Use of Exosomes for Cosmetics Applications
Abstract
1. Introduction
2. Methodology
Search Strategy
3. Results
Meta-Analysis
4. Exosomes Composition and Biomedical Applications
Exosome Growth Factors in Biomedical Applications
5. Cosmetic Applications of Exosomes
5.1. Regenerative Dermatology
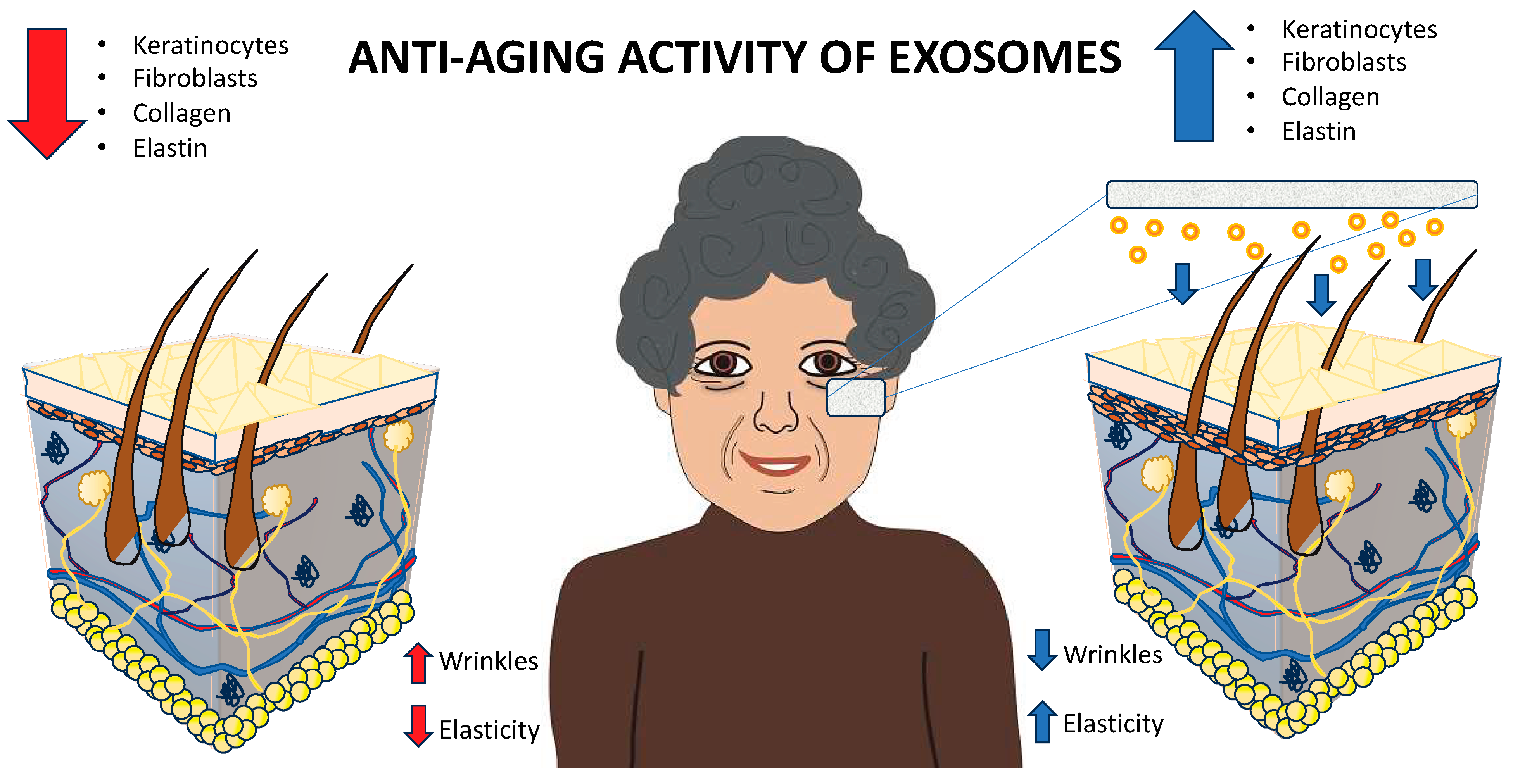
5.1.1. Geroprevention or Geroprotection (Anti-Aging)
5.1.2. Hidroregulation (Skin Hydration)
5.1.3. Facial Dyschromia (Skin Pigmentation)
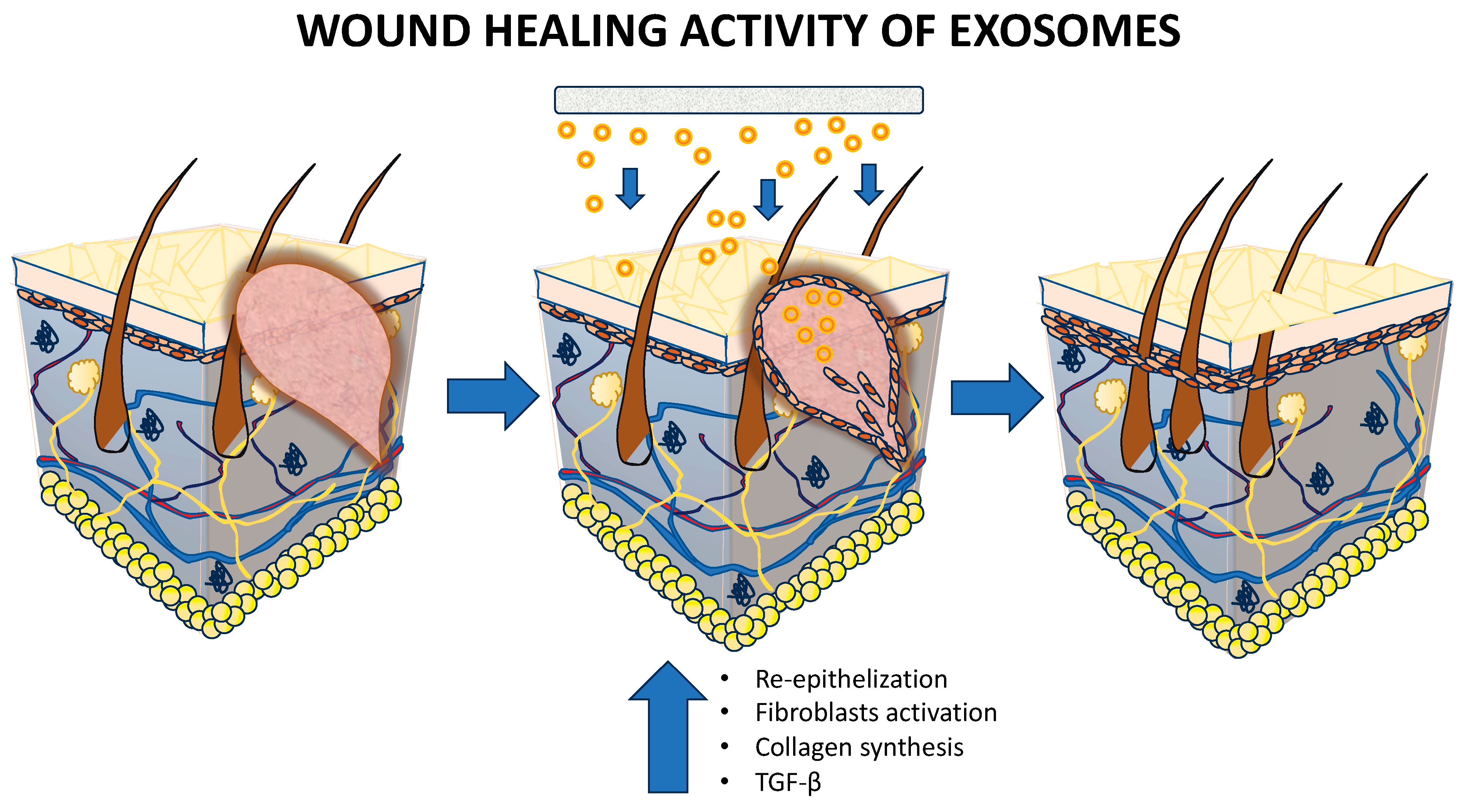
5.1.4. Wound Healing
5.1.5. Delivery of Biomolecules
5.1.6. Customized Dermatology
5.1.7. Clinical Validation
6. Limitations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Cho, B.S.; Irvine Duncan, D. Perspective Chapter: Development of Exosomes for Esthetic Use. In Physiology; Saheera, S., Ed.; IntechOpen: London, UK, 2023; Volume 20, ISBN 978-1-83768-949-1. [Google Scholar]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, Biologic Function and Clinical Potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Ha, D.H.; Kim, H.; Lee, J.; Kwon, H.H.; Park, G.-H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef] [PubMed]
- Bondhopadhyay, B.; Sisodiya, S.; Alzahrani, F.A.; Bakhrebah, M.A.; Chikara, A.; Kasherwal, V.; Khan, A.; Rani, J.; Dar, S.A.; Akhter, N.; et al. Exosomes: A Forthcoming Era of Breast Cancer Therapeutics. Cancers 2021, 13, 4672. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Wang, X.; Lu, M.; Ding, Q.; Chen, A.F.; Xiang, M.; Chen, S. iPSC-Derived Exosomes Promote Angiogenesis in Naturally Aged Mice. Aging 2023, 15, 5854. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.H.; Lee, Y.B.; Kang, D.; Choi, E.; Nam, Y.; Lee, K.H.; You, H.-J.; Kang, H.J.; An, S.H.; Jeon, H. Overcome the Barriers of the Skin: Exosome Therapy. Biomater. Res. 2021, 25, 22. [Google Scholar] [CrossRef] [PubMed]
- Benito-Martínez, S.; Zhu, Y.; Jani, R.A.; Harper, D.C.; Marks, M.S.; Delevoye, C. Research Techniques Made Simple: Cell Biology Methods for the Analysis of Pigmentation. J. Investig. Dermatol. 2020, 140, 257–268.e8. [Google Scholar] [CrossRef] [PubMed]
- Samal, S.; Dash, P.; Dash, M. Drug Delivery to the Bone Microenvironment Mediated by Exosomes: An Axiom or Enigma. Int. J. Nanomed. 2021, 16, 3509–3540. [Google Scholar] [CrossRef] [PubMed]
- Prasai, A.; Jay, J.W.; Jupiter, D.; Wolf, S.E.; El Ayadi, A. Role of Exosomes in Dermal Wound Healing: A Systematic Review. J. Investig. Dermatol. 2022, 142, 662–678.e8. [Google Scholar] [CrossRef]
- Ku, Y.C.; Omer Sulaiman, H.; Anderson, S.R.; Abtahi, A.R. The Potential Role of Exosomes in Aesthetic Plastic Surgery: A Review of Current Literature. Plast. Reconstr. Surg.-Glob. Open 2023, 11, e5051. [Google Scholar] [CrossRef] [PubMed]
- Frazier, T.; Alarcon, A.; Wu, X.; Mohiuddin, O.A.; Motherwell, J.M.; Carlsson, A.H.; Christy, R.J.; Edwards, J.V.; Mackin, R.T.; Prevost, N.; et al. Clinical Translational Potential in Skin Wound Regeneration for Adipose-Derived, Blood-Derived, and Cellulose Materials: Cells, Exosomes, and Hydrogels. Biomolecules 2020, 10, 1373. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, T.; Farooq, N.; Sharma, A.; Shiekh, P.A.; Hassan, A.; Dar, L.A.; Nazir, J.; Godha, M.; Sheikh, F.A.; Gugjoo, M.B.; et al. Exosomes in Nanomedicine: A Promising Cell-Free Therapeutic Intervention in Burn Wounds. Stem Cell Res. Ther. 2024, 15, 355. [Google Scholar] [CrossRef] [PubMed]
- Butreddy, A.; Kommineni, N.; Dudhipala, N. Exosomes as Naturally Occurring Vehicles for Delivery of Biopharmaceuticals: Insights from Drug Delivery to Clinical Perspectives. Nanomaterials 2021, 11, 1481. [Google Scholar] [CrossRef]
- Hajialiasgary Najafabadi, A.; Soheilifar, M.H.; Masoudi-Khoram, N. Exosomes in Skin Photoaging: Biological Functions and Therapeutic Opportunity. Cell Commun. Signal. 2024, 22, 32. [Google Scholar] [CrossRef] [PubMed]
- Tienda-Vázquez, M.A.; Hanel, J.M.; Márquez-Arteaga, E.M.; Salgado-Álvarez, A.P.; Scheckhuber, C.Q.; Alanis-Gómez, J.R.; Espinoza-Silva, J.I.; Ramos-Kuri, M.; Hernández-Rosas, F.; Melchor-Martínez, E.M.; et al. Exosomes: A Promising Strategy for Repair, Regeneration and Treatment of Skin Disorders. Cells 2023, 12, 1625. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal Stromal/Stem Cell (MSC)-Derived Exosomes in Clinical Trials. Stem Cell Res. Ther. 2023, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.; Thapa, N.; Chwae, Y.; Yoon, S.; Kim, B.; Lee, J.; Jang, Y.; Kim, J. Transforming Growth Factor-β Family and Stem Cell-derived Exosome Therapeutic Treatment in Osteoarthritis (Review). Int. J. Mol. Med. 2022, 49, 62. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, W.; Yao, Q.; Zhang, H.; Dong, G.; Zhang, M.; Liu, Y.; Chen, J.-K.; Dong, Z. Exosome Production and Its Regulation of EGFR during Wound Healing in Renal Tubular Cells. Am. J. Physiol.-Ren. Physiol. 2017, 312, F963–F970. [Google Scholar] [CrossRef] [PubMed]
