Abstract
Bromelain, a natural enzyme derived from pineapple, is known for its antioxidant properties, and its potential as a photoprotective agent has garnered interest in skincare applications. The primary objective of this research was to evaluate and optimize the effectiveness of bromelain-based creams in providing antioxidant and photoprotective protection against ultraviolet (UV) radiation. Antioxidant activity was assessed using the DPPH radical scavenging assay, and the Sun Protection Factor (SPF) was determined in vitro and in vivo to evaluate photoprotective activity. The results revealed that bromelain exhibited strong antioxidant activity. Photoprotection, as measured by SPF, the formulation F3, which combined bromelain with other UV filters, exhibited the highest SPF values of 22.043 ± 0.277 (in vitro) and 21.3 ± 2.901 (in vivo), indicating enhanced photoprotective efficacy. This improvement in SPF was likely due to the synergistic effect of bromelain with the UV filters Octyl Methoxycinnamate (OMC). The findings suggest a positive correlation between antioxidant activity and photoprotection, with bromelain’s antioxidant properties contributing to its overall photoprotective effect. Bromelain may be used on people without causing skin or eye irritation. This study supports the potential of bromelain-based creams as dual-action skincare formulations, offering both antioxidant and UV protection.
1. Introduction
Repeated exposure to solar Ultraviolet (UV) radiation poses significant health risks by generating Reactive Oxygen Species (ROS), which can damage essential biomolecules and induce oxidative stress [1]. UVA and UVB rays penetrate the epidermis, leading to ROS production, activation of intracellular signaling pathways, and damage to DNA, proteins, and lipids, ultimately disrupting the skin’s defense mechanisms [2]. Excessive free radical formation triggers several pathological processes, including the Mitogen-Activated Protein Kinase (MAPK) cascade and Matrix Metalloproteinase (MMP) activation, which contribute to the degradation of skin structure and function. Although the skin possesses an intrinsic antioxidant defense system to counteract oxidative stress, chronic UV exposure can overwhelm this protective capacity, resulting in detrimental effects [1].
Since the 1930s, the primary approach for preventing UV-induced skin damage has been the topical application of sunscreens [3]. Currently, the use of photoprotective agents is essential to prevent UV radiation from penetrating the skin. Sunscreen formulations contain active ingredients that function as UV absorbers, reflectors, or scatterers, such as oxybenzone, octinoxate, and homosalate. However, these chemical UV filters have been associated with adverse effects, including contact allergies, reproductive toxicity, endocrine disruption, skin irritation, and even an increased risk of skin cancer [4].
As a result, there is a growing trend toward developing safer, plant-based sunscreen formulations [5]. Botanical-derived sunscreens are generally considered less toxic and less irritating, making them increasingly popular in cosmetic formulations [6]. Several plant-derived compounds have demonstrated significant potential as natural sunscreen additives in the cosmetics industry, including Sophora japonica L. [7], Vitis vinifera L. [8], Silybum marianum L. [9], Ginkgo biloba L. [7], and Juglans regia L. [10]. Plants have evolved natural photoprotective mechanisms to mitigate oxidative stress and prevent photoinhibition, making them promising candidates for sunscreen development.
Indonesia, a region rich in biodiversity, offers vast potential for exploring plant-based photoprotective agents. One of the most widely consumed fruits in Indonesia is Ananas comosus sp., commonly known as pineapple. The country has a well-established pineapple industry, with commercial cultivation on large-scale plantations. In 2022, Indonesia produced approximately 3.2 million tons of pineapple [11]. Pineapple contains bromelain, a mixture of proteolytic enzymes and nonenzymatic bioactive compounds primarily concentrated in the stem [12]. Bromelain has been widely incorporated into skincare products due to its exfoliating properties and its ability to remove damaged skin cells [13]. Many cosmetic brands now include bromelain in their formulations for its skin renewal and anti-inflammatory benefits.
Recent research has demonstrated that bromelain exerts anti-inflammatory effects by reducing the secretion of proinflammatory cytokines, such as IL-1β, IL-6, TNF-α, and TGF-β [14,15,16]. Additionally, bromelain has been shown to attenuate H2O2-induced ROS production, suggesting its potential to mitigate oxidative stress-related skin damage. This mechanism can be exploited to protect against the harmful effects of UV exposure. Furthermore, emerging studies have highlighted bromelain’s potential role in preventing UV-induced skin damage [17].
This study aims to develop a cream formulation containing bromelain, both as a standalone active ingredient and in combination with Octyl Methoxycinnamate (OMC). The research seeks to evaluate the impact of bromelain on UV protection efficacy and antioxidant activity within the formulation. The photoprotective activity of the formulations will be assessed through in vitro and in vivo experiments using rabbit skin exposed to UVB radiation. Additionally, this study aims to determine the potential of bromelain as a natural alternative to synthetic UV filters and to assess its effectiveness in mitigating UV-induced skin damage.
2. Materials and Methods
2.1. Acquisition of Bromelain
The bromelain used in this study is an isolated compound derived from pineapple stems (Ananas comosus). The bromelain enzyme was obtained from the company Vital-chem Zhuhai Co., Ltd., Zhuhai, China.
2.2. Sunscreen Cream Formulation
The formulation of sunscreen cream was carried out by adding 3% bromelain and 5% Octyl Methoxycinnamate to the cream base. The preparation of sunscreen cream is made twice with the following formula, as depicted in Table 1.

Table 1.
Sunscreen cream formulation.
The sunscreen cream is created using a single-phase methodology. The raw materials are weighed first, then EDTA is dissolved in water and mixed with glycerin, followed by heating to 75 °C. In the oil phase, Olivem® 1000, Ammonium Polyacryloyldimethyl Taurate, caprylic capric triglyceride, and OMC are mixed and heated to 75 °C. The oil phase is then added to the water phase and stirred until a homogeneous emulsion forms. Lower the temperature of the emulsion mixture to 30 °C, then add phenoxyethanol, propanediol, and bromelain. Mix until homogeneous.
2.3. Physical Examination
The physical characteristics, such as color, smell, and consistency (visual observation), are in the final formulation.
2.4. pH Measurements
The pH was measured at room temperature (18–20 °C) using a pH meter (ADWA pH meter) by direct insertion of the electrode into the formulations placed in a beaker [18].
2.5. Centrifugation
The samples were subjected to centrifugation using the centrifuge (EBA 200Hettich, Tuttlingen, Germany) at 7000 rpm for 30 min [19].
2.6. Viscocity Measurement
Cream viscosity is determined using a Brookfield viscometer. Measurements are obtained by inserting the sample into a container and then installing it on a portable viscotester. The visibility is measured based on the stable digital display of the measuring instrument [18].
2.7. Spreadibility Test
The spreadability test was conducted by placing approximately 0.5 g of the cream in the center of a premarked circular glass plate, which is part of the spreadability apparatus. Another glass plate was placed over the sample and left undisturbed for one minute. The diameter of the spread cream was then measured. Additional weights were added incrementally until a final load of 250 g was reached, with each weight increase being 50 g per minute. The spread diameter was recorded at each increment [20].
2.8. Adhesion Test
For the adhesion test, 0.25 g of gel was placed between two glass slides and pressed with a 1 kg weight for 5 min. The slides were then placed in the adhesion test apparatus and subjected to an 80-g weight. The time required for the gel to detach from the glass slides was recorded [20].
