Acnocure, a Synergistic Anti-Microbial and Anti-Inflammatory Combination of Thymol and Curcuma Turmerones, Formulation and Time-Kill Studies Against C. acnes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material, Solvents, Chemicals, and Standards
2.2. In Vitro Studies: High Throughput Anti-Microbial Screening
Time Kill Curve Kinetics of Acnocure Combination Against C. acnes in a Cosmetic Simplex Formula
2.3. Anti-Inflammatory Efficacy Assay (AIEA): Modulation of Inflammatory Mediators by PMA and LPS Stimulated Human Macrophage-like U937 Mononuclear Cell Line
In Vitro IL-1α Induced Anti-Inflammatory Efficacy in Fibroblasts
2.4. Formulation
Formulation Process
- Cosmetic simplex placebo: without the actives;
- Cosmetic simplex 1: 0.25% thymol and 0.1% Curcuma turmerones;
- Cosmetic simplex 2: 0.1% thymol and 0.1% Curcuma turmerones.
3. Results
3.1. In Vitro Antimicrobial Activity of Thymol, Curcuma Turmerones and Acnocure
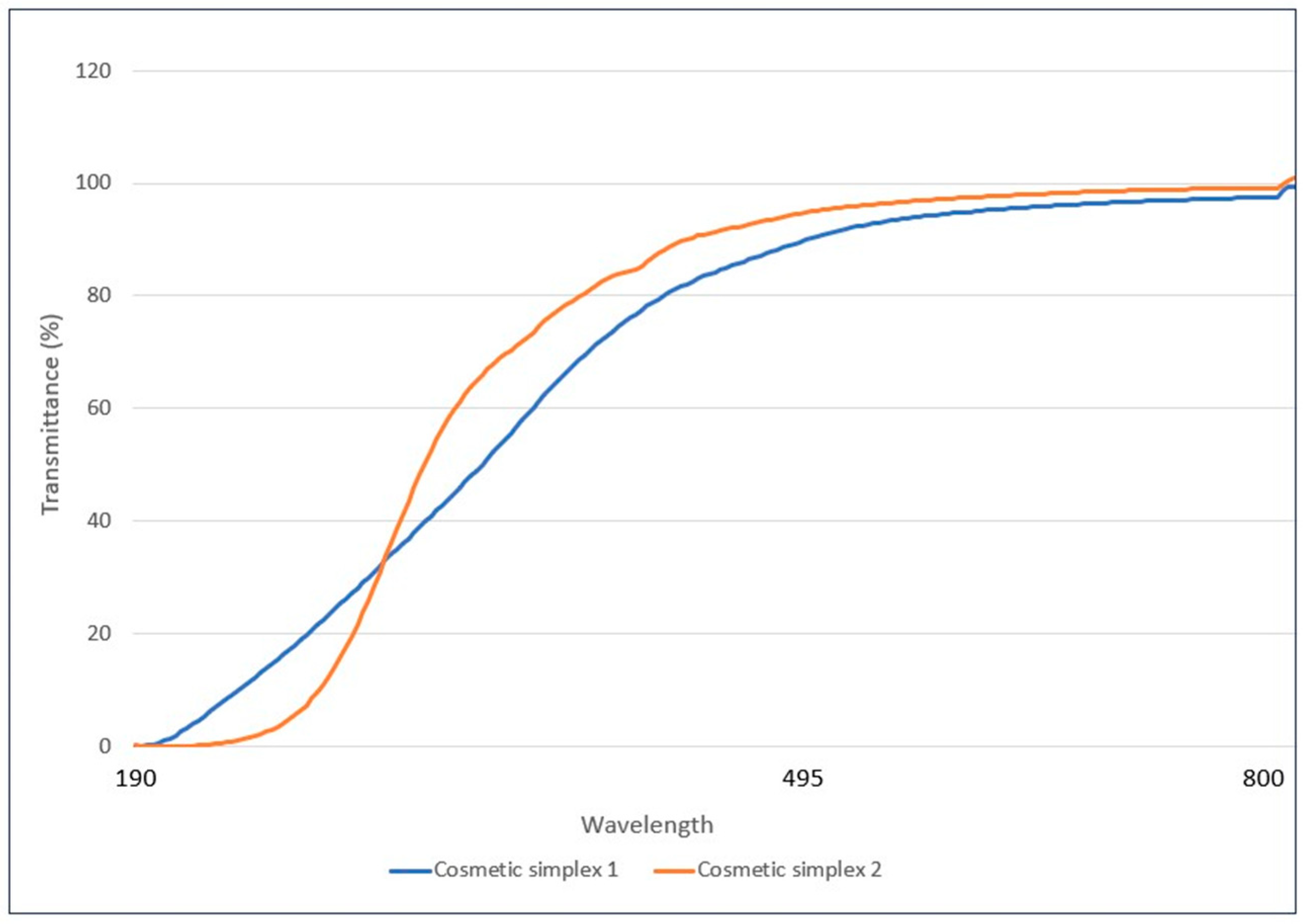
3.2. Assessment of Transparency of Simplex Formulations
- Cosmetic simplex 1;
- Cosmetic simplex 2.