- Olejarz, W.; Kubiak-Tomaszewska, G.; Chrzanowska, A.; Lorenc, T. Exosomes in Angiogenesis and Anti-Angiogenic Therapy in Cancers. Int. J. Mol. Sci. 2020, 21, 5840. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Lim, K.; Shin, Y.; Song, K.; Kang, G.-H.; Kim, D.Y.; Shin, H.-C.; Cho, S.-G. Thermostable Basic Fibroblast Growth Factor Enhances the Production and Activity of Human Wharton’s Jelly Mesenchymal Stem Cell-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 16460. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Yang, H.C.; Rhee, W.J.; Kang, H. Vascular Smooth Muscle Cell-Derived Exosomal MicroRNAs Regulate Endothelial Cell Migration Under PDGF Stimulation. Cells 2020, 9, 639. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xue, J.; Wu, Y.; Zhou, H. Therapeutic Effect of Exosomes Derived from Hepatocyte-Growth-Factor-Overexpressing Adipose Mesenchymal Stem Cells on Liver Injury. Folia Histochem. Cytobiol. 2023, 61, 160–171. [Google Scholar] [CrossRef]
- Ma, K.; Xu, H.; Zhang, J.; Zhao, F.; Liang, H.; Sun, H.; Li, P.; Zhang, S.; Wang, R.; Chen, X. Insulin-like Growth Factor-1 Enhances Neuroprotective Effects of Neural Stem Cell Exosomes after Spinal Cord Injury via an miR-219a-2-3p/YY1 Mechanism. Aging 2019, 11, 12278–12294. [Google Scholar] [CrossRef] [PubMed]
- Taha, E.A.; Ono, K.; Eguchi, T. Roles of Extracellular HSPs as Biomarkers in Immune Surveillance and Immune Evasion. Int. J. Mol. Sci. 2019, 20, 4588. [Google Scholar] [CrossRef] [PubMed]
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.M.; Sadik, M.; Alaarg, A.; Smith, C.I.E.; Lehtiö, J.; El Andaloussi, S.; et al. Cells Release Subpopulations of Exosomes with Distinct Molecular and Biological Properties. Sci. Rep. 2016, 6, 22519. [Google Scholar] [CrossRef]
- Kawamoto, E.; Park, E.J.; Shimaoka, M. Methods to Study Integrin Functions on Exosomes. In The Integrin Interactome; Vicente-Manzanares, M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; Volume 2217, pp. 265–281. ISBN 978-1-07-160961-3. [Google Scholar]
- Maji, S.; Chaudhary, P.; Akopova, I.; Nguyen, P.M.; Hare, R.J.; Gryczynski, I.; Vishwanatha, J.K. Exosomal Annexin II Promotes Angiogenesis and Breast Cancer Metastasis. Mol. Cancer Res. 2017, 15, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Névo, N.; Jouve, M.; Valenzuela, J.I.; Maurin, M.; Verweij, F.J.; Palmulli, R.; Lankar, D.; Dingli, F.; Loew, D.; et al. Specificities of Exosome versus Small Ectosome Secretion Revealed by Live Intracellular Tracking of CD63 and CD9. Nat. Commun. 2021, 12, 4389. [Google Scholar] [CrossRef]
- You, Y.; Shan, Y.; Chen, J.; Yue, H.; You, B.; Shi, S.; Li, X.; Cao, X. Matrix Metalloproteinase 13-containing Exosomes Promote Nasopharyngeal Carcinoma Metastasis. Cancer Sci. 2015, 106, 1669–1677. [Google Scholar] [CrossRef]
- Hong, C.S.; Diergaarde, B.; Whiteside, T.L. Small Extracellular Vesicles in Plasma Carry Luminal Cytokines That Remain Undetectable by Antibody-Based Assays in Cancer Patients and Healthy Donors. BJC Rep. 2024, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, R.D.; Bandari, S.K.; Vlodavsky, I. Proteases and Glycosidases on the Surface of Exosomes: Newly Discovered Mechanisms for Extracellular Remodeling. Matrix Biol. 2019, 75, 160–169. [Google Scholar] [CrossRef]
- Sreeraj, H.; AnuKiruthika, R.; Tamilselvi, K.S.; Subha, D. Exosomes for Skin Treatment: Therapeutic and Cosmetic Applications. Nano TransMed 2024, 3, 100048. [Google Scholar] [CrossRef]
- Farooq, M.; Khan, A.W.; Kim, M.S.; Choi, S. The Role of Fibroblast Growth Factor (FGF) Signaling in Tissue Repair and Regeneration. Cells 2021, 10, 3242. [Google Scholar] [CrossRef]