2.9. Cycling Test
The cycling test method is carried out by storing the preparation at 2 different temperatures in 6 cycles. The cream preparation is put into a jar and then stored in a refrigerator at a temperature of 4 °C ± 2 °C for 24 h and then transferred to an oven at a temperature of 45 °C ± 2 °C for 24 h. The storage time of the two temperatures in 2 days is considered 1 cycle. Qualitative observations are made for the occurrence of separation in the conical tube. Observations are made for 6 cycles [21].
2.10. Radical Scavenging Activity DPPH Assay
The study was conducted on bromelain enzyme and a cream containing bromelain to evaluate the impact of the formulation process on the antioxidant activity of the bromelain enzyme. The radical scavenging capacity of bromelain was evaluated by measuring the reduction in the stable free radical 2,2-Diphenyl-1-Picrylhydrazyl (DPPH). The maximum wavelength of 40 μg/mL of DPPH solution was determined at 400–800 nm. For the test, 1 g of each cream was weighed and mixed with 1.5 mL of absolute ethanol. The mixture was stirred for 5 min in a vortex, followed by 10 min of ultrasonic bath and subsequent centrifugation at 3000 rpm for 20 min. After centrifugation, 500 µL of the supernatant was combined with 500 µL of the DPPH solution (radical) or 500 µL of ethanol (control) and then incubated for 30 min at room temperature and protected from light. The absorbance of the samples was measured using a UV-VIS spectrophotometer at 517 nm. The antioxidant activity was calculated according to Equation [22]:
% of inhibition = [(ADPPH − Asample)/ADPPH] × 100%
Linear regression resulted from percent inhibition versus concentration. The concentration of a sample that was required for 50% inhibition was determined and expressed as an IC50 value [22].
2.11. Evaluation of UV Filtering Potential
The UV absorption of each sample (cream base, F1, F2, and F3) diluted with ethanol (0.2 mg/mL) was measured using UV–visible spectrophotometer (EvolutionTM 300, Thermo Fisher Scientific, Waltham, MA, USA) in a range between 290 and 320 nm, and the Sun Protection Factor (SPF) was determined using Mansur’s equation [23].
where Erythemal Effect (EE), I(l) solar intensity spectrum, Abs(l) absorbance of sunscreen product, and CF correction factor (=10). Table EE × I is presented in Table 2.
SPF value = CF × ∑Abs × EE × I

Table 2.
EE × I value.
2.12. Animal Treatment
An application letter for the ethical use of experimental animals was approved by the Research Ethics Commission (KEP) Universitas Padjadjaran in Bandung on 9 September 2024 and obtained ethical approval letter no. 979/UN6.KEP/EC/2024.
2.13. In Vivo Sunscreen Activity Test
The activity test was conducted by determining the Sun Protection Factor (SPF) against UV-B rays in vivo using six female rabbits. The rabbits’ backs were shaved and marked with an area of 2 × 2 cm, and the rabbits were sensitized, with the compound 8-MOP administered orally at a dose of 10 mg/kg body weight. Next, the Minimal Erythema Dose (MED) was determined by exposing the rabbits’ backs without treatment with UVB narrow band (Phillips PL-S 9W/01/2P 1CT/6X10BOX) at a dose range of 12.5–25 mJ/cm2. After 24 h, erythema in the irradiated areas was observed, and the Minimal Erythemal Dose (MED) value was measured. After obtaining the MED value without treatment, the sunscreen activity test was continued by applying the test cream to the rabbit’s back at a dose of 2 mg/cm2. The area was then irradiated with UV rays with additional doses until erythema appeared. The SPF value was determined by the ratio of the MED value on the sunscreen-protected skin to the MED value on the unprotected skin [24].
2.14. Irritation Test
The irritation test was conducted using female albino rabbits (Oryctolagus cuniculus). Three healthy and active rabbits were selected. In the skin irritation test, an area of 2 × 2 cm was shaved before the test began. A dose of 2 mg/cm2 cream was applied. The first area received cream as a control, the second area received cream F1, and the third area received cream F3. The treated areas on the animal’s back were covered with sterile gauze, plastic, and bandages to prevent evaporation. The coverings were sequentially removed after 3 min, 1 h, and 4 h. The responses were assessed every hour after treatment. Observations were made based on signs of erythema and edema at 1, 24, 48, 72 h, as well as on days 7 and 14 after treatment. The observation results were scored based on the severity of the skin irritation [25].
Ocular irritation potential measured with the Draize eye irritation test involves exposing the test formulation in a single dose, along with a negative control formulation (distilled water), to the eye of albino rabbits (Oryctolagus cuniculus). Irritation assessments at 1 h, 24 h, 48 h, and 72 h after cream application. The severity of irritation is assessed based on observed changes in corneal opacity, conjunctival redness, swelling, and discharge over a specified period. Scoring is performed according to a standardized numerical scale to determine the degree of irritation [26].
2.15. Statistical Analysis
The acquired data underwent a comprehensive analysis using the one-way analysis of variance (ANOVA) method. For post hoc comparisons, the multiple range tests by Duncan were selected. Subsequently, a significance threshold of p < 0.05 was employed for determining statistical significance.
3. Results
3.1. Physicochemical Properties of the Sunscreen Cream Formulation
The formulation of bromelain-containing cream must consider several key factors, including enzyme stability, pH, and temperature. It is essential to maintain the enzymatic activity and antioxidant properties of bromelain throughout the formulation process to ensure the efficacy and stability of the final product. The cream formulation is created twice to ensure accuracy. The results of making cream are shown in (Figure 1).
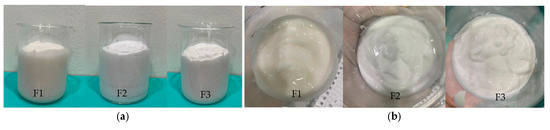
Figure 1.
Appearance of cream formulation: (a) Appearance; (b) texture and consistency.
After the sunscreen cream preparation was created, an evaluation of the cream was carried out, including pH, viscosity, centrifugation, adhesive power, and spreading power [27] with the following results in Table 3.

Table 3.
Sunscreen cream evaluation.
The organoleptic evaluation aimed to assess the sensory characteristics of the prepared cream formulations. This assessment was conducted through visual observation of the cream’s appearance, color, odor, and texture (Table 3). All sunscreen cream formulations exhibited a typical white to off-white color with a smooth texture. Formulations F1 and F2 emitted a faint plant stem aroma, whereas F3 was odorless. The color of F1 was off-white, while F2 and F3 retained a white hue. These variations are attributed to the presence of bromelain, which imparts an off-white to yellowish color and has a distinct odor, thereby influencing the final product’s overall properties.
A viscosity test was performed to evaluate the thickness and flowability of the cream formulations, ensuring ease of application. An optimal viscosity balances fluidity and thickness [28]. The viscosity results in Table 3 revealed distinct differences among the formulations, with F3 exhibiting the highest viscosity, followed by F2 and F1. The increased viscosity in F3 is attributed to the addition of OMC, a lipophilic compound that enhances viscosity and stability by reducing particle movement, thereby slowing the flow rate. Furthermore, viscosity is influenced by the active ingredient concentration; higher concentrations result in lower viscosity, as observed in the comparison between F1 and F3. Despite these variations, all formulations complied with the cream viscosity standard of 2000–50,000 cP [3].
The spreadability test was conducted to determine the ease of cream application. An optimal cream formulation should exhibit a spreadability range of 5–7 cm [29]. As shown in Table 3, all formulations met this criterion, indicating adequate spreadability. The adhesion test assessed the cream’s ability to adhere to the skin [30]. According to Indonesian regulatory standards, an ideal cream should maintain adhesion between 2 and 300 s [31]. The test showed that all formulations met the required standards for good adhesion and spreadability. The adhesion test results, as outlined in Table 3, show that all sunscreen cream formulations adhered to the skin for 180–220 s, which satisfies the standard requirements for good adhesion and spreadability.
