet, M.; Kalia, Y.N. Cutaneous Iontophoresis of μ-Conotoxin CnIIIC—A Potent Na V 1.4 Antagonist with Analgesic, Anaesthetic and Myorelaxant Properties. Int. J. Pharm. 2017, 518, 59–65. [Google Scholar] [CrossRef]
- Turner, A.; Kaas, Q.; Craik, D.J. Hormone-like Conopeptides—New Tools for Pharmaceutical Design. RSC Med. Chem. 2020, 11, 1235–1251. [Google Scholar] [CrossRef]
- Erasa XEP 30 Clinical Results. Available online: https://erasaskincare.com/pages/our-results (accessed on 1 June 2024).
- Balaev, A.N.; Okhmanovich, K.A.; Osipov, V.N. A Shortened, Protecting Group Free, Synthesis of the Anti-Wrinkle Venom Analogue Syn-Ake® Exploiting an Optimized Hofmann-Type Rearrangement. Tetrahedron Lett. 2014, 55, 5745–5747. [Google Scholar] [CrossRef]
- Pennington, M.W.; Czerwinski, A.; Norton, R.S. Peptide Therapeutics from Venom: Current Status and Potential. Bioorg. Med. Chem. 2018, 26, 2738–2758. [Google Scholar] [CrossRef] [PubMed]
- dsm.com. An Effective Synthetic Peptide Ingredient Found in the Venom of the Temple Viper 2024. Available online: https://www.dsm.com/personal-care/en_US/products/skin-bioactives/syn-ake.html# (accessed on 1 June 2024).
- Gok, B.; Budama-Kilinc, Y.; Kecel-Gunduz, S. Anti-Aging Activity of Syn-Ake Peptide by in Silico Approaches and in Vitro Tests. J. Biomol. Struct. Dyn. 2024, 42, 5015–5029. [Google Scholar] [CrossRef] [PubMed]
- Vasudeva, N.; Sharma, S.K. Biologically Active Compounds from the Genus Hibiscus. Pharm. Biol. 2008, 46, 145–153. [Google Scholar] [CrossRef]
- Shammi, S.J.; Islam, R.; Ashraf-Uz-Zaman, R.M.; Alam, B. Comparative Pharmacological Studies of Abelmoschuse Sculentus Linn. Fruits and Seeds. Glob. J. Pharmacol. 2014, 8, 98–106. [Google Scholar]
- Irene, F. MYOXINOLTM REGIME DELIVERS VISIBLE WRINKLE REDUCTION THAT GETS BETTER & BETTER*. Available online: https://ireneforteskincare.com/pages/clinical-trials (accessed on 1 June 2024).
- Global Anti-Aging Market 2024–2033. Available online: https://www.custommarketinsights.com/report/anti-aging-market/#:~:text=The%20size%20of%20the%20global,7.5%25%20between%202022%20and%202030 (accessed on 1 June 2024).
- Transparecy Market Research Cosmetic Skin Care Market. Available online: https://www.transparencymarketresearch.com/cosmetic-skin-care-market.html (accessed on 1 June 2024).
- Cosmetic Peptide Manufacturing Market Outlook from 2024 to 2034. Available online: https://www.futuremarketinsights.com/reports/cosmetic-peptide-manufacturing-market (accessed on 1 June 2024).
- Global Syn-Ake Market Size, Scope And Forecast Report. Available online: https://www.marketresearchintellect.com/product/global-syn-ake-market/ (accessed on 1 June 2024).
- Irene Forte, Hibiscus Night Cream. Myoxinol Acts in a Similar Way to Injectables in Reducing Lines. Available online: https://lampoonmagazine.com/article/2022/07/22/irene-forte-hibiscus/ (accessed on 1 June 2024).
- Clean Beauty Market Set to Reach $8.10 Billion by 2023: Rising Consumer Awareness Drives Demand–ResearchAndMarkets.Com. Available online: https://www.businesswire.com/news/home/20231106992010/en/Clean-Beauty-Market-Set-to-Reach-8.10-Billion-by-2023-Rising-Consumer-Awareness-Drives-Demand---ResearchAndMarkets.com (accessed on 1 June 2024).