- McGraw, I.T.; Wilson, E.E.; Behfar, A.; Paradise, C.R.; Rohrich, R.J.; Wyles, S.P. Evolving Role of Exosomes in Plastic and Reconstructive Surgery and Dermatology. Plast. Reconstr. Surg.-Glob. Open 2024, 12, e6061. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Z.; Wang, Y.; Zhou, K.; Li, H.; Bi, S.; Wang, Y.; Wu, W.; Huang, Y.; Peng, B.; et al. Bioengineered MSC-Derived Exosomes in Skin Wound Repair and Regeneration. Front. Cell Dev. Biol. 2023, 11, 1029671. [Google Scholar] [CrossRef] [PubMed]
- Proffer, S.L.; Paradise, C.R.; DeGrazia, E.; Halaas, Y.; Durairaj, K.K.; Somenek, M.; Sivly, A.; Boon, A.J.; Behfar, A.; Wyles, S.P. Efficacy and Tolerability of Topical Platelet Exosomes for Skin Rejuvenation: Six-Week Results. Aesthetic Surg. J. 2022, 42, 1185–1193. [Google Scholar] [CrossRef]
- Zhang, B.; Gong, J.; He, L.; Khan, A.; Xiong, T.; Shen, H.; Li, Z. Exosomes Based Advancements for Application in Medical Aesthetics. Front. Bioeng. Biotechnol. 2022, 10, 1083640. [Google Scholar] [CrossRef]
- Thakur, A.; Shah, D.; Rai, D.; Parra, D.C.; Pathikonda, S.; Kurilova, S.; Cili, A. Therapeutic Values of Exosomes in Cosmetics, Skin Care, Tissue Regeneration, and Dermatological Diseases. Cosmetics 2023, 10, 65. [Google Scholar] [CrossRef]
- Baumann, L.; Bernstein, E.F.; Weiss, A.S.; Bates, D.; Humphrey, S.; Silberberg, M.; Daniels, R. Clinical Relevance of Elastin in the Structure and Function of Skin. Aesthetic Surg. J. Open Forum 2021, 3, ojab019. [Google Scholar] [CrossRef]
- Al-Atif, H. Collagen Supplements for Aging and Wrinkles: A Paradigm Shift in the Field of Dermatology and Cosmetics. Dermatol. Pract. Concept. 2022, 12, e2022018. [Google Scholar] [CrossRef]
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.F.M.R.; Motta, A.; Fauzi, M.B. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef] [PubMed]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Zhang, Y.; Su, Z.; Wu, S.; Li, Y.; Yi, J.; Lai, W.; Chen, J.; Zheng, Y. hMSC Exosomes as a Novel Treatment for Female Sensitive Skin: An in Vivo Study. Front. Bioeng. Biotechnol. 2022, 10, 1053679. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-K.; Zhong, L.; Santiago, J. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef]
- Muthu, S.; Bapat, A.; Jain, R.; Jeyaraman, N.; Jeyaraman, M. Exosomal Therapy—A New Frontier in Regenerative Medicine. Stem Cell Investig. 2021, 8, 7. [Google Scholar] [CrossRef]
- Miller, J.; Chen, G.; Hayag, M.V. A Review of Exosomes in Regenerative Cosmetic Dermatology. Mol. Front. J. 2023, 7, 64–70. [Google Scholar] [CrossRef]
- Vyas, K.S.; Kaufman, J.; Munavalli, G.S.; Robertson, K.; Behfar, A.; Wyles, S.P. Exosomes: The Latest in Regenerative Aesthetics. Regen. Med. 2023, 18, 181–194. [Google Scholar] [CrossRef]
- Dal’Forno-Dini, T.; Birck, M.S.; Rocha, M.; Bagatin, E. Exploring the Reality of Exosomes in Dermatology. An. Bras. Dermatol. 2024, 100, 121–130. [Google Scholar] [CrossRef]
- Taub, A.F. Regenerative Topical Skincare: Stem Cells and Exosomes. Front. Med. 2024, 11, 1443963. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Ji, Y.; Zhang, X.; Shu, S.; Wu, Z. Characterization of pH- and Thermosensitive Hydrogel as a Vehicle for Controlled Protein Delivery. J. Pharm. Sci. 2011, 100, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Cai, J.; Wei, Y.; Jiang, Z.; Desjardins, H.E.; Adams, A.E.; Li, S.; Kao, H.-K.; Guo, L. Exosomes Are Comparable to Source Adipose Stem Cells in Fat Graft Retention with Up-Regulating Early Inflammation and Angiogenesis. Plast. Reconstr. Surg. 2019, 144, 816e–827e. [Google Scholar] [CrossRef]
- Li, K.; Zhou, P.; Guo, Y.; Xu, T.; Lin, S.; Lin, S.; Ji, C. Recent Advances in Exosomal Non-coding RNA-based Therapeutic Approaches for Photoaging. Ski. Res. Technol. 2023, 29, e13463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guan, J.; Niu, X.; Hu, G.; Guo, S.; Li, Q.; Xie, Z.; Zhang, C.; Wang, Y. Exosomes Released from Human Induced Pluripotent Stem Cells-Derived MSCs Facilitate Cutaneous Wound Healing by Promoting Collagen Synthesis and Angiogenesis. J. Transl. Med. 2015, 13, 49. [Google Scholar] [CrossRef]