3.2. Formulation Cream Stability
The stability of the cream formulations was assessed using the 6-cycle freeze-thaw method. One cycle consisted of 24 h at 4 ± 2 °C, followed by 24 h at 40 ± 2 °C [16]. The results indicated that no significant changes were observed in the pH, organoleptic properties, spreadability, adhesion, or viscosity following the completion of the freeze-thaw cycles, suggesting that the formulations maintain their stability under these conditions (Table 4).

Table 4.
Freeze thaw stability.
The results of the pH evaluation at the freeze-thaw test data analysis using one-way ANOVA showed a significant difference in all formulas (sig > 0.05). It showed that all formulas are not stable in periods of storage with extreme temperatures based on pH values. This can be caused by the influence of bromelain, which is decomposed by high temperatures during the manufacturing or storage process that produces acidic or basic substances, so this can affect the pH value.
Based on one-way ANOVA analysis, it was presented that F1 has a significance value (sig > 0.05). Temperature can affect the viscosity, where high temperatures can reduce viscosity, conversely, low temperatures can increase viscosity [22].
3.3. Antioxidant Activity
The DPPH solution has a maximum absorbance of 517 nm. This wavelength corresponds to the green region of the spectrum (500–520 nm) in terms of transmitted color [32]. The DPPH assay is a quick, sensitive, and straightforward method for evaluating the antioxidant activity of plant extracts [33]. This activity is observed through the color change in the DPPH solution, which shifts from purple to yellow. The extent of this color change correlates with the number of moles of the stabilized molecule present [34]. Results were expressed as the percentage of inhibition, and the calculated IC50 values were 3.180 mg/mL for bromelain enzyme and 4.004 mg/mL for cream containing bromelain. A lower IC50 value indicates higher antioxidant potency [33]. The study’s results revealed a dose-dependent increase in inhibition percentage against DPPH radicals, encompassing concentrations from 100 to 500 μg/mL. Notably, the most pronounced antioxidant activity was observed at the concentration of 500 μg/mL, as depicted in Table 5.

Table 5.
Antioxidant activity of the enzyme bromelain and ascorbic acid.
3.4. Photoprotection Activity
Bromelain’s SPF value was measured to assess its UV protection capacity. The evaluation was conducted at three concentrations: 10 g/L, 20 g/L, and 30 g/L. The results indicated that bromelain at 30 g/L exhibited the highest SPF value of 7.34. However, this SPF value is considered insufficient for effective UV protection. Therefore, in this study, bromelain will be combined with Octyl Methoxycinnamate (OMC) to enhance its photoprotective efficacy and achieve a higher SPF. Notably, molecules with an SPF value of 15 or greater are highly desirable in the development of sunscreen formulations [35].
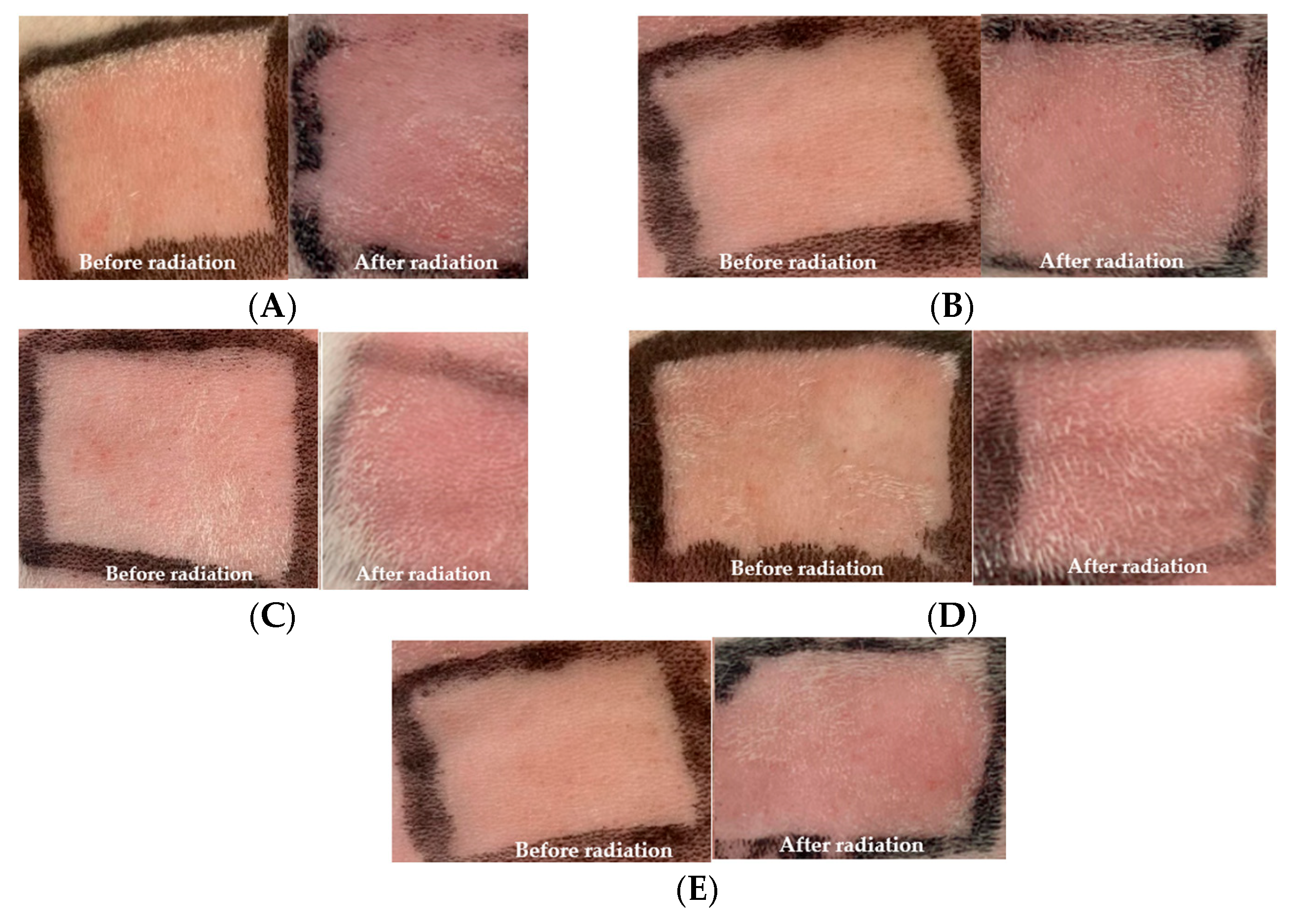
Photoprotective activity was measured by in vitro and in vivo methods. For in vitro, the SPF was evaluated by the methodology developed by the Mansur method. For in vivo studies using rabbits, the UVB radiation on the back skin of the rabbit is provided in Figure 2.
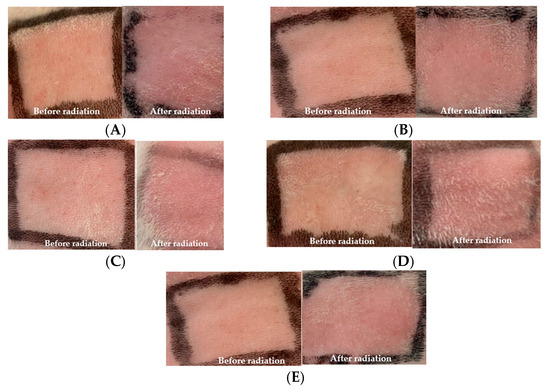
Figure 2.