3.3. Time-Kill Curves of Cutibacterium acnes on Exposure to Acnocure Simplex Formulations
3.4. Anti-Inflammatory Efficacy Assay (AIEA): COX-2 and NF-κB Inflammatory Pathways
In Vitro IL-1α-Induced Anti-Inflammatory Study in Fibroblasts
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuchayi, M.S.; Makrantonaki, E.; Ganceviciene, R.; Dessinioti, C.; Feldman, S.R.; Zouboulis, C.C. Acne vulgaris. Nat. Rev. Dis. Primers 2015, 1, 15029. [Google Scholar] [CrossRef] [PubMed]
- Yentzer, B.A.; Hick, J.; Reese, E.L.; Uhas, A.; Feldman, S.R.; Balkrishnan, R. Acne vulgaris in the United States: Descriptive epidemiology. Cutis 2010, 86, 94. [Google Scholar] [PubMed]
- McConnell, R.C.; Fleischer, A.B.; Williford, P.M.; Feldman, S.R. Most topical tretinoin treatment is for acne vulgaris through the age of 44 years: An analysis of the National Ambulatory Medical Care Survey. J. Am. Acad. Dermatol. 1998, 38, 221. [Google Scholar] [CrossRef] [PubMed]
- Bernales, S.A. Acne vulgaris: Role of the immune system. Int. J. Dermatol. 2021, 60, 1076. [Google Scholar] [CrossRef]
- Cong, T.X.; Hao, D.; Wen, X.; Li, X.; He, G.; Jiang, X. From pathogenesis of acne vulgaris to anti-acne agents. Arch. Dermatol. Res. 2019, 311, 337. [Google Scholar] [CrossRef] [PubMed]
- Barnard, E.; Shi, B.; Kang, D.; Craft, N.; Li, H. The balance of metagenomic elements shapes the skin microbiome in acne and health. Sci. Rep. 2016, 6, 39491. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.H.; Kwon, H.H. What’s new in the physiopathology of acne? Br. J. Dermatol. 2015, 172, 13. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Byun, E.J.; Kim, H.S. Potential role of the microbiome in acne: A comprehensive review. J. Clin. Med. 2019, 8, 987. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Dagnelie, M.A.; Khammari, A.; Corvec, S. The Skin Microbiome: A New Actor in Inflammatory Acne. Am. J. Clin. Dermatol. 2020, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bhushan, R. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945. [Google Scholar] [CrossRef]
- Amed, I.; Berg, A.; Hudson, S.; Weaver, K.K.; Pacchia, M.L. The Beauty Market in 2023: A Special State of Fashion Report. Available online: https://www.mckinsey.com/industries/retail/our-insights/the-beauty-market-in-2023-a-special-state-of-fashion-report (accessed on 22 December 2024).