- Wan, R.; Hussain, A.; Behfar, A.; Moran, S.L.; Zhao, C. The Therapeutic Potential of Exosomes in Soft Tissue Repair and Regeneration. Int. J. Mol. Sci. 2022, 23, 3869. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; He, Y.; Chen, S.; Zhang, D.; Yu, Y.; Fan, C. Macrophage-Derived miRNA-Containing Exosomes Induce Peritendinous Fibrosis after Tendon Injury through the miR-21-5p/Smad7 Pathway. Mol. Ther.-Nucleic Acids 2019, 14, 114–130. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wang, Q.; Jiang, D. Extracellular Vesicles from Bone Marrow-Derived Multipotent Mesenchymal Stromal Cells Regulate Inflammation and Enhance Tendon Healing. J. Transl. Med. 2019, 17, 211. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Xu, M.; Bai, J.; Lin, J.; Yu, B.; Liu, Y.; Guo, X.; Shen, J.; Sun, H.; Hao, Y.; et al. Tenocyte-Derived Exosomes Induce the Tenogenic Differentiation of Mesenchymal Stem Cells through TGF-β. Cytotechnology 2019, 71, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Z.-P.; Xu, C.; Guo, A. TGF-Β1-Containing Exosomes Derived from Bone Marrow Mesenchymal Stem Cells Promote Proliferation, Migration and Fibrotic Activity in Rotator Cuff Tenocytes. Regen. Ther. 2020, 15, 70–76. [Google Scholar] [CrossRef]
- Li, M.; Jia, J.; Li, S.; Cui, B.; Huang, J.; Guo, Z.; Ma, K.; Wang, L.; Cui, C. Exosomes Derived from Tendon Stem Cells Promote Cell Proliferation and Migration through the TGF β Signal Pathway. Biochem. Biophys. Res. Commun. 2021, 536, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, M.; Shi, M.; Zhang, T.; Lu, W.; Yang, S.; Cui, Q.; Li, Z. Adipose-Derived Mesenchymal Stromal Cell-Derived Exosomes Promote Tendon Healing by Activating Both SMAD1/5/9 and SMAD2/3. Stem Cell Res. Ther. 2021, 12, 338. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Wang, Y.; Wang, Z.; Thoreson, A.R.; Jacobson, D.S.; Amadio, P.C.; Behfar, A.; Moran, S.L.; Zhao, C. A Novel Engineered Purified Exosome Product Patch for Tendon Healing: An Explant in an Ex Vivo Model. J. Orthop. Res. 2021, 39, 1825–1837. [Google Scholar] [CrossRef]
- Dilsiz, N. A Comprehensive Review on Recent Advances in Exosome Isolation and Characterization: Toward Clinical Applications. Transl. Oncol. 2024, 50, 102121. [Google Scholar] [CrossRef] [PubMed]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and Maintenance of Skin Barrier Function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef]
- Guillot, A.J.; Martínez-Navarrete, M.; Garrigues, T.M.; Melero, A. Skin Drug Delivery Using Lipid Vesicles: A Starting Guideline for Their Development. J. Control. Release 2023, 355, 624–654. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic Acid: A Key Molecule in Skin Aging. Dermato-Endocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Bahr, M.M.; Amer, M.S.; Abo-El-Sooud, K.; Abdallah, A.N.; El-Tookhy, O.S. Preservation Techniques of Stem Cells Extracellular Vesicles: A Gate for Manufacturing of Clinical Grade Therapeutic Extracellular Vesicles and Long-Term Clinical Trials. Int. J. Vet. Sci. Med. 2020, 8, 1–8. [Google Scholar] [CrossRef]
- Wang, D.-Q.; Li, X.; Zhang, R.-Y.; Yuan, C.; Yan, B.; Humbert, P.; Quan, Z.-X. Effects of Investigational Moisturizers on the Skin Barrier and Microbiome Following Exposure to Environmental Aggressors: A Randomized Clinical Trial and Ex Vivo Analysis. J. Clin. Med. 2023, 12, 6078. [Google Scholar] [CrossRef]
- Yoo, K.; Thapa, N.; Lee, J.; Jang, Y.; Lee, J.O.; Kim, J. Dermal Fibroblast Cell-derived Exosomes for Atopic Dermatitis: In-vitro Test. Ski. Res. Technol. 2023, 29, e13382. [Google Scholar] [CrossRef] [PubMed]
- Quiñones-Vico, M.I.; Sanabria-de La Torre, R.; Sánchez-Díaz, M.; Sierra-Sánchez, Á.; Montero-Vílchez, T.; Fernández-González, A.; Arias-Santiago, S. The Role of Exosomes Derived from Mesenchymal Stromal Cells in Dermatology. Front. Cell Dev. Biol. 2021, 9, 647012. [Google Scholar] [CrossRef]
- Dong, J.; Wu, B.; Tian, W. How to Maximize the Therapeutic Effect of Exosomes on Skin Wounds in Diabetes Mellitus: Review and Discussion. Front. Endocrinol. 2023, 14, 1146991. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Qin, X.; He, J.; Wang, X.; Wang, J.; Yang, R.; Chen, X. The Functions and Clinical Application Potential of Exosomes Derived from Mesenchymal Stem Cells on Wound Repair: A Review of Recent Research Advances. Front. Immunol. 2023, 14, 1256687. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.; Qin, F.; Chen, J. Exosomes: A Promising Avenue for Cancer Diagnosis beyond Treatment. Front. Cell Dev. Biol. 2024, 12, 1344705. [Google Scholar] [CrossRef]
- Gui, Q.; Ding, N.; Yao, Z.; Wu, M.; Fu, R.; Wang, Y.; Zhao, Y.; Zhu, L. Extracellular Vesicles Derived from Mesenchymal Stem Cells: The Wine in Hebe’s Hands to Treat Skin Aging. Precis. Clin. Med. 2024, 7, pbae004. [Google Scholar] [CrossRef]