Observation of erythema (A) without treatment; (B) treatment with base cream; (C) treatment with F1; (D) treatment with F2; (E) treatment with F3.
The SPF values obtained through in vitro and in vivo methods are presented in Table 6 and Figure 3. The Sun Protection Factor (SPF) of each formulation was evaluated using both methodologies, as summarized in Table 6. The findings revealed that the cream base exhibited a relatively low SPF value, with an in vitro SPF of 1.125 ± 0.109 and an in vivo SPF of 6.34 ± 1.068. In contrast, formulations containing bromelain (F1, F2, and F3) demonstrated significantly higher SPF values, indicating enhanced photoprotective potential.

Table 6.
SPF Value in vitro.
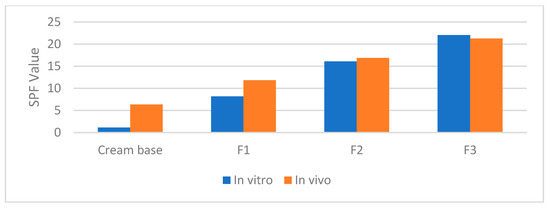
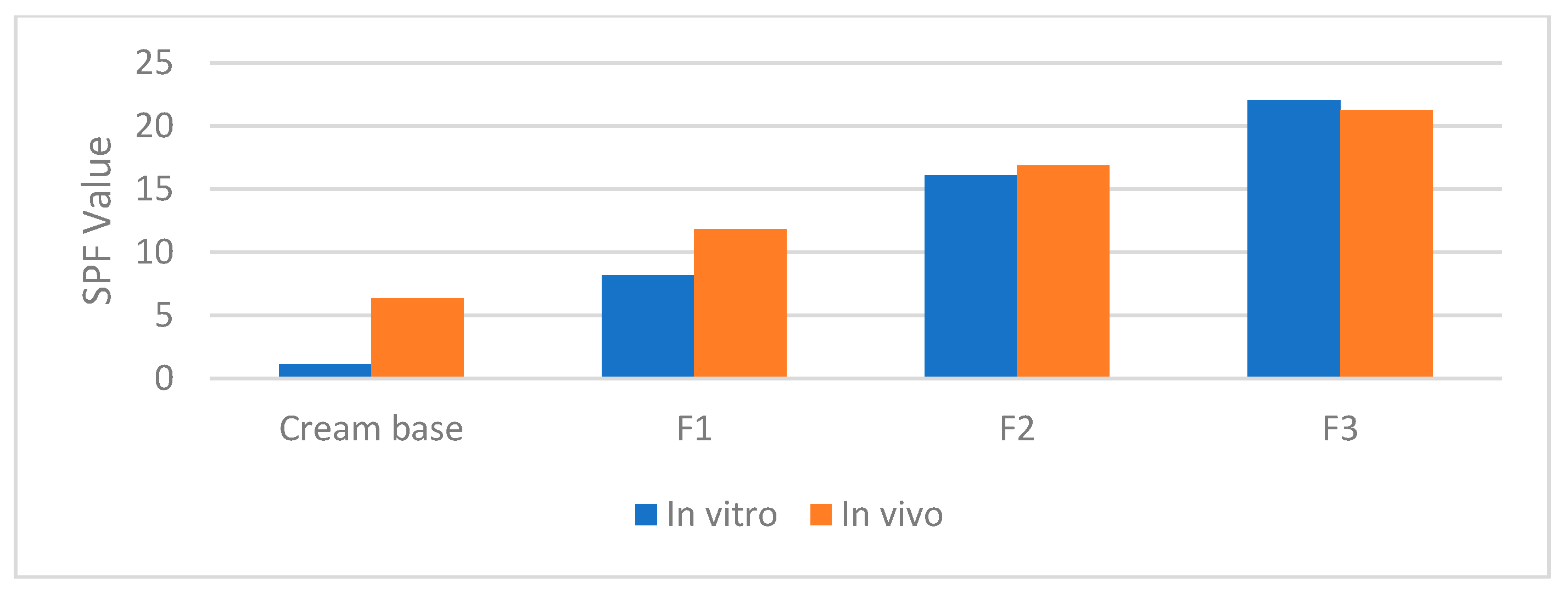
Figure 3.
Correlation in vitro and in vivo SPF value.
Formulation F1 showed an in vitro SPF of 8.180 ± 0.181 and an in vivo SPF of 11.83 ± 1.879, representing a notable improvement compared to the base cream. Formulation F2 exhibited an in vitro SPF of 16.082 ± 0.349 and an in vivo SPF of 16.87 ± 2.799, further enhancing sun protection efficacy. Among the tested formulations, F3 demonstrated the highest SPF values, with an in vitro SPF of 22.043 ± 0.277 and an in vivo SPF of 21.3 ± 2.901, indicating the most effective sun protection. These results suggest that the incorporation of bromelain into sunscreen formulations contributes to a substantial improvement in UV protection.
3.5. Dermal and Ocular Irritation Assesment
3.5.1. Dermal Irritation
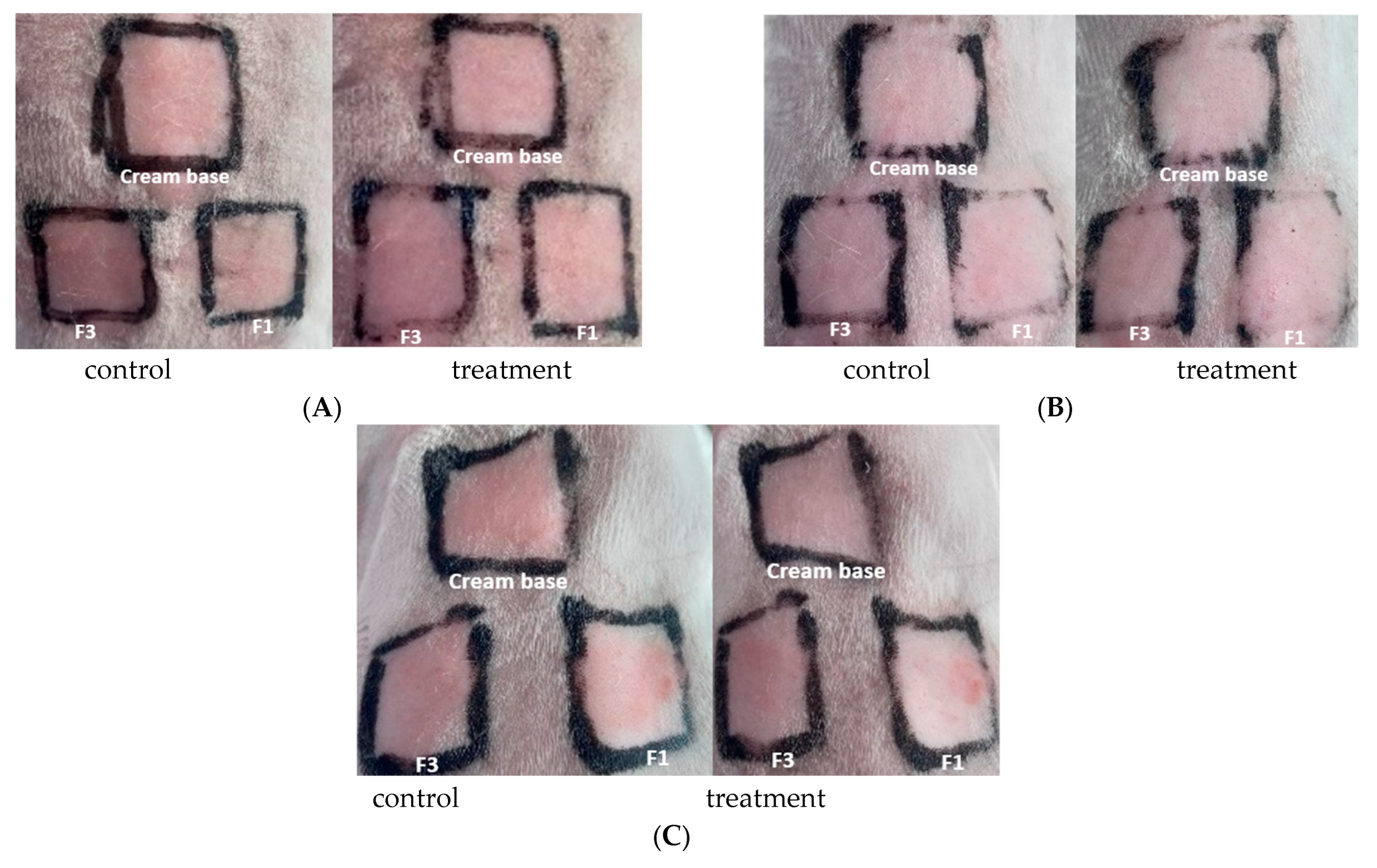
The irritation test was conducted on sunscreen creams containing bromelain, specifically on F1 (bromelain 3%) and F3 (OMC + bromelain 3%), with the following observation results, as presented in Figure 4.