- Borris, R.P. Natural products research: Perspectives from a major pharmaceutical company. J. Ethnopharmacol. 1996, 51, 29. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.W. Plant derived antimalarial agents: New leads and challenges. Phytochem. Rev. 2005, 4, 55. [Google Scholar] [CrossRef]
- Marquez-Valadez, B.; Maldonado, P.D.; Galvan-Arzate, S.; Méndez-Cuesta, L.A.; Pérez-De La Cruz, V.; Pedraza-Chaverrí, J.; Chánez-Cárdenas, M.E.; Santamaría, A. Alpha-mangostin induces changes in glutathione levels associated with glutathione peroxidase activity in rat brain synaptosomes. Nutr. Neurosci. 2012, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Xin, Y.; Guo, Y.; Diao, Y.; Kou, X.; Luo, L.; Yin, Z. Ampelopsin reduces endotoxic infammation via repressing ROS-mediated activation of PI3K/Akt/NF-κB signaling pathways. Int. Immunopharmacol. 2012, 12, 278. [Google Scholar] [CrossRef]
- Etti, I.C.; Abdullah, R.; Kadir, A.; Hashim, N.M.; Yeap, S.K.; Imam, M.U.; Ramli, F.; Malami, I.; Lam, K.L.; Etti, U.; et al. The molecular mechanism of the anticancer effect of Artonin E in MDA-MB 231 triple negative breast cancer cells. PLoS ONE 2017, 12, e0182357. [Google Scholar] [CrossRef]
- Li, C.G.; Yan, L.; Jing, Y.Y.; Xu, L.H.; Liang, Y.D.; Wei, H.X.; Hu, B.; Pan, H.; Zha, Q.B.; Ouyang, D.Y.; et al. Berberine augments ATP induced infammasome activation in macrophages by enhancing AMPK signaling. Oncotarget 2017, 8, 95. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in liver diseases: A systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients 2018, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Umesalma, S.; Sudhandiran, G. Differential inhibitory effects of the polyphenol ellagic acid on infammatory mediators NF-κB, iNOS, COX-2, TNF-α, and IL-6 in 1, 2-dimethylhydrazine-induced rat colon carcinogenesis. Basic Clin. Pharmacol. Toxicol. 2010, 107, 650. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhang, H.; Cai, X.; Shang, Y. Epigallocatechin3-gallate inhibits infammation and epithelialmesenchymal transition through the PI3K/AKT pathway via upregulation of PTEN in asthma. Int. J. Mol. Med. 2018, 41, 818. [Google Scholar]
- Soleymani, S.; Farzaei, M.H.; Zargaran, A.; Niknam, S.; Rahimi, R. Promising plant-derived secondary metabolites for treatment of acne vulgaris: A mechanistic review. Arch. Dermatol. Res. 2020, 312, 5. [Google Scholar] [CrossRef]
- Amiri, H. Essential oils composition and antioxidant properties of three Thymus species. Evid. Based Complement. Alternat. Med. 2012, 2012, 728065. [Google Scholar] [CrossRef] [PubMed]
- Bairwa, R.; Sodha, R.S.; Rajawat, B.S. Trachyspermum ammi. Pharmacogn. Rev. 2012, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadi, M.; Riahi-Madvar, A.; Hadian, J.; Rezaee, F.; Rafiee, R. In vitro cytotoxic and antimicrobial activity of essential oil from Satureja sahendica. Toxicol. Environ. Chem. 2012, 94, 1735. [Google Scholar] [CrossRef]
- Markovic, P.; Chatzopoulou, P.; Siljegovic, J.; Nikolic, M.; Glamoclija, J.; Ciric, A.; Soković, M. Chemical analysis and antimicrobial activities of the essential oils of Satureja thymbra L. and Thymbra spicata L. and their main components. Arch. Biol. Sci. 2011, 63, 457. [Google Scholar] [CrossRef]
- Khajeh, M.; Yamini, Y.; Sefidkon, F.; Bahramifar, N. Comparison of essential oil composition of Carum copticum obtained by supercritical carbon dioxide extraction and hydrodistillation methods. Food Chem. 2004, 86, 587. [Google Scholar] [CrossRef]
- Ghosheh, O.A.; Houdi, A.A.; Crooks, P.A. High performance liquid chromatographic analysis of the pharmacologically active quinones and related compounds in the oil of the black seed (Nigella sativa L.). J. Pharm. Biomed. Anal. 1999, 19, 757. [Google Scholar] [CrossRef] [PubMed]