- Cicero, A.L.; Delevoye, C.; Gilles-Marsens, F.; Loew, D.; Dingli, F.; Guéré, C.; André, N.; Vié, K.; Van Niel, G.; Raposo, G. Exosomes Released by Keratinocytes Modulate Melanocyte Pigmentation. Nat. Commun. 2015, 6, 7506. [Google Scholar] [CrossRef] [PubMed]
- Takano, K.; Hachiya, A.; Murase, D.; Tanabe, H.; Kasamatsu, S.; Takahashi, Y.; Moriwaki, S.; Hase, T. Quantitative Changes in the Secretion of Exosomes from Keratinocytes Homeostatically Regulate Skin Pigmentation in a Paracrine Manner. J. Dermatol. 2020, 47, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Akhtar, S.; Prabhu, K.S.; Zarif, L.; Khan, R.; Alam, M.; Buddenkotte, J.; Ahmad, A.; Steinhoff, M.; Uddin, S. Exosomes: Emerging Diagnostic and Therapeutic Targets in Cutaneous Diseases. Int. J. Mol. Sci. 2020, 21, 9264. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Feng, H.; Zeng, H.; Wu, Y.; Zhang, Q.; Yu, J.; Hou, K.; Wu, M. Exosomes: The Emerging Mechanisms and Potential Clinical Applications in Dermatology. Int. J. Biol. Sci. 2024, 20, 1778–1795. [Google Scholar] [CrossRef]
- Bento-Lopes, L.; Cabaço, L.C.; Charneca, J.; Neto, M.V.; Seabra, M.C.; Barral, D.C. Melanin’s Journey from Melanocytes to Keratinocytes: Uncovering the Molecular Mechanisms of Melanin Transfer and Processing. Int. J. Mol. Sci. 2023, 24, 11289. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.R.; Ho, T.; Abdel-Malek, Z.A. Participation of Keratinocyte- and Fibroblast-derived Factors in Melanocyte Homeostasis, the Response to UV, and Pigmentary Disorders. Pigment. Cell Melanoma Res. 2021, 34, 762–776. [Google Scholar] [CrossRef]
- Wang, T.; Gao, H.; Wang, D.; Zhang, C.; Hu, K.; Zhang, H.; Lin, J.; Chen, X. Stem Cell-derived Exosomes in the Treatment of Melasma and Its Percutaneous Penetration. Lasers Surg. Med. 2023, 55, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Han, Z.; Song, D.; Peng, Y.; Xiong, M.; Chen, Z.; Duan, S.; Zhang, L. Engineered Exosome for Drug Delivery: Recent Development and Clinical Applications. Int. J. Nanomed. 2023, 18, 7923–7940. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Pang, Y.; He, Q.; Song, Z.; Xie, X.; Zeng, J.; Guo, J. Exosome-Derived microRNAs: Emerging Players in Vitiligo. Front. Immunol. 2024, 15, 1419660. [Google Scholar] [CrossRef]
- Moghassemi, S.; Dadashzadeh, A.; Sousa, M.J.; Vlieghe, H.; Yang, J.; León-Félix, C.M.; Amorim, C.A. Extracellular Vesicles in Nanomedicine and Regenerative Medicine: A Review over the Last Decade. Bioact. Mater. 2024, 36, 126–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H.; Wang, J. Exosomes as a Novel Pathway for Regulating Development and Diseases of the Skin (Review). Biomed. Rep. 2018, 8, 207–214. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, T.; Xue, Y.; Zhan, B.; Lai, Z.; Huang, W.; Peng, X.; Zhou, Y. Research Progress of Extracellular Vesicles and Exosomes Derived from Mesenchymal Stem Cells in the Treatment of Oxidative Stress-Related Diseases. Front. Immunol. 2023, 14, 1238789. [Google Scholar] [CrossRef] [PubMed]
- Buschow, S.I.; Nolte‘t Hoen, E.N.M.; Van Niel, G.; Pols, M.S.; Ten Broeke, T.; Lauwen, M.; Ossendorp, F.; Melief, C.J.M.; Raposo, G.; Wubbolts, R.; et al. MHC II in Dendritic Cells Is Targeted to Lysosomes or T Cell-Induced Exosomes Via Distinct Multivesicular Body Pathways. Traffic 2009, 10, 1528–1542. [Google Scholar] [CrossRef]
- Hussain, M.W.A.; Jahangir, S.; Ghosh, B.; Yesmin, F.; Anis, A.; Satil, S.N.; Anwar, F.; Rashid, M.H. Exosomes for Regulation of Immune Responses and Immunotherapy. J. Nanotheranostics 2022, 3, 55–85. [Google Scholar] [CrossRef]
- Tutuianu, R.; Rosca, A.-M.; Iacomi, D.M.; Simionescu, M.; Titorencu, I. Human Mesenchymal Stromal Cell-Derived Exosomes Promote In Vitro Wound Healing by Modulating the Biological Properties of Skin Keratinocytes and Fibroblasts and Stimulating Angiogenesis. Int. J. Mol. Sci. 2021, 22, 6239. [Google Scholar] [CrossRef]
- Foo, J.B.; Looi, Q.H.; How, C.W.; Lee, S.H.; Al-Masawa, M.E.; Chong, P.P.; Law, J.X. Mesenchymal Stem Cell-Derived Exosomes and MicroRNAs in Cartilage Regeneration: Biogenesis, Efficacy, miRNA Enrichment and Delivery. Pharmaceuticals 2021, 14, 1093. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, Y.; Yu, A.; Zhang, Z.; Deng, Z.; Xiong, K.; Wang, Q.; Zhang, J. Therapeutic Role of Exosomes and Conditioned Medium in Keloid and Hypertrophic Scar and Possible Mechanisms. Front. Physiol. 2023, 14, 1247734. [Google Scholar] [CrossRef]