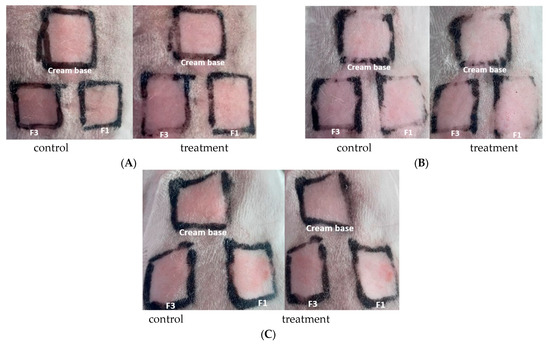
Figure 4.
Observation of dermal irritation test (A) rabbit 1; (B) rabbit 2; (C) rabbit 3.
In the dermal irritation test, on the cream base, F1 and F3 with a dose of 0.5 mL on rabbit 1, rabbit 2, and rabbit 3, after treatment, were observed at 1 h, 24 h, 48 h, and up to 72 h, and did not show any erythema, eschar, or edema compared to the control, thus receiving a score of 0, as shown in Table 7.

Table 7.
Skin irritation score.
3.5.2. Ocular Irritation
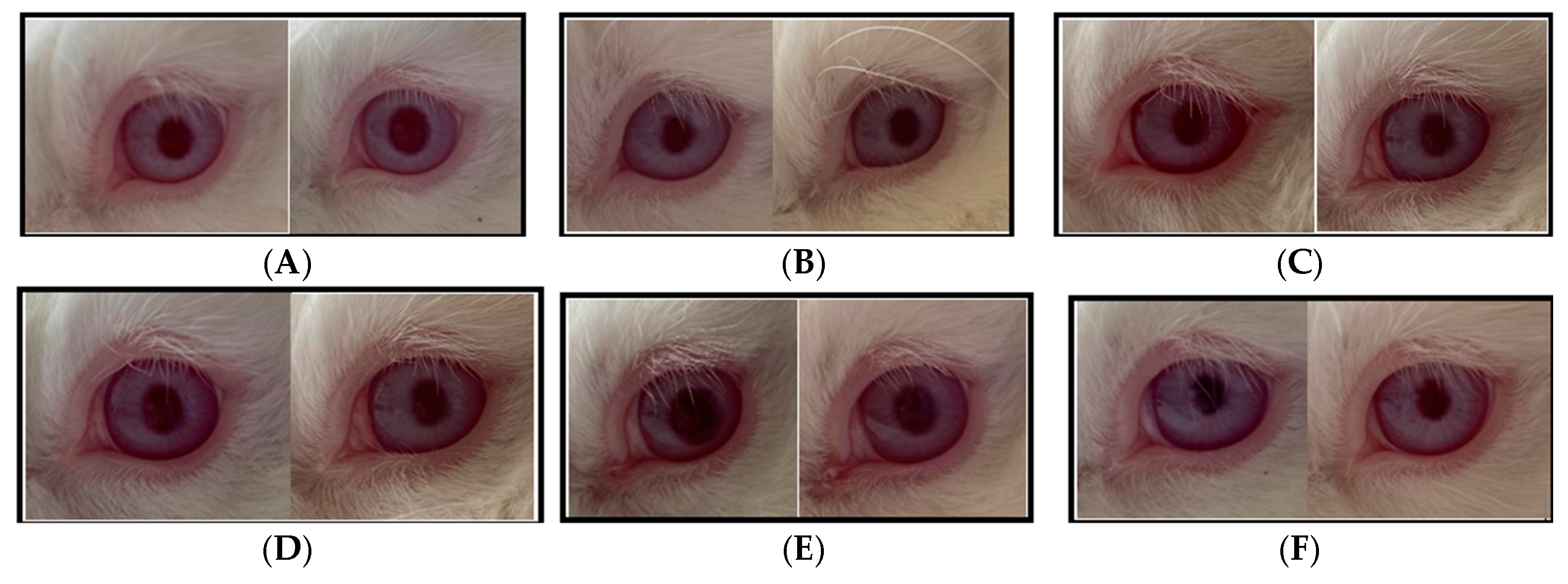
The irritation test was conducted on sunscreen creams containing bromelain, specifically F1 (bromelain 3%) and F3 (OMC + bromelain 3%), with the following observation results, as presented in Figure 5.
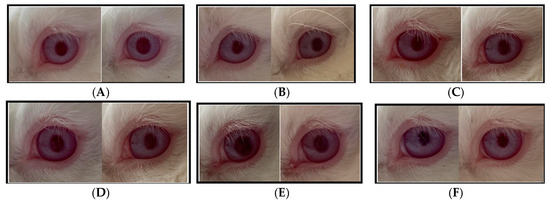
Figure 5.
Observation of the ocular irritation test (A) test animal 1 was administered F1; (B) test animal 2 was administered F1; (C) test animal 3 was administered F1; (D) test animal 1 was administered F3; (E) test animal 2 was administered F3; (F) test animal 3 was administered F3.
Based on the results of the ocular irritation test, on the cream base, F1 and F3 with a dose of 0.1 mL showed no reactions from the beginning of the application, as observed from the symptoms in the cornea, iris, conjunctiva, and chemosis areas. The cornea area appeared clear and not cloudy. The iris was normal and the iris lines were clearly visible. Furthermore, there was no redness in the conjunctiva membrane and eyelid, nor swelling in the eyelids or chemosis. The eyes did not produce excessive tears and were not sensitive to light. Based on the symptoms observed, there were no noticeable changes in the eyes of the test animals after the application, thus the score given from 1 h, 24 h, 48 h, 72 h, and up to day 21 was 0, similar to the control eyes. According to Figure 5 there was no difference between before treatment (control) and after the bromelain cream treatment.
