Abstract
Hyaluronic acid (HA) content in the skin progressively decreases with age; thus, its supplementation—either topical, oral, and by subcutaneous injection—represents a first-line intervention to ameliorate skin aging signs. The present multicenter randomized placebo-controlled trial (RCT) evaluated the skin antiaging efficacy of an In&Out treatment, i.e., a concomitant topical, through a cosmetic cream, and the oral administration of specifically designed HAs, full-spectrum HAs (FS-HAs), in a multiethnic population using instrumental measurements and clinical assessments. The efficacy of FS-HAs was also evaluated in groups receiving FS-HA in a single administration route and in the presence of the placebo in the counterpart route. The above treatment scheme was applied for 56 days to 88 adult subjects, equally divided into four groups. Treatments containing at least one FS-HA molecule showed progressive and significant intragroup ameliorations of all the instrumental skin parameters evaluated. The In&Out treatment resulted in a greater improvement with respect to the two other active groups and in significant intergroup differences with respect to its placebo counterpart. FS-HA treatments resulted, as well, in the significant improvements of clinical parameters that correlated with the subjects’ appreciation recorded by a self-assessment, hence confirming that the In&Out administration of FS-HA represents an interesting approach to counteract skin aging signs.
1. Introduction
The human skin, located at the body’s interface, undergoes continuous changes throughout our life, driven by intrinsic aging and by extrinsic aging, which significantly concur to alter its morphology and functionality, ultimately resulting in what is known as aged skin [1,2]. Intrinsic aging is determined by chronological and genetic factors, while extrinsic aging is shaped by a range of external influences, collectively referred to as the “exposome”, including pollution, UV exposure (photoaging), diet, lifestyle, and overall health conditions [3,4]. The occurrence of dryness, roughness, wrinkles, and pigmented spots and a decreased barrier function are the main histological changes resulting from alterations in the skin’s structure and function and are hallmark signs of skin aging [5,6,7,8]. Aged skin is characterized by a decline in its key structural components, including collagen and elastin, which form and maintain its 3D structure, and hyaluronic acid (HA), a major component of the extracellular matrix (ECM) that ensures the moisturization and firmness of the ECM due to its high hydrophilic properties [9,10].
As collagen and elastin content decreases with age, the skin gradually loses its mechanical tension [6]. Furthermore, in senile skin, HA entirely disappears in the epidermis [10,11] while it is still present in the dermis, and it also becomes more tissue-associated, a condition that may decrease its hygroscopic ability with the consequence of a loss of skin moisture [10,12]. Therefore, hyaluronic acid (HA) plays a significant role in antiaging skin treatments due to its ability to restore moisture in the skin’s layers, promote endogenous collagen synthesis through its physicochemical properties, stimulate dermal fibroblasts, and provide antioxidant effects [13]. Additionally, the signaling properties of HA fragments, produced via hyaluronidase-mediated degradation or oxidative damage [14,15,16], further highlight the importance of the polymer’s dimensional characteristics in determining its biological functions [17,18]. Indeed, the biological effects of HA depend on the size of the polymer, resulting in differentiated performances. Therefore, its dimensional characteristics should be carefully evaluated when investigating HA’s biological effects.
Since hyaluronic acid (HA) content in the skin is closely associated with skin quality and appearance, and the concepts of skincare, wellness, and antiaging are integral to daily life, HA supplementation remains a key strategy in antiaging skin treatments [13,19]. This is primarily achieved through topical application via cosmetic formulations [20,21,22], intra-dermal injection of HA fillers [23], and systemic administration through dietary supplements [24,25] or by combining topical and oral administration [26,27]. In this context, full-spectrum hyaluronans (FS-HAs) represent a promising advancement in HA treatments. FS-HAs are second-generation hyaluronans produced using an innovative technology known as HA Tech 2.0® [28]. This technology enables the precise modulation of various parameters during the fermentation process, resulting in tailored products composed of polymers with a spectrum of sizes that closely resemble the molecular profile of the targeted application area. Unlike commercially available products with a narrow peak distribution, full-spectrum hyaluronans (FS-HAs) exhibit a broader range of peaks. This enables them to encompass a wider spectrum of molecular weights, enhancing their biological activity and providing more diverse signaling capabilities for cellular responses.
In this study, we aimed at evaluating the antiaging efficacy of the following two commercially available FS-HA ingredients manufactured by ROELMI HPC, Origgio (VA), Italy: ExceptionHYAL® Star for oral administration and PrincipHYAL® Cube3 for topic use. FS-HAs have been specifically developed and characterized to be used both in cosmetic and nutraceutical products, demonstrating effectiveness in improving the signs of skin aging, as demonstrated by randomized clinical trials (RCTs) [25,26]. In a previous RCT, the effect on skin aging of a combined treatment of a cosmetic product, containing PrincipHYAL® Difference, and a food supplement, containing ExceptionHYAL® Star, on a Caucasian population was evaluated [26]. This present In&Out study aimed to evaluate the skin antiaging efficacy of a combined treatment of a cosmetic product containing a newly developed FS-HA, i.e., PrincipHYAL® Cube3, and a food supplement containing the previous ExceptionHYAL® Star, with respect to a placebo treatment without any of the two FS-HAs in a multiethnic adult population for a period of 56 days. The antiaging effect of treatments individually using the two FS–HAs in combination with the placebo in their counterpart administration route was also evaluated.