- Sousa, P.; Lopes, B.; Sousa, A.C.; Moreira, A.; Coelho, A.; Alvites, R.; Alves, N.; Geuna, S.; Maurício, A.C. Advancements and Insights in Exosome-Based Therapies for Wound Healing: A Comprehensive Systematic Review (2018–June 2023). Biomedicines 2023, 11, 2099. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Li, J.; He, Y.; Xu, W.; Liu, S.; Li, Y.; Chen, Y.; Li, B. Engineering Multifunctional Films Based on Metal-Phenolic Networks for Rational pH-Responsive Delivery and Cell Imaging. ACS Biomater. Sci. Eng. 2016, 2, 317–325. [Google Scholar] [CrossRef]
- Liu, Q.; Li, S.; Dupuy, A.; Mai, H.L.; Sailliet, N.; Logé, C.; Robert, J.-M.H.; Brouard, S. Exosomes as New Biomarkers and Drug Delivery Tools for the Prevention and Treatment of Various Diseases: Current Perspectives. Int. J. Mol. Sci. 2021, 22, 7763. [Google Scholar] [CrossRef]
- Zubarev, I.; Vladimirtsev, D.; Vorontsova, M.; Blatov, I.; Shevchenko, K.; Zvereva, S.; Lunev, E.A.; Faizuloev, E.; Barlev, N. Viral Membrane Fusion Proteins and RNA Sorting Mechanisms for the Molecular Delivery by Exosomes. Cells 2021, 10, 3043. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.O.; He, M. Unlocking the Power of Exosomes for Crossing Biological Barriers in Drug Delivery. Pharmaceutics 2021, 13, 122. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kim, M.S. Using Exosomes, Naturally-Equipped Nanocarriers, for Drug Delivery. J. Control. Release 2015, 219, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in Drug Delivery Systems, Challenges and Future Directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Torres-Martinez, E.J.; Cornejo Bravo, J.M.; Serrano Medina, A.; Pérez González, G.L.; Villarreal Gómez, L.J. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef]
- Villarreal-Gómez, L.J.; Cornejo-Bravo, J.M.; Vera-Graziano, R.; Grande, D. Electrospinning as a Powerful Technique for Biomedical Applications: A Critically Selected Survey. J. Biomater. Sci. Polym. Ed. 2016, 27, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Varshney, A.; Bajaj, R.; Pokharkar, V. Exosomes as New Generation Vehicles for Drug Delivery: Biomedical Applications and Future Perspectives. Molecules 2022, 27, 7289. [Google Scholar] [CrossRef]
- Rodriguez, C.; Porcello, A.; Chemali, M.; Raffoul, W.; Marques, C.; Scaletta, C.; Lourenço, K.; Abdel-Sayed, P.; Applegate, L.A.; Pelissier Vatter, F.; et al. Medicalized Aesthetic Uses of Exosomes and Cell Culture-Conditioned Media: Opening an Advanced Care Era for Biologically Inspired Cutaneous Prejuvenation and Rejuvenation. Cosmetics 2024, 11, 154. [Google Scholar] [CrossRef]
- Burke, J.; Kolhe, R.; Hunter, M.; Isales, C.; Hamrick, M.; Fulzele, S. Stem Cell-Derived Exosomes: A Potential Alternative Therapeutic Agent in Orthopaedics. Stem Cells Int. 2016, 2016, 5802529. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chu, X.; Zhang, X.; Li, X.; Zheng, J.; Li, S.; Li, N.; Sherazi, T.A.; Zhang, S. The Effect of Polymer Backbones and Cation Functional Groups on Properties of Anion Exchange Membranes for Fuel Cells. J. Membr. Sci. 2020, 603, 118025. [Google Scholar] [CrossRef]
- Suh, J.H.; Joo, H.S.; Hong, E.B.; Lee, H.J.; Lee, J.M. Therapeutic Application of Exosomes in Inflammatory Diseases. Int. J. Mol. Sci. 2021, 22, 1144. [Google Scholar] [CrossRef]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Liaw, W.-S.; Chen, C.-A.; Zhou, Q.A. Exosomes─Nature’s Lipid Nanoparticles, a Rising Star in Drug Delivery and Diagnostics. ACS Nano 2022, 16, 17802–17846. [Google Scholar] [CrossRef]
- Besse, B.; Charrier, M.; Lapierre, V.; Dansin, E.; Lantz, O.; Planchard, D.; Le Chevalier, T.; Livartoski, A.; Barlesi, F.; Laplanche, A.; et al. Dendritic Cell-Derived Exosomes as Maintenance Immunotherapy after First Line Chemotherapy in NSCLC. OncoImmunology 2016, 5, e1071008. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Wei, D.; Wu, Z.; Zhou, X.; Wei, X.; Huang, H.; Li, G. Phase I Clinical Trial of Autologous Ascites-Derived Exosomes Combined With GM-CSF for Colorectal Cancer. Mol. Ther. 2008, 16, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-Preconditioned Mesenchymal Stromal Cells Modify Macrophage Polarization for Resolution of Chronic Inflammation via Exosome-Shuttled Let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.-C.; Guo, S.-C.; Li, M.; Ke, Q.-F.; Guo, Y.-P.; Zhang, C.-Q. Chitosan Wound Dressings Incorporating Exosomes Derived from MicroRNA-126-Overexpressing Synovium Mesenchymal Stem Cells Provide Sustained Release of Exosomes and Heal Full-Thickness Skin Defects in a Diabetic Rat Model. Stem Cells Transl. Med. 2017, 6, 736–747. [Google Scholar] [CrossRef]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019, 2019, 7132708. [Google Scholar] [CrossRef]