4. Discussion
4.1. Characteristic Sunscreen Cream
In this study, the oil-in-water (O/W) cream was chosen as a sunscreen dosage form because it has advantages, such as being comfortable to use, easy to apply, nonsticky, and easy to wash with water compared to ointment or paste preparations [36]. In cream preparations, an emulsifier or surfactant is required to stabilize the emulsion. Emulsifiers play a crucial role in stabilizing sunscreen formulations by ensuring uniform dispersion of oil- and water-soluble ingredients [37]. In this study, Olivem® 1000 was used as the primary emulsifier due to its ability to form a stable oil-in-water (O/W) emulsion, which enhances the spreadability, absorption, and consistency of the cream. Olivem® 1000, composed of cetearyl olivate and sorbitan olivate, mimics the skin’s lipid structure, promoting better skin compatibility and moisture retention [38]. This emulsifier was selected to improve the overall stability and efficacy of the bromelain-containing sunscreen, ensuring homogeneity in the formulation while maintaining optimal SPF performance.
During storage, F1, F2, and F3 have consistent physical appearances. The odor and color of each formula did not change significantly during storage. The pH measurement aims to determine the preparation’s pH value in order to meet the pH requirements of topical preparations. A good topical preparation must have a pH between 4.5 and 6.5 [39]. A very low pH or acidic preparation can irritate the skin, whereas a high pH or alkaline preparation can make the skin scaly [40]. According to Table 8, all formulas met the requirements for the pH of the cream preparation, which ranged from 4.5 to 6.5, and there was no statistically significant difference between each formula.

Table 8.
Ocular Irritation Score.
4.2. Antioxidant Activity Assay
The IC50 value of the bromelain enzyme indicates that it exhibited a stronger antioxidant effect, as it required a lower concentration to inhibit 50% of DPPH radicals, in contrast to the cream formulations indicating that their antioxidant potency was lower than pure bromelain enzyme. This difference may be attributed to the potential dilution or modification of bromelain’s activity during the formulation process, including interactions with other excipients that may affect its stability, bioavailability, or efficacy in scavenging DPPH radicals. These results highlight that the formulation of bromelain into cream may alter its antioxidant potential, reducing its efficacy compared to the enzyme in its pure form. Despite this, the cream formulations still exhibited antioxidant activity, particularly at higher concentrations, as demonstrated by the dose-dependent increase in inhibition.
Plant-based antioxidants, such as bromelain, help protect the body from diseases caused by free radicals with few or no adverse effects. These antioxidants reduce oxidative stress by halting the oxidative chain reaction, thereby preventing further oxidative damage [41].
4.3. Photoprotective and Antioxidant Activity of Bromelain
There are both in vitro and in vivo methods for determining if SPFs are valuable, with in vivo testing considered the gold standard for regulatory purposes. Although human-based SPF testing is more commonly used, rabbit skin testing remains a valuable model for the preliminary evaluation of sunscreen formulations. SPF values obtained from rabbit skin testing can be adjusted using in vitro methods, such as UV spectrophotometry, which helps refine SPF estimations and verify the accuracy of rabbit skin test data. Our findings indicate that SPF values from in vivo testing did not significantly differ from those obtained through in vitro methods, supporting the reliability of the rabbit model.
Bromelain, as a proteolytic enzyme, has a specific subclass called cysteine proteinases. Cysteine proteinases are a group of enzymes that use a cysteine residue in their active site to catalyze the breakdown of proteins [42]. They can help control the rate of apoptosis in response to UV damage, ensuring that damaged cells are removed while preventing excessive or unnecessary cell death. This regulation of cell death may contribute to skin cell survival following UV exposure, reducing the overall damage to the skin [43,44,45]. While some apoptosis is protective, excessive cell death can lead to skin thinning and an increased risk of skin cancer. Cysteine proteinases have been shown to have antioxidant properties, helping to scavenge ROS and reduce oxidative stress. By neutralizing harmful free radicals, cysteine proteinases may protect skin cells from oxidative damage and reduce the long-term risks of UV-induced skin damage [46].
Bromelain exerts anti-inflammatory effects against UVB-induced skin damage by inhibiting NF-κB activation, thereby reducing proinflammatory cytokines (TNF-α, IL-6, IL-1β) and suppressing COX-2/PGE2 pathways that cause erythema [14,15]. It also modulates the MAPK signaling pathway, specifically by downregulating p38 and JNK, which mitigates oxidative stress and prevents excessive inflammation. Additionally, bromelain inhibits MMPs (MMP-1, MMP-9) to protect collagen from UV-induced degradation, while its antioxidant activity neutralizes ROS, reducing DNA damage and photoaging [47,48]. These combined mechanisms make bromelain a potential natural photoprotective and anti-inflammatory agent for skincare applications. This mechanism is relevant to other research that reported polysaccharide from the flower bud of Sophora japonica L. decreased ROS generation and down-regulated the expression of phosphor-JNK and phosphor-p38 MAPK proteins significantly in UVB-irradiated cells [7], and other plant-based, such as Juglans regia L. and Perilla frutescens, reduced UV-induced ROS production [10].
Bromelain exhibited potent antioxidant activity, with an IC50 value of 4.296 µg/mL, categorizing it as a highly effective free radical scavenger. This strong antioxidative capacity enhances the photoprotective effects of UV filters by reducing lipid peroxidation, preventing DNA damage, and stabilizing cellular structures against oxidative stress. Specifically, higher antioxidant activity, as indicated by lower IC50 values, corresponded with increased SPF values. This finding aligns with the well-established role of antioxidants in neutralizing free radicals and mitigating oxidative stress, both of which are key contributors to UV-induced skin damage [46]. The synergistic interaction between bromelain and UV filters further supports its potential as a functional ingredient in sunscreen formulations, providing both antioxidative and photoprotective benefits.
4.4. Synergistic Effect of Bromelain Combination with UV Filter
These findings demonstrate that the incorporation of bromelain into the cream formulations significantly enhances SPF values in both in vitro and in vivo evaluations, with the highest protection observed in the bromelain and OMC combination (F3). The addition of bromelain to UV filters resulted in greater UV absorption, indicating a synergistic effect that enhances sunscreen efficacy. Bromelain, an enzyme with potent antioxidant and photoprotective properties, contributes to improved sunscreen stability and effectiveness. The observed increase in SPF values in formulations with higher antioxidant activity suggests that bromelain mitigates oxidative damage induced by UV radiation [49]. Moreover, bromelain-containing formulations exhibited significantly higher SPF values compared to the base cream, highlighting its additional photoprotective benefits beyond its antioxidant action.
The antioxidant and anti-inflammatory properties of bromelain play a critical role in enhancing skin protection. By reducing oxidative stress and modulating inflammatory pathways, bromelain offers comprehensive defense against UV-induced cellular damage. This dual mechanism not only prevents oxidative deterioration but also minimizes inflammation and erythema, common effects of sun exposure. These findings underscore the potential of bromelain as a valuable bioactive ingredient in sunscreen formulations, providing enhanced UV protection and skin health benefits.
4.5. Irritation Potential of Bromelain-Containing Formulation
The irritation tests conducted include dermal and ocular irritation. This is because sunscreen cream products are generally applied to the facial skin and around the eye area, so their safety needs to be tested. The irritation test for the cream containing 3% bromelain on rabbits showed no irritation on the skin or eyes, indicating that the sunscreen cream containing bromelain is safe to use and is nonirritating. These results are in line with research conducted by Thomas et al., which demonstrated that the bromelain enzyme does not irritate the skin and even has good wound healing activity [50].