2. Materials and Methods
2.1. Study Design
This multicenter, randomized, placebo-controlled, parallel-group clinical study was conducted at Complife sites in Italy and China. Caucasian and South American/African participants were enrolled at Complife Italia facilities in San Martino Siccomario (Pavia, Italy) and Rende (Cosenza, Italy), while Asian participants were enrolled at Complife (Beijing, China) Testing Technology. The study lasted 56 days, with clinical visits scheduled at baseline (D0) and after 14 (D14), 28 (D28), and 56 (D56) days of product use. Throughout the study, the participants maintained a daily diary to record treatment compliance, product tolerability, and dietary habits. All study procedures were conducted in accordance with the World Medical Association’s (WMA) Declaration of Helsinki and its subsequent amendments. The study protocol was approved by the “Independent Ethical Committee for Non-Pharmacological Clinical Trials” on 11 November 2022 (ref. no. 2022/07). The trial was registered in the ISRCTN registry (ISRCTN15779299, https://doi.org/10.1186/ISRCTN15779299) and submitted on 22 December 2022. All participants were informed about the study’s nature, purpose, benefits, and potential risks. They provided written informed consent and signed a release form permitting the publication of photographs prior to undergoing any study-related procedures.
2.2. Subjects and Compliance to Treatment
Eligible participants were healthy adults aged 34 to 65 years, including Caucasian (n = 24), South American/African (n = 20), and Asian (n = 44) men and women, all presenting mild-to-moderate signs of skin aging (e.g., skin roughness, wrinkles, dull, or uneven skin tone) and eyebags (with a subpopulation of at least 16 subjects per group). Exclusion criteria included acute, chronic, or progressive illnesses that could interfere with study outcomes or pose risks to the participant, planned hospitalization during the study period, pharmacological treatments incompatible with study requirements, allergies or sensitivities to cosmetic products, drugs, patches, medical devices, or investigational products, as well as pregnancy or breastfeeding (for women). Furthermore, subjects were prohibited from any exposure to both natural and artificial sunlight throughout the entire testing period and were required to refrain from using any products other than the ones being tested.
Compliance with treatment was assessed by counting and recording the remaining pills in each bottle after 28 and 56 days of treatment, with a compliance threshold set at ≥80%.
2.3. Interventions
The active cosmetic cream (AC) had the following composition (INCI-EU nomenclature): Aqua, Tripelargonin, Neopentyl Glycol Dipelargonate, Polyglyceryl-3-Stearate, Triolein, C10-18 Triglycerides, Cetearyl Alcohol, Glyceryl Dioleate, Sodium Hyaluronate (Principhyal® Cube3, 0.5% w/w), Sunflower Seed Oil Glycerides, Hydroxyethylcellulose, Caprylyl Glycol, Ethylhexylglycerin, O-Cymen-5-ol, Parfum. The placebo cosmetic cream (PC) had the same composition as AC, except for sodium hyaluronate. The active food supplement (AF) contained 200 mg ExceptionHYAL® Star, 90 mg maltodextrin, and 30 mg magnesium stearate. The placebo food supplement (PF) was a capsule containing 290 mg maltodextrin and 30 mg magnesium stearate.
The frequency of use was one capsule per day (taken in the morning) and two daily applications of the cosmetic cream (morning and evening).
Participants were randomized into treatment groups using a computer-generated, restricted, and balanced randomization list (1:1:1:1 ratio) created with PASS 11 (version 11.0.8, PASS, LLC, Kaysville, UT, USA) based on the “Wey’s urn” algorithm. The randomized treatment groups were as follows: the PC-AF group applied the PC cream and took the active food supplement containing ExceptionHYAL® Star; the AC-PF group applied the AC cream containing PrincipHYAL® Cube3 and took the placebo food supplement; the AC-AF group applied the cosmetic cream containing PrincipHYAL® Cube3 and took the food supplement containing ExceptionHYAL® Star; and the PC-PF group applied the placebo cosmetic cream and took the placebo food supplement.
2.4. Blinding and Randomisation
The jars containing the cosmetic products and the tubes containing the food supplements were identical, differing only by batch numbers. A technician not involved in the study assembled the product combinations (cosmetic and food supplement) according to their batch numbers and placed them in boxes labeled with numerical codes. Each numerical code corresponded to a treatment combination from the randomization list. The randomization list was securely concealed in sequentially numbered, sealed, and opaque envelopes. Neither the participants nor the study personnel were informed of the group assignments, ensuring blinding throughout the study.
2.5. Primary and Secondary Objectives
The primary objective of the study was to evaluate the antiaging efficacy of the treatments through instrumental measurements of skin parameters, including skin profilometry (wrinkles), moisturization, brightness, elasticity, and firmness. The secondary objective focused on a smaller subset of Caucasian participants to assess treatment efficacy in terms of skin thickness and density using instrumental measurements. Additionally, clinical evaluations were conducted to assess “crow’s feet” wrinkles, nasolabial folds, eyebags, and skin homogeneity, both directly on participants and through the analysis of digital photographs taken throughout the study.
2.6. Outcomes
Clinical and instrumental evaluations were carried out by a dermatologist under controlled environmental conditions (temperature 22 °C ± 2 °C and relative humidity 50 ± 10%). Participants were allowed to acclimatize for 15–20 min before each visit to ensure accurate measurements.
2.6.1. Instrumental Measurements
Skin profilometry was assessed in the periorbital area (“crow’s feet” wrinkles area) using the PRIMOSCR Small Field device (Canfield Scientific Europe, BV, Utrecht, The Netherlands), an optical 3D non-contact skin measurement system based on structured light projection. Skin roughness and smoothness were assessed using the Ra and Rz parameters, which represent the average surface roughness and the average depth of roughness, respectively.