- Didri, N.; Dubreuil, L.; Pinkas, M. Activity of thymol, carvacrol, cinnamaldehyde and eugenol on oral bacteria. Pharm. Acta Helv. 1994, 69, 25. [Google Scholar] [CrossRef]
- Aeschbach, R.; Loliger, J.; Scott, B.C.; Murcia, A.; Butler, J.; Halliwell, M.; Aruoma, O.I. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem. Toxicol. 1994, 32, 31. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.L. Antifungal action and antiaflatoxigenic properties of some essential oil constituents. Lett. Appl. Microbiol. 1994, 19, 110. [Google Scholar] [CrossRef] [PubMed]
- Yanishlieva, N.V.; Marinovaa, E.M.; Gordon, M.H.; Raneva, V.G. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 1999, 64, 59. [Google Scholar] [CrossRef]
- Frank, J.; McEnroe, W.F. Structures and synthesis of some new antibacterial sesquiterpenoids from the gorgonian coral Pseudopterogorgia rigida. Tetrahedron 1978, 34, 1661. [Google Scholar]
- Itokawa, H.; Shi, Q.; Akiyama, T.; Morris-Natschke, S.L.; Lee, K.H. Recent advances in the investigation of curcuminoids. Chin. Med. 2008, 17, 11. [Google Scholar] [CrossRef]
- Hwang, J.K.; Sim, J.S.; Baek, N.I.; Pyun, Y.R. Xanthorrhizol: A potential antibacterial agent from Curcuma xanthorrhiza against Streptococcus mutans. Planta Med. 2000, 66, 196. [Google Scholar] [CrossRef]
- Chun, K.S.; Sohn, Y.; Kim, H.S.; Kim, O.H.; Park, K.K.; Lee, J.M.; Lee, J.; Lee, J.Y.; Moon, A.; Lee, S.S.; et al. Anti-tumor promoting potential of naturally occurring diarylheptanoids structurally related to curcumin. Mutat. Res. 1999, 428, 49. [Google Scholar] [CrossRef] [PubMed]
- Imtiyaz, A.S.; Venkatesalu, V. Botany, traditional uses, phytochemistry and pharmacological properties of Saussurea costus—An endangered plant from Himalaya—A review. Phytochem. Lett. 2022, 47, 140. [Google Scholar]
- Bokshan, S.L.; Gomez, J.R.; Chapin, K.C.; Green, A.; Paxton, E.S. Reduced Time to Positive Cutibacterium acnes Culture Utilizing a Novel Incubation Technique: A Retrospective Cohort Study. J. Shoulder Elb. Arthroplast. 2019, 3, 2471549219840823. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Eskin, N.A.M.; Iranshahy, M.; Fazly, B.B.S. Phytochemicals: A Promising Weapon in the Arsenal against Antibiotic-Resistant Bacteria. Antibiotics 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, J.; De Bont, J.A.M.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201. [Google Scholar] [CrossRef] [PubMed]
- Yousfi, F.; Abrigach, F.; Petrovic, J.D.; Sokovic, M.; Ramdani, M. Phytochemical screening and evaluation of the antioxidant and antibacterial potential of Zingiber officinale extracts. S. Afr. J. Bot. 2021, 142, 433. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174. [Google Scholar] [CrossRef] [PubMed]
- Poudel, D.K.; Ojha, P.K.; Rokaya, A.; Satyal, R.; Satyal, P.; Setzer, W.N. Analysis of Volatile Constituents in Curcuma Species, viz. C. aeruginosa, C. zedoaria, and C. longa, from Nepal. Plants 2022, 26, 1932. [Google Scholar]
- Yasni, S.; Imaizumi, K.; Sin, K.; Sugano, M.; Nonaka, G.; Sidik, P. Identification of an active principle in essential oils and hexane-soluble fractions of Curcuma xanthorrhiza Roxb. showing triglyceride-lowering action in rats. Food Chem. Toxicol. 1994, 32, 273. [Google Scholar] [CrossRef] [PubMed]
- Uehara, S.; Yasuda, I.; Takeya, K.; Itokawa, H. Terpenoids and curcuminoids of the rhizoma of Curcuma xanthorrhiza roxb. J. Pharm. Soc. Jpn. 1992, 112, 817. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.H.; Kim, Y.; Lee, S.K. Sesquiterpenoids from the rhizome of Curcuma zedoaria. Arch. Pharm. Res. 2001, 24, 424. [Google Scholar] [CrossRef]
- Martins, A.P.; Salgueiro, L.; Goncalves, M.J.; da Cunha, A.P.; Vila, R.; Canigueral, S.; Mazzoni, V.; Tomi, F.; Casanova, J. Essential oil composition and antimicrobial activity of three Zingiberaceae from S.Tome E principe. Planta Med. 2001, 67, 580. [Google Scholar] [CrossRef] [PubMed]