5. Conclusions
The present study demonstrated the importance and interest in using bromelain in sunscreen preparations with incorporated UVB filter OMC (Octyl Methoxycinnamate), as it was observed that SPF and antioxidant activity increased, leading to greater skin protection. The antioxidant activity and SPF are correlated with reducing oxidative stress and modulating the anti-inflammatory response. Bromelain cream 3% did not irritate the skin or eyes of rabbits, suggesting that it may be safe for human use without causing irritation. These results provide a basis for further development of bromelain-based formulations with the dual benefits of antioxidant protection and enhanced photoprotection against UV-induced damage. However, to obtain more clinically relevant and translatable results, future studies should include in vivo testing on human skin to further validate the photoprotective efficacy and safety of bromelain-based formulations. Such studies will provide more comprehensive insights into their real-world application and effectiveness in preventing UV-induced damage.
Author Contributions
Conceptualization, Z.M. and S.R.M.; methodology, S.R.M., C.K.K. and Z.M.; software, Z.M.; validation, S.R.M. and Z.M.; formal analysis, S.R.M. and Z.M.; investigation, S.R.M. and C.K.K.; resources, S.R.M., C.K.K. and Z.M.; data curation, Z.M. writing—original draft preparation, Z.M.; writing—review and editing, S.R.M.; supervision, S.R.M. and S.S.; funding acquisition, S.R.M. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This review is funded by the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia (Grant number 4002/UN6.3.1/PT.00/2024) and the APC was funded by Academic Leadership Grant Univeristas Padjadjaran of Prof. Sriwidodo (Grant number 1556/UN6.3.1/PT.00/2024).
Institutional Review Board Statement
This study did not involve human subjects but used animal models. The animal experiments were approved by the Research Ethics Committee of Universitas Padjadjaran no. 979/UN6.KEP/EC/2024.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Katiyar, S.K.; Elmets, C.A. Green tea polyphenolic antioxidants and skin photoprotection (Review). Int. J. Oncol. 2001, 18, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Debacq-Chainiaux, F.; Leduc, C.; Verbeke, A.; Toussaint, O. UV, stress and aging. Dermatoendocrinology 2012, 4, 236–240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruszkiewicz, J.A.; Pinkas, A.; Ferrer, B.; Peres, T.V.; Tsatsakis, A.; Aschner, M. Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol. Rep. 2017, 4, 245–259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shusterove, L.; Romero, J. The Importance of Coral Reefs and How to Ensure Their Longevity, a One Health Approach. VIP 2020, 12. Available online: https://scholarworks.boisestate.edu/vip_2020/12 (accessed on 1 May 2024).
- Napagoda, M.T.; Malkanthi, B.M.A.S.; Abayawardana, S.A.K.; Qader, M.M.; Jayasunghe, L. Photoprotective potential in some medicinalplants used to treat skin diseases in Sri Lanka. BMC Compl. Altern. Med. 2016, 16, 479. [Google Scholar] [CrossRef]
- Rabinovich, L.; Kazlouskaya, V. Herbal sunprotection agents: Human studies. Clin. Dermatol. 2018, 36, 369–375. [Google Scholar] [CrossRef]
- Li, L.Y.; Huang, T.; Lan, C.; Ding, H.; Yan, C.; Dou, Y. Protective effect of polysaccharide from Sophora japonica L. flower buds against UVB radiation in a human keratinocyte cell line (HaCaT cells). J. Photochem. Photobiol. B Biol. 2019, 191, 135–142. [Google Scholar] [CrossRef]
- Cefali, L.C.; Ataide, J.A.; Oliveria Sousa, I.M. In vitro solar protection factor, antioxidant activity, and stability of a topical formulation containing Benitaka grape (Vitis vinifera L.) peel extract. Nat. Prod. Res. 2020, 34, 2677–2682. [Google Scholar] [CrossRef]
- Vostálová, J.; Tinková, E.; Biedermann, D.; Kosina, P.; Ulrichová, J.; Rajnochová Svobodová, A. Skin protective activity of silymarin and its flavonolignans. Molecules 2019, 24, 1022. [Google Scholar] [CrossRef]
- Muzaffer, U.; Paul, V.I.; Prasad, N.R. Protective effect of Juglans regia L. against ultraviolet B radiation induced inflammatory responses in human epidermal keratinocytes. Phytomedicine 2018, 42, 100–111. [Google Scholar] [CrossRef]
- Badan Pusat Statistik. Fruit Plant Production in 2021–2022. Available online: https://www.bps.go.id/id/statistics-table/2/NjIjMg==/produksi-tanaman-buah-buahan.html (accessed on 1 May 2024).
- Arshad, Z.I.M.; Amid, A.; Yusof, F.; Jaswir, I.; Ahmad, K.; Loke, S.P. Bromelain: An overview of industrial application and purification strategies. Appl. Microbiol. Biotechnol. 2014, 98, 7283–7297. [Google Scholar] [CrossRef] [PubMed]
- Harats, M.; Haik, J.; Cleary, M.; Vashurin, I.; Aviv, U.; Kornhaber, R. A Retrospective Review of an Off-label Bromelain-based Selective Enzymatic Debridement (Nexobrid(R)) in the Treatment of Deep, Partial, and Full Thickness Burns and Hard to Heal Wounds. Isr. Med. Assoc. J. 2020, 22, 83–88. [Google Scholar] [PubMed]
- Badriyya, E.; Salman Pratiwi, A.R.; Dillasamola, D.; Aldi, Y.; Husni, E. Topical AntiInflammatory Activity of Bromelain. Pharmacogn. J. 2020, 12, 1586–1593. [Google Scholar] [CrossRef]
- Bakare, A.O.; Owoyele, B.V. Bromelain reduced pro-inflammatory mediators as a common pathway that mediate antinociceptive and anti-anxiety effects in sciatic nerve ligated Wistar rats. Sci. Rep. 2021, 11, 289. [Google Scholar] [CrossRef]
- El-Demerdash, F.M.; Hussien, D.M.; Ghanem, N.F.; Al-Farga, A.M. Bromelain Modulates Liver Injury, Hematological, Molecular, and Biochemical Perturbations Induced by Aluminum via Oxidative Stress Inhibition. Biomed. Res. Int. 2022, 1, 5342559. [Google Scholar] [CrossRef]
- Mori, A.; Lee, W.R. Protective effect of bromelain and pineapple extracts on UV-induced damage in human skin cells. J. Emerg. Investig. 2023, 6, 1–6. [Google Scholar] [CrossRef]
- Esprendor, R.V.F.; Raiser, A.L.; Torres, M.P.R.; Ribeiro, E.B.; Nogueira, R.M.; Andrighetti, C.R.; ValladÃ, D.M. Development and stability study of products containing cupuaçu butter. Sci. Electron. Arch. 2019, 12, 77–85. [Google Scholar]
- Sambasivarao, A.; Rao, C.S.; dan Reddy, H.M. Accelerated stability testing of dosages forms as per international conference of hormozination (ICH) guidelines. World J. Pharm. Med. Res. 2016, 2, 99–103. [Google Scholar]