Skin moisturization was measured on the cheekbone using the Corneometer® CM 825 (Courage + Khazaka, electronic GmbH, Köln, Germany). Skin brightness (i.e., the ability of the skin to reflect the light) was measured, on the cheekbone, as the 8° gloss value (i.e., specularly reflected light) by using a spectrophotometer/colorimeter CM-700D (Konica Minolta, Milan, Italy). Skin thickness (dermis and epidermis), dermis thickness, and the dermis density were measured on the cheekbone area by the ultrasound technique using a DUB® SkinScanner75 system (EOTECH SAS, Marcoussis, France) equipped with a high-frequency (50 MHz) probe. The ultrasound measurement was carried out on a subpopulation (5 subjects for each group).
Digital pictures of the face were taken by VISIA® CR (Canfield Scientific Europe, BV, Utrecht, The Netherlands) under standardize positioning and lighting conditions.
2.6.2. Clinical Analysis
The clinical scoring of “crow’s feet” wrinkles (0: no wrinkles to 6: remarkable wrinkles), nasolabial folds (0: no fold to 5: remarkable fold), and eyebags (0: no eyebags to 7: remarkable eyebags) was performed by the site dermatologists using visual scales from the Skin Aging Atlas Vol. 1—Caucasian Type [29], and the Skin Aging Atlas. Volume 2, Asian Type [30]. Half-point increments were permitted. The clinical scoring of eyebags was conducted on a subpopulation consisting of 16 subjects per group.
Skin homogeneity was clinically evaluated using an 11-point visual analog scale (VAS), where 0 indicated uneven skin tone, 1–3 represented moderately uneven skin tone, 4–6 indicated mildly uneven skin tone, and 7–10 represented even skin tone.
2.6.3. Self-Assessment Questionnaire
Participants were asked to provide their feedback on the treatments by completing a questionnaire addressing perceived efficacy, satisfaction, tolerance, and their intention to purchase or recommend the products. Responses were categorized as “completely agree”, “agree”, “disagree”, or “completely disagree”. For analysis, the responses “completely agree” and “agree” were combined and considered as positive feedback from responders.
2.7. Statistical Analysis
Sample size calculation was based on the results of a previous study [26]. Based on the study output, a sample size by 20 subjects per group was obtained. The sample size analysis was carried out with PASS 11 statistical software (version 11.0.8, PASS, LLC, Kaysville, UT, USA).
Intragroup (vs. baseline) statistical analysis was conducted on raw data using repeated measures ANOVA (RM-ANOVA), followed by the Tukey–Kramer post hoc test for normally distributed data. For non-normally distributed data, the Friedman test was applied, followed by the Tukey–Kramer post hoc test. Intergroup (vs. treatments) statistical analysis was conducted on percentage variations using one-way ANOVA for normally distributed data. For non-normally distributed data, the Kruskal–Wallis one-way ANOVA was applied.
Variations were considered statistically significant when the p value was <0.05. All the statistical analysis were performed using NCSS 10 software (vers. 10.0.7; NCSS, LLC. Kaysville, UT, USA).
3. Results
3.1. Participant Characteristics, Tolerability, and Compliance with Treatment
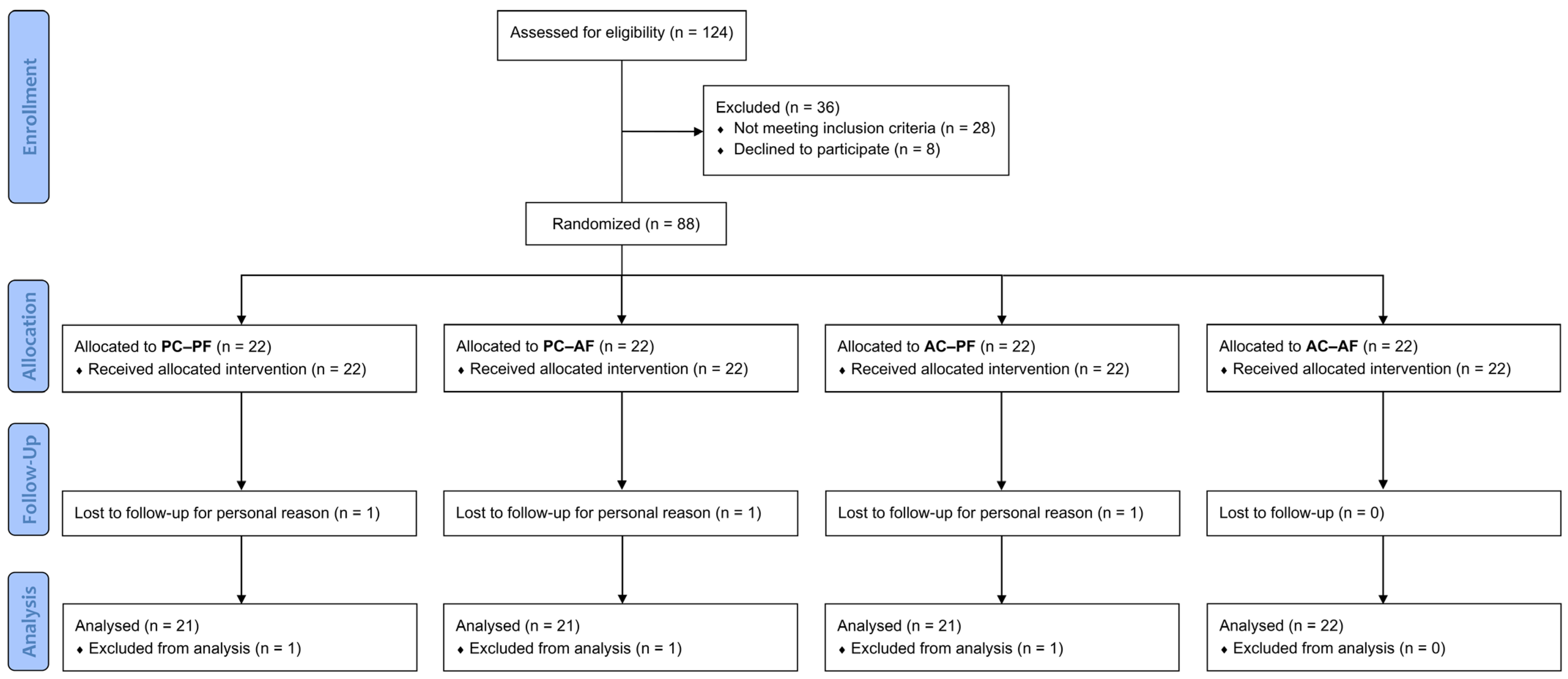
The study screened 124 subjects, of whom 28 did not meet the inclusion criteria, and 8 declined to participate. A total of 88 subjects (n = 88) were successfully randomized into four groups, with 22 subjects in each group. The per-protocol (PP) population consisted of 85 subjects, as 3 participants dropped out of the study for personal reasons (independent from products intake/application). The participant flow chart is presented in Figure 1.