- Ciosek, J. Understanding of transmittance. Proc. SPIE-Int. Soc. Opt. Eng. 2003, 5064, 287–294. [Google Scholar]
- Hwang, J.K.; Shim, J.S.; Pyun, Y.R. Antibacterial activity of xanthorrhizol from Curcuma xanthorrhiza against oral pathogens. Fitoterapia 2000, 71, 321. [Google Scholar] [CrossRef]
- Stein Gold, L.F. What’s new in acne and inflammation. J. Drugs Dermatol. 2013, 12, s67. [Google Scholar]
- Weiss, J.S. Messages from molecules: Deciphering the code. J. Drugs Dermatol. 2013, 12, s70. [Google Scholar]
- Bellew, S.; Thiboutot, D.; Del Rosso, J.Q. Pathogenesis of acne vulgaris: What’s new, what’s interesting and what may be clinically relevant. J. Drugs Dermatol. 2011, 10, 582. [Google Scholar]
- Del Bufalo, A.; Bernad, J.; Dardenne, C.; Verda, D.; Meunier, J.R.; Rousset, F.; Martinozzi-Teissier, S.; Pipy, B. Contact sensitizers modulate the arachidonic acid metabolism of PMA-differentiated U-937 monocytic cells activated by LPS. Toxicol. Appl. Pharmacol. 2011, 256, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-L.; Lu, Z.-Y.; Zhang, X.; Liu, W.-W.; Yao, G.-D.; Liu, X.-L.; Liu, W.; Wu, Q.-J.; Hayashi, T.; Yamato, M.; et al. Gelatin promotes cell aggregation and pro-inflammatory cytokine production in PMA-stimulated U937 cells by augmenting endocytosis-autophagy pathway. Int. J. Biochem. Cell Biol. 2018, 95, 132. [Google Scholar] [CrossRef]
- Vassiliou, E.; Awoleye, O.; Davis, A.; Mishra, S. Anti-Inflammatory and Antimicrobial Properties of Thyme Oil and Its Main Constituents. Int. J. Mol. Sci. 2023, 24, 6936. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, F.; Xu, Y.; Zhang, J.; Qi, J.; Liu, X.; Wang, R. Thymol alleviates lipopolysaccharide-stimulated inflammatory response via downregulation of RhoA-mediated NF-κB signalling pathway in human peritoneal mesothelial cells. Eur. J. Pharmacol. 2018, 833, 210. [Google Scholar] [CrossRef]
- Kwon, H.I.; Jeong, N.H.; Kim, S.Y.; Kim, M.H.; Son, J.H.; Jun, S.H.; Kim, S.; Jeon, H.; Kang, S.C.; Kim, S.H.; et al. Inhibitory effects of thymol on the cytotoxicity and inflammatory responses induced by Staphylococcus aureus extracellular vesicles in cultured keratinocytes. Microb. Pathog. 2019, 134, 103603. [Google Scholar] [CrossRef] [PubMed]
- Sköld, K.; Twetman, S.; Hallgren, A.; Yucel-Lindberg, T.; Modéer, T. Effect of a chlorhexidine/thymol-containing varnish on prostaglandin E2 levels in gingival crevicular fluid. Eur. J. Oral Sci. 1998, 106, 571. [Google Scholar] [CrossRef] [PubMed]
- Marsik, P.; Kokoska, L.; Landa, P.; Nepovim, A.; Soudek, P.; and Vanek, T. In vitro inhibitory effects of thymol and quinones of Nigella sativa seeds on cyclooxygenase-1- and -2-catalyzed prostaglandin E2 biosyntheses. Planta Med. 2005, 71, 739. [Google Scholar] [CrossRef]
- Cordier-Dirikoc, S.; Pedretti, N.; Garnier, J.; Clarhaut-Charreau, S.; Ryffel, B.; Morel, F.; Bernard, F.X.; Hamon de Almeida, V.; Lecron, J.C.; Jégou, J.F. Dermal fibroblasts are the key sensors of aseptic skin inflammation through interleukin 1 release by lesioned keratinocytes. Front. Immunol. 2022, 13, 984045. [Google Scholar] [CrossRef] [PubMed]