- Barthe, M.; Bavoux, C.; Finot, F.; Mouche, I.; Cuceu-Petrenci, C.; Forreryd, A.; Chérouvrier Hansson, A.; Johansson, H.; Lemkine, G.F.; Thénot, J.-P.; et al. Safety Testing of Cosmetic Products: Overview of Established Methods and New Approach Methodologies (NAMs). Cosmetics 2021, 8, 50. [Google Scholar] [CrossRef]
- Norouzi, F.; Aghajani, S.; Vosoughi, N.; Sharif, S.; Ghahremanzadeh, K.; Mokhtari, Z.; Verdi, J. Exosomes Derived Stem Cells as a Modern Therapeutic Approach for Skin Rejuvenation and Hair Regrowth. Regen. Ther. 2024, 26, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Su, P.; Zhao, F.; Zhang, Q.; Huang, X.; He, C.; Wu, Q.; Wang, Z.; Ma, J.; Wang, Z. Adipose Mesenchymal Stem Cell-Derived Exosomes Promote Skin Wound Healing in Diabetic Mice by Regulating Epidermal Autophagy. Burn. Trauma 2024, 12, tkae001. [Google Scholar] [CrossRef]
- De, A.; Chakraborty, D.; Agarwal, I.; Sarda, A. Present and Future Use of Exosomes in Dermatology. Indian J. Dermatol. 2024, 69, 461–470. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Yoo, S.M.; Park, H.H.; Lim, H.J.; Kim, Y.-L.; Lee, S.; Seo, K.-W.; Kang, K.-S. Exosomes Derived from Human Umbilical Cord Blood Mesenchymal Stem Cells Stimulates Rejuvenation of Human Skin. Biochem. Biophys. Res. Commun. 2017, 493, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Zhang, Q.; Hu, W.; Zhao, C.; Lv, W.; Yi, Y.; Wang, Y.; Tang, H.; Wu, M.; Wu, Y. The Novel Mechanisms and Applications of Exosomes in Dermatology and Cutaneous Medical Aesthetics. Pharmacol. Res. 2021, 166, 105490. [Google Scholar] [CrossRef]
- Palakurthi, S.S.; Shah, B.; Kapre, S.; Charbe, N.; Immanuel, S.; Pasham, S.; Thalla, M.; Jain, A.; Palakurthi, S. A Comprehensive Review of Challenges and Advances in Exosome-Based Drug Delivery Systems. Nanoscale Adv. 2024, 6, 5803–5826. [Google Scholar] [CrossRef] [PubMed]
- McMahon, R.P.; Kelly, D.L.; Boggs, D.L.; Li, L.; Hu, Q.; Davis, J.M.; Carpenter, W.T. Feasibility of Reducing the Duration of Placebo-Controlled Trials in Schizophrenia Research. Schizophr. Bull. 2008, 34, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tsai, T.; Lee, C. Regulation of Exosomes as Biologic Medicines: Regulatory Challenges Faced in Exosome Development and Manufacturing Processes. Clin. Transl. Sci. 2024, 17, e13904. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.A.; Chan, L.K.W.; Hung, L.C.; Phoebe, L.K.W.; Park, Y.; Yi, K.-H. Clinical Applications of Exosomes: A Critical Review. Int. J. Mol. Sci. 2024, 25, 7794. [Google Scholar] [CrossRef]
- Sasaki, G.H. Plastic Surgery Update on the Mesenchymal Stem-Cell Derived Extracellular Vesicles towards Cell-Free Therapeutic Applications. OALib 2021, 8, 1–24. [Google Scholar] [CrossRef]


| Year | Exosomes | Regenerative Dermatology | Antiaging | Skin Hydration | Skin Pimentation | Wound Healing | Delivery of Biomolecules | Total (Year) |
|---|---|---|---|---|---|---|---|---|
| 2024 | 4982 | 26 | 25 | 2 | 7 | 309 | 31 | 5382 |
| 2023 | 5099 | 17 | 11 | 2 | 4 | 282 | 28 | 5443 |
| 2022 | 5226 | 15 | 11 | 1 | 2 | 258 | 30 | 5543 |
| 2021 | 5075 | 11 | 4 | 1 | 1 | 190 | 27 | 5309 |
| 2020 | 4299 | 5 | 4 | 1 | 4 | 145 | 22 | 4480 |
| 2019 | 3281 | 4 | 4 | 0 | 2 | 92 | 15 | 3398 |
| 2018 | 2573 | 1 | 3 | 0 | 1 | 61 | 10 | 2649 |
| 2017 | 2040 | 0 | 0 | 0 | 1 | 41 | 13 | 2095 |
| 2016 | 1560 | 1 | 0 | 0 | 1 | 36 | 4 | 1602 |
| 2015 | 1126 | 1 | 0 | 0 | 1 | 20 | 5 | 1153 |
| 2014 | 912 | 0 | 0 | 0 | 0 | 4 | 2 | 918 |
| Total | 36,173 | 81 | 62 | 7 | 24 | 1438 | 187 | 37,972 |
| Biomolecule Type | Molecule | Application | Reference |
|---|---|---|---|
| Growth Factors | Transforming Growth Factor-β (TGF-β) | Promotes wound healing, tissue remodeling, and immune regulation | [19] |
| Epidermal Growth Factor (EGF) | Stimulates cell proliferation and skin regeneration | [20] | |
| Vascular Endothelial Growth Factor (VEGF) | Enhances angiogenesis and vascular repair | [21] | |
| Fibroblast Growth Factor (FGF) | Supports fibroblast activity, wound healing, and skin elasticity | [22] | |