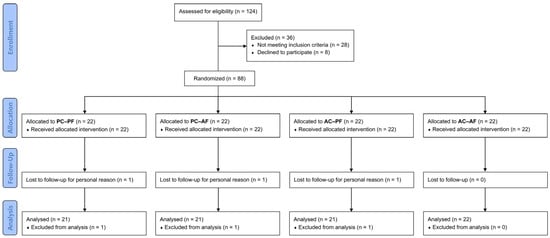
Figure 1.
Participants flow diagram.
Each group included 6 Caucasian, 5 South American/African, and 10 Asian participants, aged 35 to 65 years, all exhibiting clinical signs of mild-to-moderate aging and eyebags (15 subjects per group).
No significant intergroup differences were observed at baseline for any of the primary outcomes, confirming unbiased randomization and homogeneity across the four groups (Table 1).

Table 1.
Demographics and baseline characteristics of study participants.
Both the active and placebo products were well-tolerated, with no adverse events reported by either the participants or the investigators. Overall tolerability was confirmed by 100% of the participants for each product.
The compliance to treatment for each group was ≥80% (min. 93% and max. 100%).
3.2. Primary Endpoints
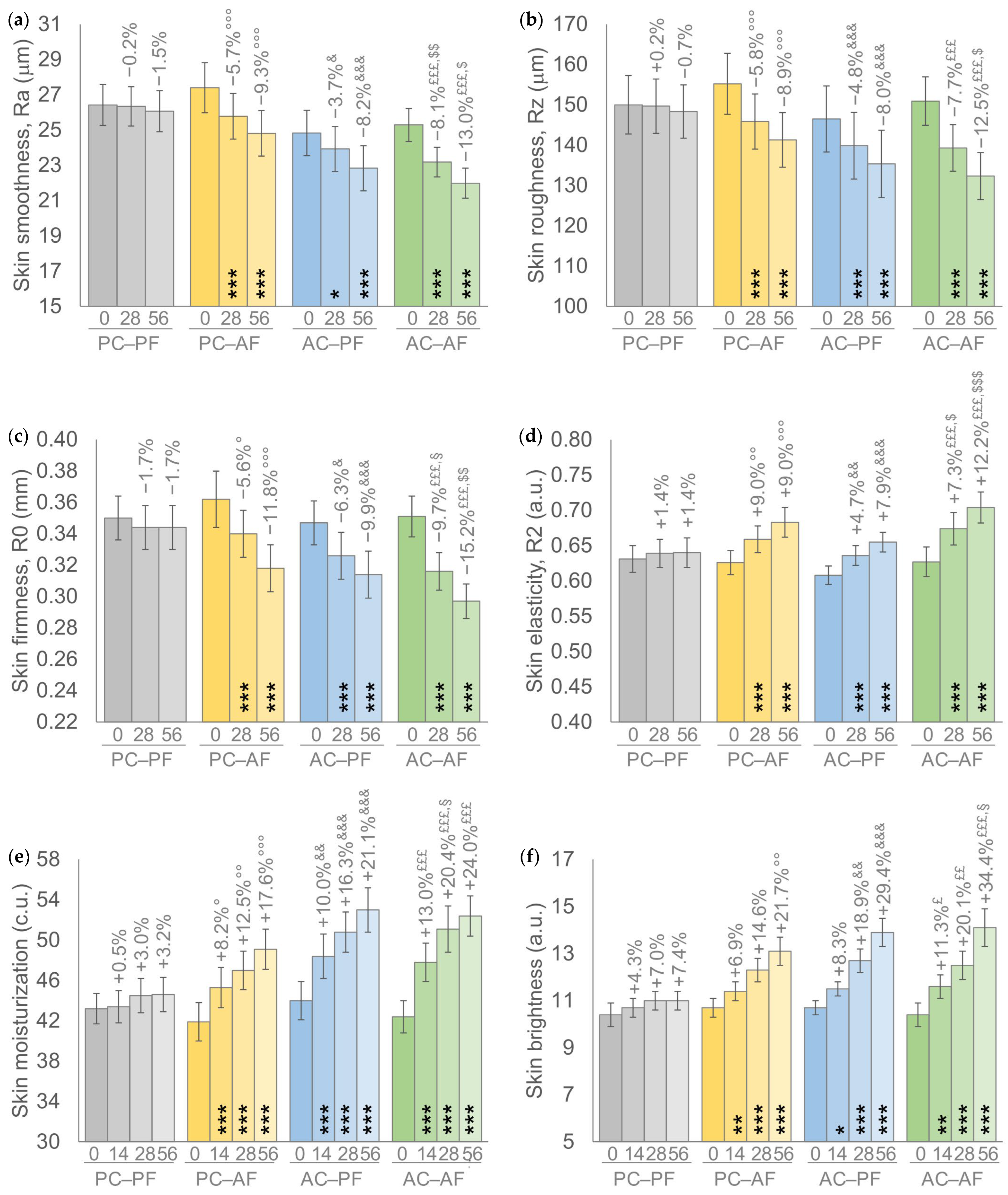
Results achieved by the treatment scheme showed a similar trend in all primary endpoints; a progressive and statistically significant intragroup improvement of all analyzed parameters was recorded in groups receiving a treatment containing at least one FS-HA molecule (Figure 2). Furthermore, the percentages of improvement achieved by the AC-AF group, i.e., the In&Out treatment, with respect to the basal values were higher compared to the other two active groups and statistically significant (p < 0.001) for all parameters throughout the study, except for skin brightness at D14 (p < 0.01). Similar percentages of improvement with respect to their basal value were recorded in the other two groups receiving one FS-HA molecule, with a significant (p < 0.001) intragroup variation for all parameters with the exception of the Ra parameter at D28 and skin brightness at D14 for the AC-PF group (p < 0.05 for both) and of skin brightness at D14 for the PC-AF group (p < 0.01).
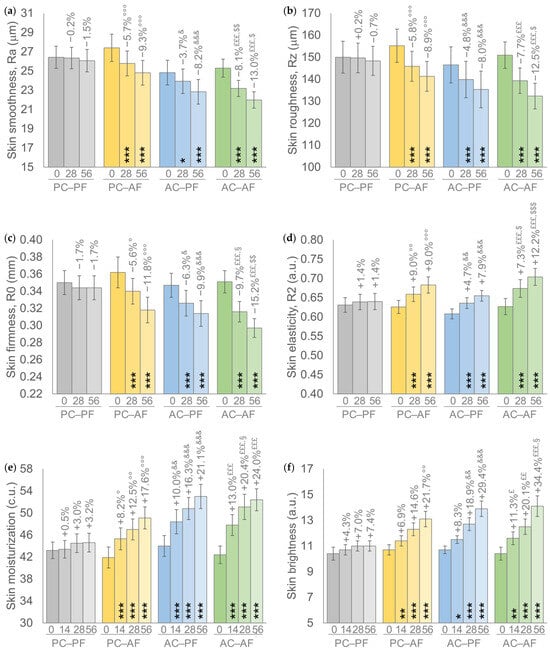
Figure 2.
(a) Skin smoothness. (b) Skin roughness. (c) Skin firmness. (d) Skin elasticity. (e) Skin moisturization. (f) Skin brightness. Data are mean ± SEM. The intragroup (vs. baseline) statistical analysis is reported inside the bars by the symbol *, while the intergroup (active vs. placebo) statistical analysis is reported above the bars by the symbol ° (PC-AF vs. PC-PF), & (AC-PF vs. PC-PF), £ (AC-AF vs. PC-PF), § (PC-AF vs. AC-AF), $ (AC-PF vs. AC-AF) as follows: 1 symbol p < 0.05, 2 symbols p < 0.01, 3 symbols p < 0.001.