- Kupper, T.S.; Ballard, D.W.; Chua, A.O.; McGuire, J.S.; Flood, P.M.; Horowitz, M.C.; Langdon, R.; Lightfoot, L.; Gubler, U. Human keratinocytes contain mRNA indistinguishable from monocyte interleukin 1 alpha and beta mRNA. Keratinocyte epidermal cell-derived thymocyte-activating factor is identical to interleukin 1. J. Exp. Med. 1986, 164, 2095. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Ahmad, A.S.; Glushakov, A.V.; Chambers, C.; Doré, S. Putative role of prostaglandin receptor in intracerebral hemorrhage. Front. Neurol. 2012, 3, 145. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Han, I.H.; Lee, H.R.; Lee, H.M. Prostaglandin E2 Induces IL-6 and IL-8 Production by the EP Receptors/Akt/NF-κB Pathways in Nasal Polyp-Derived Fibroblasts. Allergy Asthma Immunol. Res. 2014, 6, 449. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhu, F.; Konstantopoulos, K. Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-kappaB activation. Am. J. Physiol. Cell Physiol. 2010, 298, C1445. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Hong, C.H.; Huh, S.K.; Kim, S.S.; Oh, O.J.; Min, H.Y.; Park, K.K.; Choong, W.Y.; Hwang, J.K. Suppressive effect of natural sesquiterpenoids on inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) activity in mouse macrophage cells. J. Environ. Pathol. Toxicol. Oncol. 2002, 21, 141. [Google Scholar] [CrossRef]


| Strains (Culture Collection Number) | Targeted Disorder | Culture/Enumeration Media | Diluent for Inoculum Preparation | Test Media |
|---|---|---|---|---|
| Cutibacterium acnes ATCC 6919 | Acne | Trypto-casein-soy agar (TSA) | Tryptone salt broth | Trypto-casein-soy broth (TSB) |
| Staphylococcus aureus (ATCC 6538) | Atopy | TSA agar | Tryptone salt broth | Trypto-casein-soy broth (TSB) |
| Staphylococcus epidermidis (ATCC 12228) | Acne | TSA agar | Tryptone salt broth | Trypto-casein-soy broth (TSB) |
| Corynebacterium freneyi (CIP 52.16) | Armpit odor | TSA agar | Tryptone salt broth | Trypto-casein-soy broth (TSB) |
| Compounds | C. acnes ATCC 6919 | S. aureus CIP 4.83 | S. epidermidis CIP 68.21 | Corynebacterium freneyi ATCC 7711 |
|---|---|---|---|---|
| MIC (mg/mL) | MIC (mg/mL) | MIC (mg/mL) | MIC (mg/mL) | |
| Thymol | 0.43 | 0.29 | 0.47 | 0.58 |
| Curcuma turmerones | 0.37 | 0.28 | 0.51 | 0.34 |
| Acnocure | 0.32 | 0.26 | 0.47 | 0.11 |
| Name of Sample | Maximum Transmittance (%) | Wavelength (nm) |
|---|---|---|
| Cosmetic simplex 1 | 100 | 753 |
| Cosmetic simplex 2 | 99.634 | 740 |
| Compounds | Anti-Inflammatory IC5O µg/mL | ||||
|---|---|---|---|---|---|
| 1L-1β | IL-6 | IL-8 | TNF-α | PGE-2 | |
| Thymol | 175 | <100 | NA | NA | <1 |
| Turmerones | NA | NA | NA | NA | <1 |
| Acnocure | NA | NA | NA | NA | <1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pannakal, S.T.; Prasad, A.; Phadke, S.; Sanyal, A.; Butti, S.; Khodr, A.; Morain, C.; Agnaou, R.; Shariff, R.; Benazzouz, A.; et al. Acnocure, a Synergistic Anti-Microbial and Anti-Inflammatory Combination of Thymol and Curcuma Turmerones, Formulation and Time-Kill Studies Against C. acnes. Cosmetics 2025, 12, 37. https://doi.org/10.3390/cosmetics12020037
Pannakal ST, Prasad A, Phadke S, Sanyal A, Butti S, Khodr A, Morain C, Agnaou R, Shariff R, Benazzouz A, et al. Acnocure, a Synergistic Anti-Microbial and Anti-Inflammatory Combination of Thymol and Curcuma Turmerones, Formulation and Time-Kill Studies Against C. acnes. Cosmetics. 2025; 12(2):37. https://doi.org/10.3390/cosmetics12020037
Chicago/Turabian StylePannakal, Steve Thomas, Arpita Prasad, Snehal Phadke, Aryasekhar Sanyal, Srinu Butti, Ahmad Khodr, Cynthia Morain, Reda Agnaou, Rezwan Shariff, Adrien Benazzouz, and et al. 2025. "Acnocure, a Synergistic Anti-Microbial and Anti-Inflammatory Combination of Thymol and Curcuma Turmerones, Formulation and Time-Kill Studies Against C. acnes" Cosmetics 12, no. 2: 37. https://doi.org/10.3390/cosmetics12020037
APA StylePannakal, S. T., Prasad, A., Phadke, S., Sanyal, A., Butti, S., Khodr, A., Morain, C., Agnaou, R., Shariff, R., Benazzouz, A., Patil, K., Chawda, K., John, S., Roy, D., & Sharma, V. (2025). Acnocure, a Synergistic Anti-Microbial and Anti-Inflammatory Combination of Thymol and Curcuma Turmerones, Formulation and Time-Kill Studies Against C. acnes. Cosmetics, 12(2), 37. https://doi.org/10.3390/cosmetics12020037