| Platelet-Derived Growth Factor (PDGF) | Aids in tissue repair and fibroblast recruitment | [23] | |
| Hepatocyte Growth Factor (HGF) | Encourages cell motility, proliferation, and tissue repair | [24] | |
| Insulin-Like Growth Factor (IGF) | Stimulates cell growth and regeneration | [25] | |
| Proteins | Heat Shock Proteins (HSPs) | Assist in protein folding, stress responses, and cell survival | [33] |
| Alix and TSG101 | Exosomal marker proteins involved in exosome biogenesis | [34] | |
| Integrins | Mediate cell adhesion and signaling, influencing tissue repair and immune responses | [35] | |
| Annexins | Facilitate membrane fusion and trafficking | [36] | |
| CD63, CD9, and CD81 | Surface markers and tetraspanins involved in exosome structure and cell targeting | [37] | |
| Collagen and Elastin Precursors | Contribute to skin structure and elasticity | [15] | |
| Matrix Metalloproteinases (MMPs) | Regulate extracellular matrix remodeling | [38] | |
| Other Bioactive Molecules | Cytokines | Such as IL-6 and TNF-α, modulate inflammation and immune responses | [39] |
| Enzymes | Play roles in extracellular matrix remodeling and signaling | [40] |
| Parameter | [27] | [88] | [9] |
|---|---|---|---|
| Focus | MSC-derived exosomes | Immune cell-derived exosomes | Role of exosomes in rodents |
| Mechanism | Angiogenesis, cell proliferation, ECM remodeling, inflammation modulation | Immune activation, antigen presentation, anti-tumor effects | microRNA signaling, wound closure, scar reduction |
| Clinical Implications | Regenerative medicine for wound healing | Immune therapy and anti-tumor potential | Standardized protocols for wound healing studies |
| Challenges | Targeting and retention issues | Defining precise immune responses | Translating rodent data to humans |
| Solutions | Bioengineering customization | Protein packaging for immune modulation | Pre-clinical guidance for clinical studies |
| Exosomes | Isolation/Purification | Source Cell-Type/Application Route | Size | Administration | In Vitro/In Vivo Assessment | Application | Ref. |
|---|---|---|---|---|---|---|---|
| MART-1 peptide-loaded exosomes | Ultrafiltration/UC sucrose cushion | Dendritic cell EVs derived from monocytes | 50–150 nm | Intradermal injection | In vitro/ In vivo | Wound healing and regenerative management | [109] |
| Autologous ascites-derived exosomes combined with GM-CSF | Sucrose/D2O density gradient ultracentrifugation | Dendritic cells (Dex), tumor cells (Tex), and malignant effusions | 60–90 nm | Subcutaneous immunization | In vivo | Colorectal Cancer | [110] |
| LPS-preconditioned MSC-derived exosomes (LPS pre-Exo) | Gradient centrifugation method | Human umbilical cord tissue | 40–90 nm | Intracutaneous injection | In vitro/ In vivo | Chronic inflammation and wound healing | [111] |
| Chitosan Wound Dressings Incorporating Exosomes Derived from MicroRNA-126-Overexpressing Synovium Mesenchymal Stem Cells | Gradient centrifugation method | Biopsies of synovial membrane | 30–150 nm | Pressure dressing | In vitro/ In vivo | Heal Full-Thickness Skin Defects in a Diabetic | [112] |
| Bone marrow from human jaw and iliac crest | Gradient centrifugation method | Human monocytes | 20–200 nm | Intravenous administration | In vitro/ In vivo | Cutaneous Wound Healing | [113] |
| Platelet-derived exosome | Gradient centrifugation method | Human platelets | 40–250 nm | Topical treatment | In vivo | Skin rejuvenation | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villarreal-Gómez, L.J.; Origel-Lucio, S.; Hernández-Hernández, D.A.; Pérez-González, G.L. Use of Exosomes for Cosmetics Applications. Cosmetics 2025, 12, 9. https://doi.org/10.3390/cosmetics12010009
Villarreal-Gómez LJ, Origel-Lucio S, Hernández-Hernández DA, Pérez-González GL. Use of Exosomes for Cosmetics Applications. Cosmetics. 2025; 12(1):9. https://doi.org/10.3390/cosmetics12010009
Chicago/Turabian StyleVillarreal-Gómez, Luis Jesús, Sergio Origel-Lucio, Daniela Alejandra Hernández-Hernández, and Graciela Lizeth Pérez-González. 2025. "Use of Exosomes for Cosmetics Applications" Cosmetics 12, no. 1: 9. https://doi.org/10.3390/cosmetics12010009
APA StyleVillarreal-Gómez, L. J., Origel-Lucio, S., Hernández-Hernández, D. A., & Pérez-González, G. L. (2025). Use of Exosomes for Cosmetics Applications. Cosmetics, 12(1), 9. https://doi.org/10.3390/cosmetics12010009