A statistically significant intergroup difference with respect to the placebo group, i.e., PC-PF, was recorded by groups receiving at least one active treatment; a p < 0.001 was recorded by the In&Out (AC-AF) treatment for all parameters throughout the study, except for skin brightness at D14 (p < 0.05) and at D28 (p < 0.01); at least a p < 0.05 was recorded by the other two groups receiving one HA molecule, except for skin brightness at D14 and D28 for PC-AF and at D14 for AC-PF.
Furthermore, the In&Out (AC-AF) treatment resulted in statistically significant intergroup differences either with respect to AC-PF treatment for skin smoothness (Ra) at D28 (p < 0.01) and at D56 (p < 0.05), for skin roughness (Rz) at D56 (p < 0.05), for skin firmness (R0) at D56 (p < 0.01), and for skin elasticity (R2) at D28 (p < 0.05) and at D56 (p < 0.001), either with respect to PC-AF for skin firmness (R0) at D28 (p < 0,05), for skin moisturization at D28 (p < 0.05), and skin brightness at D56 (p < 0.05). No statistically significant (p > 0.05) intergroup differences were recorded between the two groups that received one FS-HA molecule.
3.3. Secondary Endpoints
The results of the secondary endpoints (skin thickness, dermis thickness, and dermis density, Table 2) showed—in accordance to the treatment scheme—a similar trend as for primary endpoints- A progressive and statistically significant intragroup improvement was recorded throughout the study in all groups receiving a treatment containing at least one FS-HA molecule, except for the AC-PF group at D28 for all these three parameters. The In&Out treatment showed—throughout the study—higher percentages of intragroup improvement for all three parameters compared to the other two active groups. PC-AF group showed a profile similar to the In&Out treatment in terms of significant intragroup difference for all parameters, even to slightly lower percentages of improvement. No relevant and significant intragroup difference was recorded in the placebo group.