- Garg, A.; Anggarwal, D.; Garg, S.; dan Singla, A.K. Spreading of Semisolid Formulation: An Update. Pharm. Technol. 2022, 26, 84–104. [Google Scholar]
- Suciati, T.; Aliyandi, A.; Satrialdi. Development of transdermal nanoemulsion formulation for simultaneous delivery of protein vaccine and Artin-M adjuvant. Int. J. Pharm. Pharm. Sci. 2014, 6, 536–541. [Google Scholar]
- Saptarini, N.M.; Rahayu, D.; Herawati, I.E. Antioxidant Activity of Crude Bromelain of Pineapple (Ananas comosus (L.) Merr) Crown from Subang District, Indonesia. J. Pharm. Bioallied. Sci. 2019, 11 (Suppl. S4), S551–S555. [Google Scholar] [CrossRef] [PubMed]
- Mansur, J.S.; Breder, M.N.; Mansur, M.C.; Azulay, R.D. Determination of sun protection factor by spectrophotometry. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Elcistia, R.; Zulkarnain, A.K. Formula Optimization of O/W Cream Combination of Oxybenzone and Titanium Dioxide and Its In Vivo Activity Testing. Maj. Farm. 2018, 14, 63–78. [Google Scholar]
- OECD. Test No. 404: Acute Dermal Irritation/Corrosion, OECD Guidelines for the Testing of Chemicals, Section 4; Organisation for Economic Cooperation and Development (OECD) Publishing: Paris, France, 2015. [Google Scholar]
- OEDa. Test No. 405: Acute Eye Irritation/Corrosion, OECD Guidelines for the Testing of Chemicals, Section 4; Organisation for Economic Cooperation and Development (OECD) Publishing: Paris, France, 2021. [Google Scholar]
- Smith, E.W.; Maibach, H.I.; Surber, C. Use of Emulsions as Topical Drug Delivery Systems. In Pharmaceutical Emulsions and Suspensions; Nielloud, F., Marti-Mestres, G., Eds.; Marcel Dekker: New York, NY, USA, 2000; pp. 259–270. [Google Scholar]
- Dwi Saryanti, D.K.K. Optimization of the M/A Cream Formula from Kepok Banana Peel Extract (Musa acuminata L.). Department of Pharmaceutical Technology. J. Ris. Kefarmasian Indones. 2019, 1, 19. [Google Scholar]
- Mugitasari, D.E.; Rahmawati, B. Formulation of a cream with Morinda citrifolia L. leaf extract as a ultraviolet protection preparation. J. Keperawatan Dan Kesehat. Masy. 2020, 9, 109–119. [Google Scholar] [CrossRef]
- Mailana, D.; Nuryanti, H. Antioxidant Cream Formulation of Ethanolic Extract from Avocado Leaves (Persea americana Mill.). Acta Pharm. Indones. 2016, 4, 7–15. [Google Scholar]
- Badan Standarisasi Nasional. 16-4399-1996; Sunscreen Formulation. National Standardization Agency: Jakarta, Indonesia, 1996. [Google Scholar]
- Chen, X.; Liang, L.; Han, C. LWT—Food Science and Technology Borate suppresses the scavenging activity of gallic acid and plant polyphenol extracts on DPPH radical: A potential interference to DPPH assay. LWT Food Sci. Technol. 2020, 131, 109769. [Google Scholar] [CrossRef]
- Olugbami, J.; Gbadegesin, M.; Odunola, O. In vitro evaluation of the antioxidant potential, phenolic and flavonoid contents of the stem bark ethanol extract of Anogeissus leiocarpus. Afr. J. Med. Med. Sci. 2015, 43 (Suppl. S1), 101–109. [Google Scholar]
- Subramanian, R.; Subbramaniyan, P.; Raj, V. Antioxidant activity of the stem bark of Shorea roxburghii and its silver reducing power. SpringerPlus 2013, 2, 28. [Google Scholar] [CrossRef]
- Kora’c, R.R.; Khambholja, K.M. Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn. Rev. 2011, 5, 164–173. [Google Scholar] [CrossRef]
- Sharon, N.; Anam, S.; Yuliet. Formulation of Antioxidant Cream from Ethanol Extract of Wild Garlic (Eleutherine palmifolia L. Merr). Online J. Nat. Sci. 2013, 2, 111–122. [Google Scholar]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement Strategies for Transdermal Drug Delivery Systems: Current Trends and Applications. Drug Deliv. Transl. Res. 2021, 12, 758–791. [Google Scholar] [CrossRef]
- Hallstar. Olivem® 1000 Product Brochure, Hallstar Beauty. 2024. Available online: https://www.hallstarbeauty.com/?s=olivem+1000 (accessed on 14 August 2024).
- Luki’c, M.; Panteli’c, I.; Savi’c, S.D. Towards Optimal pH of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Biro, T.; Olah, A.; Toth, B.I.; Szollosi, A.G. Endogenous Factors That Can Influence Skin pH. In pH of the Skin: Issues and Challenges; 2018; Volume 54, pp. 54–63. [Google Scholar]
- Lee, J.H.; Lee, J.B.; Lee, J.T.; Park, H.R.; Kim, J.B. Medicinal effects of bromelain (Ananas comosus) targeting oral environment as an antioxidant and anti-inflammatory agent. J. Food Nutr. Res. 2018, 6, 773–784. [Google Scholar] [CrossRef]
- Agrawal, P.; Nikhade, P.; Patel, A.; Mankar, N.; Sedani, S. Bromelain: A Potent Phytomedicine. Cureus 2022, 14, e27876. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verma, S.; Dixit, R.; Pandey, K.C. Cysteine Proteases: Modesof Activation and Future Prospectsas Pharmacological Targets. Front. Pharmacol. 2016, 7, 107. [Google Scholar] [CrossRef]
- Takahashi, H.; Komatsu, N.; Ibe, M.; Yamamoto, A.I.; Hashimoto, Y.; Iizuka, H. Cystatin A suppresses ultraviolet B-induced apoptosis of keratinocytes. J. Dermatol. Sci. 2007, 46, 179–187. [Google Scholar] [CrossRef]
- Monteiro, A.C.; Schmitz, V.; Svensjo, E.; Gazzinelli, R.T.; Almeida, I.C.; Todorov, A.; de Arruda, L.B.; Torrecilhas, A.C.T.; Pesquero, J.B.; Morrot, A.; et al. Cooperative Activation of TLR2 and Bradykinin B2 Receptor Is Required for Induction of Type 1 Immunity in a Mouse Model of Subcutaneous Infection by Trypanosoma cruzi. J. Immunol. 2006, 177, 6325–6335. [Google Scholar] [CrossRef]
- Chanchal, D.; Swarnlata, S. Herbal photoprotective formulations and their evaluation. Open Nat. Prod. J. 2009, 2, 71–76. [Google Scholar] [CrossRef]
- Pothacharoen, P.; Chaiwongsa, R.; Chanmee, T.; Insuan, O.; Wongwichai, T.; Janchai, P.; Vaithanomsat, P. Bromelain Extract Exerts Antiarthritic Effects via Chondroprotection and the Suppression of TNF-α–Induced NF-κB and MAPK Signaling. Plants 2021, 10, 2273. [Google Scholar] [CrossRef]
- Manosroi, A.; Chankhampan, C.; Manosroi, W.; Manosroi, J. Toxicity reduction and MMP-2 stimulation of papain and bromelain loaded in elastic niosomes. J. Biomed. Nanotechnol. 2012, 8, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.A.; Taupik, M.; Djuwarno, E.N.; Papeo, R.P.; Djunaidi, N.N. Burn Wound Healing Test of Bromelain Enzyme Gel Using Carbopol 940 In Vivo. J. Syifa Sci. Clin. Res. 2023, 5, 232–244. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).