- Campa, M.; Baron, E. Anti-Aging Effects of Select Botanicals: Scientific Evidence and Current Trends. Cosmetics 2018, 5, 54. [Google Scholar] [CrossRef]


| Name | Brand Name | Source/ Origin | Mechanism of Action | Duration of Effect | Clinical Study Findings |
|---|---|---|---|---|---|
| Botulinum Toxin Injection | Botox, Dysport, Xeomin | Clostridium botulinum | Inhibits ACh 1 release by cleaving SNAP-25 2, blocking muscle contractions | 3–4 months | 80% reduction in wrinkle appearance within one week; effects last 3–4 months |
| Botulinum Toxin Topical Formulations | Topical Botox Gel | Botulinum toxin type A | Inhibits ACh release by targeting SNAP-25, blocking muscle contractions | Continuous use | Nanoparticle-based formulations: 25% reduction after 4 weeks; Liposomal delivery: 30% improvement after 8 weeks |
| Argireline | Acetyl Hexapeptide-8 | Synthetic peptide | Inhibits SNARE 3 complex assembly, blocking neurotransmitter release | Continuous use | Reduced wrinkle depth by up to 30% after 30 days |
| Snap-8 | Acetyl Octapeptide-3 | Synthetic peptide | Extends Argireline action, inhibiting SNARE complex | Continuous use | Reduced wrinkle depth by up to 38% after 28 days |
| Leuphasyl | Pentapeptide-18 | Synthetic peptide | Modulates muscle contraction by blocking calcium channels, reducing ACh release | Continuous use | Reduced wrinkle depth by up to 24% after 28 days |
| Vialox | Pentapeptide-3 | Synthetic peptide | Acts as a competitive antagonist at ACh postsynaptic membrane receptors, inhibiting muscle contraction | Continuous use | Reduced skin roughness by 47% and wrinkle depth by 49% after 28 days |
| XEP-30 and XEP-018 | μ-conotoxin CnIIIC | Synthetic peptide derived from marine cone snail venom | Blocks ACh release by targeting NaV1.4 4 sodium channels, mimicking botulinum toxin | Continuous use | Reduced wrinkle depth by up to 48% after 30 days |
| Syn-Ake | Dipeptide Diaminobutyroyl Benzylamide Diacetate | Synthetic (Snake venom mimic) | Antagonizes muscle nAChRs 5 and modulates GABAA 6 receptors | Continuous use | Reduced wrinkle size by up to 52% after 28 days |
| Myoxinol | Hibiscus esculentus extract | Natural extract | Inhibits muscle contractions via interaction with GABA 7 receptors, enhancing GABAergic transmission | Continuous use | Reduced wrinkle depth by up to 26% after 3 weeks |
| Peptide/Extract | Brand/Company | City, Country |
|---|---|---|
| Argireline® Amplified peptide solution Argireline® peptide solution C Argireline® YOUth peptide Argirelox™ peptide solution Inyline® peptide solution SNAP-8™ peptide solution C Leuphasyl Argirelox Inyline | Lipotec | Barcelona, Spain |
| Vialox SYN-Ake | DSM-Firmenich | Heerlen, Netherlands |
| XEP-30 XEP-018 | Erasa XEP-30 | New York, United States |
| Myoxinol | BASF | Monheim, Germany |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.M.; Yi, E.-J.; Jin, X.; Zheng, Q.; Park, S.-J.; Yi, G.-S.; Yang, S.-J.; Yi, T.-H. Sustainable Dynamic Wrinkle Efficacy: Non-Invasive Peptides as the Future of Botox Alternatives. Cosmetics 2024, 11, 118. https://doi.org/10.3390/cosmetics11040118
Nguyen TTM, Yi E-J, Jin X, Zheng Q, Park S-J, Yi G-S, Yang S-J, Yi T-H. Sustainable Dynamic Wrinkle Efficacy: Non-Invasive Peptides as the Future of Botox Alternatives. Cosmetics. 2024; 11(4):118. https://doi.org/10.3390/cosmetics11040118
Chicago/Turabian StyleNguyen, Trang Thi Minh, Eun-Ji Yi, Xiangji Jin, Qiwen Zheng, Se-Jig Park, Gyeong-Seon Yi, Su-Jin Yang, and Tae-Hoo Yi. 2024. "Sustainable Dynamic Wrinkle Efficacy: Non-Invasive Peptides as the Future of Botox Alternatives" Cosmetics 11, no. 4: 118. https://doi.org/10.3390/cosmetics11040118
APA StyleNguyen, T. T. M., Yi, E.-J., Jin, X., Zheng, Q., Park, S.-J., Yi, G.-S., Yang, S.-J., & Yi, T.-H. (2024). Sustainable Dynamic Wrinkle Efficacy: Non-Invasive Peptides as the Future of Botox Alternatives. Cosmetics, 11(4), 118. https://doi.org/10.3390/cosmetics11040118