Table 2.
Secondary endpoints. Instrumental data are expressed as mean ± SEM and percentage of variation. Clinical scores are expressed as mean ± SEM and the percentage of subjects who showed clinical improvement. The intragroup (vs. baseline) statistical analysis is denoted by the symbol *, while the intergroup statistical analysis is denoted by the symbols ° (PC-AF vs. PC-PF), & (AC-PF vs. PC-PF), £ (AC-AF vs. PC-PF), § (PC-AF vs. AC-AF), $ (AC-PF vs. AC-AF) as follows: 1 symbol p < 0.05, 2 symbols p < 0.01, 3 symbols p < 0.001. Symbol # indicates the statistical significance of the clinical improvement and was recorded in a percentage higher than 50% of subjects.
Statistically significant intergroup differences with respect to placebo (PC-PF) were recorded by In&Out treatment for all instrumental parameters throughout the study and by PC-AF for dermis thickness at D56. No statistically significant intergroup differences were recorded between the three active groups, except for the In&Out treatment with respect to AC-PF for dermis density at D56.
The results of clinical evaluations showed a similar trend (Table 2); a progressive improvement of the clinical scores in all groups receiving a treatment containing at least one FS-HA molecule, which resulted in a significant intragroup difference starting at D28 by all three active treatments for “crow’s feet” wrinkles and for eyebags’ appearance and by the In&Out and AC-PF treatments for nasolabial scores that—instead—did not show a significant intragroup difference in the PC-AF group throughout the study. A significant intragroup improvement was recorded for skin evenness by all active treatments, starting at D14 by the In&Out and by the AC-PF treatments and at D28 in the PC-AF group.
As the positive effect of the testing products confirmed whether a statistically significant improvement of the measured parameter is associated to an improvement of the clinical assessment in more than 50% of the subjects, all active treatments resulted as effective in ameliorating all dermatological features analyzed. Furthermore, In&Out treatment resulted as effective in a larger number of subjects and at D28 for all clinical parameters, except for nasolabial folds, for which it was effective at D56.
No significant intergroup differences were recorded between all groups in terms of crow’s feet wrinkles, nasolabial folds, skin evenness, and eyebags’ appearance, except for eyebags’ appearance by the In&Out treatment with respect to placebo at D56 (p < 0.05).
3.4. Self-Assessment Questionnaire
The percentages of positive answers collected from the subjects through the SA questionnaire showed a trend similar to the one achieved in the instrumental measurements and in the clinical assessments, i.e., subjects from the In&Out group expressed higher and increasing percentages of positive answers to all questions (94.3% at D56) with respect to the other two active groups (85.7% and 87.7% at D56). Interestingly, subjects from the placebo group also expressed—already at D14—a remarkable mean percentage of positive answers. This result could be related to the continuative usage of a base cosmetic cream without any peculiar cosmetic effect, except for its emollient effect, which could improve the skin barrier. Nevertheless, the percentages of positive answers in the placebo group were similar throughout the study.
4. Discussion
Facial skin aging is a complex and unavoidable biological process, affected by both genetic and extrinsic factors, that modifies the “aesthetic” interface that introduces us to other persons and forms the basis of the first impressions of a person’s health and beauty. As aged skin is characterized by a progressive decrement of HA content [10,11], one of the most common and straightforward approaches to counteract such decrement has always been to replenish skin HA content, mainly through the topical administration of HA by cosmetics [20,21,22] but also by intradermal injections [23] and, more recently—and with an increasing trend—through food supplements [24,25], as well as through the combination of both the topical and the oral administration route [26,27].
In the present clinical study, two innovative HA molecules, FS-HAs, specifically characterized for the topical (PrincipHYAL® Cube3) and for the oral (ExceptionHYAL® Star) administration route, were found to be effective in eliciting an overall amelioration of facial skin parameters in a multiethnic and multigender population, administered either alone or in a combined treatment (In&Out). This clinical study confirmed the results obtained in the previous one conducted on an adult female Caucasian population that was administered with two FS-HAs (PrincipHYAL® Difference and ExceptionHYAL® Star, characterized for topical and oral administrations) [26].
PrincipHYAL® Cube3 is a new and specifically designed FS-HA that replaces the FS-HA PrincipHYAL® Difference as the active component of the cosmetic cream and differs from the last one in terms of molecular weight range, as follows: 50–3000 kDa for the PrincipHYAL® Cube3 and 50–2500 kDa for PrincipHYAL® Difference.
The treatment scheme of the present study, characterized by a longer treatment period (8 weeks of product administration compared to the 4 weeks studied previously) and the inclusion of a placebo group for both routes of administration, resulted in a progressive improvement of all instrumental measurements in the groups that received at least one active FS-HA molecule with significant differences both intragroup and intergroup vs. placebo (PC-PF). The comparison between oral and topical treatment showed a particular pattern; the oral active treatment, i.e., PC-AF, containing ExceptionHYAL® Star, gave more effective results with respect to the topical active treatment, i.e., AC-PF, in ameliorating the skin parameters that refer to smoothness, roughness, firmness, and elasticity; whereas, the topical active treatment, containing PrincipHYAL® Cube3, achieved a greater improvement in terms of skin moisturization and skin brightness with respect to the oral active treatment.
However, the In&Out treatment achieved the greatest improvement of the investigated skin features and reported significant intergroup differences in terms of skin firmness at D28 compared to the oral FS-HA treatment and in all instrumental parameters, compared to topical treatment, particularly in terms of skin moisturization at D28 and skin brightness at D56.
No significant intergroup differences between the treatments containing only one active FS-HA molecule were detected. It can be supposed that supplying HA through both administration routes can result in an additive and/or faster response compared to the single administration.
The results of the instrumental secondary endpoints showed a similar trend with a progressive improvement on all three active treatments. Indeed, the In&Out treatment reported the following: a greater improvement with respect to the oral active, which in turn, showed better performances if compared to the topical one; a significant intergroup difference with respect to the topical active treatment in terms of dermis density; and a significant difference from placebo in all the evaluated parameters. Moreover, oral active treatment showed a significant intergroup difference in terms of dermis thickness with respect to the placebo treatment at D56.
The results of the secondary clinical endpoints confirmed the greater effectiveness of the In&Out treatment compared to the other two active treatments by reporting a significant improvement as early as D28 for “crow’s feet” wrinkles, skin evenness, and eyebags’ appearance.
No relevant changes in instrumental parameters were recorded in the placebo group throughout the study, thus confirming that the formulative composition of the cosmetic cream used as a placebo does not have a remarkable cosmetic effect and indicating that the improvement recorded could be attributed to the FS-HAs administration.
Furthermore, the oral administration of ExceptionHYAL® Star, either alone as in the PC-AF treatment or in combination with PrincipHYAL® Cube3 as in the In&Out treatment, resulted in a progressive improvement of the instrumental facial skin parameters up to an extended duration of 8 weeks compared to the 4 weeks of the previous study. This outcome suggests that an oral treatment leads to an important long-lasting effect, which was only hypothesized in the previous study by observing the results after 1 month of treatment.
The administration of PrincipHYAL® Cube3 led instead to important results on skin moisturization and evenness as early as 14 days. This finding of faster results achieved by PrincipHYAL® Cube3 compared to those achieved with PrincipHYAL® Difference, although preliminary, suggests that the modulation of HA molecular weight of using this new technology could represent an interesting approach in using HAs. Therefore, by combining the rapid effects of the cosmetic product with the long-term benefits of the dietary supplement, significant outcomes can be achieved in a shorter period through an In&Out treatment, with effects that are sustained over time.
5. Conclusions
The results of the present study indicated that—as far as the replenish of aged facial skin is concerned—the holistic approach pursued with the present In&Out treatment, i.e., based on the combined topical and oral administration of proper HAs, has a relevant role in improving the appearance and beauty of facial skin and leads to better results than a single topical or oral treatment. This study demonstrates the efficacy of FS-HA products in addressing antiaging concerns, as evidenced by the progressive intragroup improvement in skin parameters observed with the new PrincipHYAL® Cube3, both alone and in combination with the active nutraceutical HA. Additionally, it confirms the effectiveness of the In&Out treatment in a multiethnic and gender-diverse population, broadening its benefits beyond the Caucasian women cohort previously studied.
Furthermore, the addition of a follow-up period to an In&Out treatment would be an appropriate choice for future studies, as it would make it possible to monitor how long the treatment results in an instrumental and/or in a perceived effect. This would meet market demands for treatments with a long-lasting effect.
Author Contributions
Conceptualization, V.N. and F.T.; methodology, V.N.; validation, V.N., F.T. and G.R.; formal analysis, E.S.; investigation, G.R.; resources, V.N.; data curation, V.N. and F.T.; writing—original draft preparation, V.N. and F.T.; writing—review and editing, V.N., F.T. and G.R.; visualization, V.N. and E.S.; supervision, V.N. and G.R.; project administration, E.S.; funding acquisition, V.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded (funding no. IT0005078/22) by ROELMI HPC Srl, Origgio, Italy. The APC was funded by ROELMI HPC S.r.l., Origgio, Italy.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the “Independent Ethical Committee for Non-Pharmacological Clinical trials” on 11 November 2022 (ref. 2022/07). The trial was registered in the ISRCTN registry (ISRCTN15779299, https://doi.org/10.1186/ISRCTN15779299) and submitted on 22 December 2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available, since they are the property of the sponsor of the study (ROELMI HPC S.r.l., Origgio, Italy).
Acknowledgments
Authors would like to express their gratitude to the Complife Italia and China staff, who contributed to the study and recruited the subjects, for their professionalism and support during study development.
Conflicts of Interest
All authors were employed by the company Complife Italia. All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Tobin, D.J. Introduction to Skin Aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Wong, Q.Y.A.; Chew, F.T. Defining Skin Aging and Its Risk Factors: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 22075. [Google Scholar] [CrossRef]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Fisher, G.J. Natural and Sun-Induced Aging of Human Skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hong, Y.; Kim, M. Structural and Functional Changes and Possible Molecular Mechanisms in Aged Skin. Int. J. Mol. Sci. 2021, 22, 12489. [Google Scholar] [CrossRef]
- Genovese, L.; Corbo, A.; Sibilla, S. An Insight into the Changes in Skin Texture and Properties Following Dietary Intervention with a Nutricosmeceutical Containing a Blend of Collagen Bioactive Peptides and Antioxidants. Skin. Pharmacol. Physiol. 2017, 30, 146–158. [Google Scholar] [CrossRef]
- Kawada, C.; Kimura, M.; Masuda, Y.; Nomura, Y. Orally Administered Hyaluronan Affects Skin Dryness and Epidermal Thickening in Photoaged Hairless Mice. Biosci. Biotechnol. Biochem. 2016, 80, 1192–1195. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and Extrinsic Factors in Skin Ageing: A Review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- Tanaka, M.; Yamamoto, Y.; Misawa, E.; Nabeshima, K.; Saito, M.; Yamauchi, K.; Abe, F.; Furukawa, F. Effects of Aloe Sterol Supplementation on Skin Elasticity, Hydration, and Collagen Score: A 12-Week Double-Blind, Randomized, Controlled Trial. Skin. Pharmacol. Physiol. 2016, 29, 309–317. [Google Scholar] [CrossRef]
- Göllner, I.; Voss, W.; von Hehn, U.; Kammerer, S. Ingestion of an Oral Hyaluronan Solution Improves Skin Hydration, Wrinkle Reduction, Elasticity, and Skin Roughness: Results of a Clinical Study. J. Evid. Based Complement. Altern. Med. 2017, 22, 816–823. [Google Scholar] [CrossRef]
- Longas, M.O.; Russell, C.S.; He, X.Y. Evidence for Structural Changes in Dermatan Sulfate and Hyaluronic Acid with Aging. Carbohydr. Res. 1987, 159, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.; Maibach, H.I. Hyaluronan in Skin: Aspects of Aging and Its Pharmacologic Modulation. Clin. Dermatol. 2008, 26, 106–122. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic Acid: A Key Molecule in Skin Aging. Dermato-Endocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef]
- Bastow, E.R.; Byers, S.; Golub, S.B.; Clarkin, C.E.; Pitsillides, A.A.; Fosang, A.J. Hyaluronan Synthesis and Degradation in Cartilage and Bone. Cell Mol. Life Sci. 2008, 65, 395–413. [Google Scholar] [CrossRef]
- Cyphert, J.M.; Trempus, C.S.; Garantziotis, S. Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int. J. Cell Biol. 2015, 2015, 563818. [Google Scholar] [CrossRef]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular Size-Dependent Signaling and Biological Functions in Inflammation and Cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic Acid: A Review on Its Biology, Aspects of Drug Delivery, Route of Administrations and a Special Emphasis on Its Approved Marketed Products and Recent Clinical Studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef]
- Pavicic, T.; Gauglitz, G.G.; Lersch, P.; Schwach-Abdellaoui, K.; Malle, B.; Korting, H.C.; Farwick, M. Efficacy of Cream-Based Novel Formulations of Hyaluronic Acid of Different Molecular Weights in Anti-Wrinkle Treatment. J. Drugs Dermatol. 2011, 10, 990–1000. [Google Scholar]
- Bravo, B.; Correia, P.; Gonçalves Junior, J.E.; Sant’Anna, B.; Kerob, D. Benefits of Topical Hyaluronic Acid for Skin Quality and Signs of Skin Aging: From Literature Review to Clinical Evidence. Dermatol. Ther. 2022, 35, e15903. [Google Scholar] [CrossRef] [PubMed]
- Nobile, V.; Buonocore, D.; Michelotti, A.; Marzatico, F. Anti-Aging and Filling Efficacy of Six Types Hyaluronic Acid Based Dermo-Cosmetic Treatment: Double Blind, Randomized Clinical Trial of Efficacy and Safety. J. Cosmet. Dermatol. 2014, 13, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Ghatge, A.S.; Ghatge, S.B. The Effectiveness of Injectable Hyaluronic Acid in the Improvement of the Facial Skin Quality: A Systematic Review. Clin. Cosmet. Investig. Dermatol. 2023, 16, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-R.; Wang, R.-P.; Zhang, L.; Fan, Y.; Luan, J.; Liu, Z.; Yuan, C. Oral Administration of Hyaluronic Acid to Improve Skin Conditions via a Randomized Double-Blind Clinical Test. Skin. Res. Technol. 2023, 29, e13531. [Google Scholar] [CrossRef]
- Michelotti, A.; Cestone, E.; De Ponti, I.; Pisati, M.; Sparta, E.; Tursi, F. Oral Intake of a New Full-Spectrum Hyaluronan Improves Skin Profilometry and Ageing: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Eur. J. Dermatol. 2021, 31, 798–805. [Google Scholar] [CrossRef]
- Carlomagno, F.; Roveda, G.; Michelotti, A.; Ruggeri, F.; Tursi, F. Anti-Skin-Aging Effect of a Treatment with a Cosmetic Product and a Food Supplement Based on a New Hyaluronan: A Randomized Clinical Study in Healthy Women. Cosmetics 2022, 9, 54. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic Acid, a Promising Skin Rejuvenating Biomedicine: A Review of Recent Updates and Pre-Clinical and Clinical Investigations on Cosmetic and Nutricosmetic Effects. Int. J. Biol. Macromol. 2018, 120 Pt B, 1682–1695. [Google Scholar] [CrossRef]
- Masi, S. The 2.0 Full Spectrum Hyaluronans Technology to Improve Bioavailability and Efficacy Performance. Agro Food Ind. Hi-Tech. 2020, 31, 21–25. [Google Scholar]
- Skin Aging Atlas Vol 1—Caucasian Type—Bazin, Roland: 9782354030018—AbeBooks. Available online: https://www.abebooks.it/9782354030018/Skin-aging-atlas-vol-caucasian-2354030010/plp (accessed on 13 August 2024).
- Skin Aging Atlas. Volume 2, Asian Type–ScienceOpen. Available online: https://www.scienceopen.com/book?vid=f6dca7dc-5326-4d53-ba08-461ab32cf306 (accessed on 13 August 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).