Development and Characterization of 3D Senescent Models Mimicking Skin Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Reconstructed Human Epidermis (RHE)
2.3. Reconstructed Human Skin (RHS)
2.4. Histological and Immunostaining Analysis
2.5. RHE Permeability Measurements
2.6. Total RNA Extraction and Quantitative RT-qPCR
2.7. Differentially Expressed Genes
2.8. Statistical Analysis
3. Results
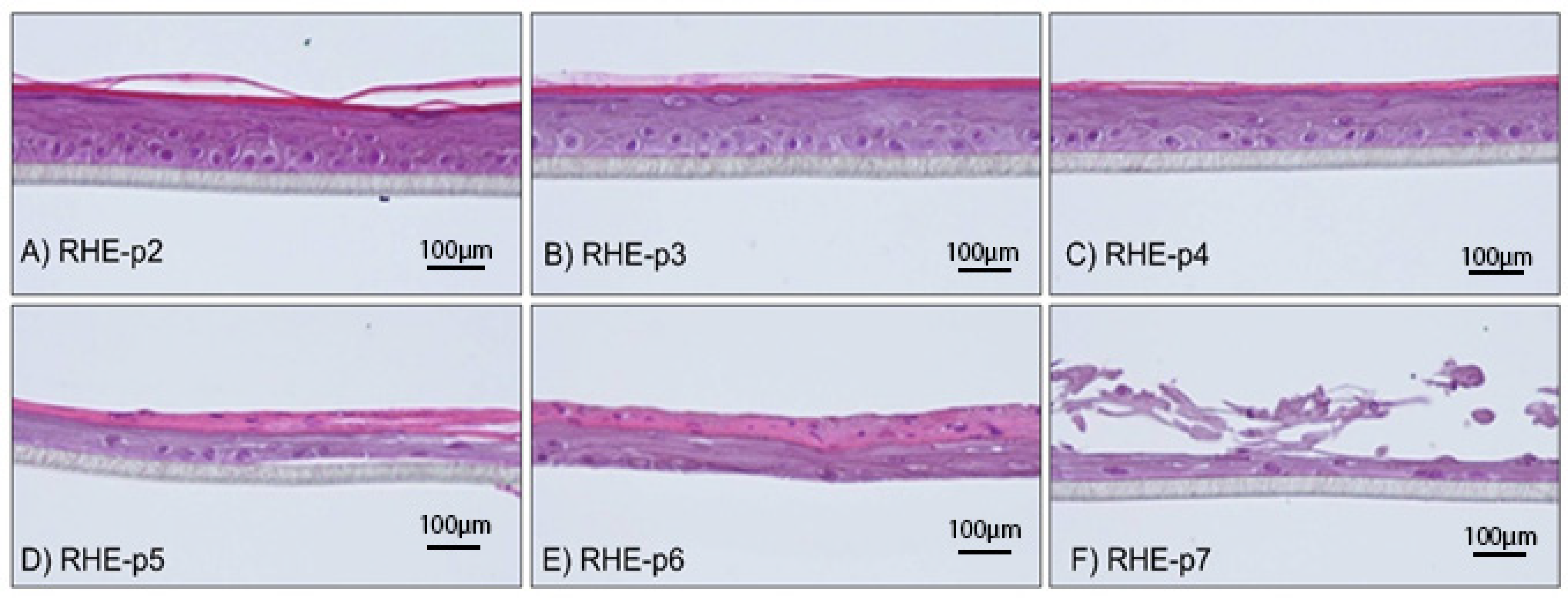
3.1. Morphological Analysis of RHEs Constructed from NHEKs Subjected to Early to Late Passages
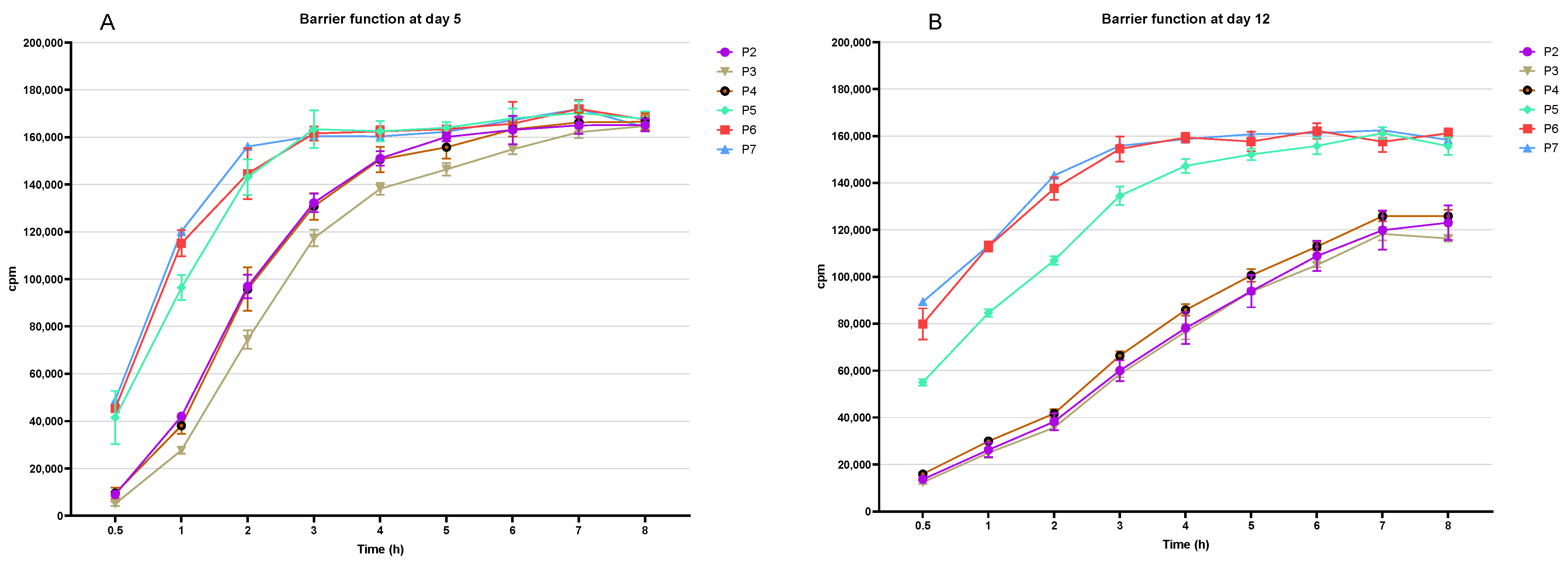
3.2. Assessment of the Epidermal Barrier Function of the RHE-p2 to RHE-p7
3.3. Analysis of Gene Expression of Epidermal Markers in RHE-p2 to RHE-p7
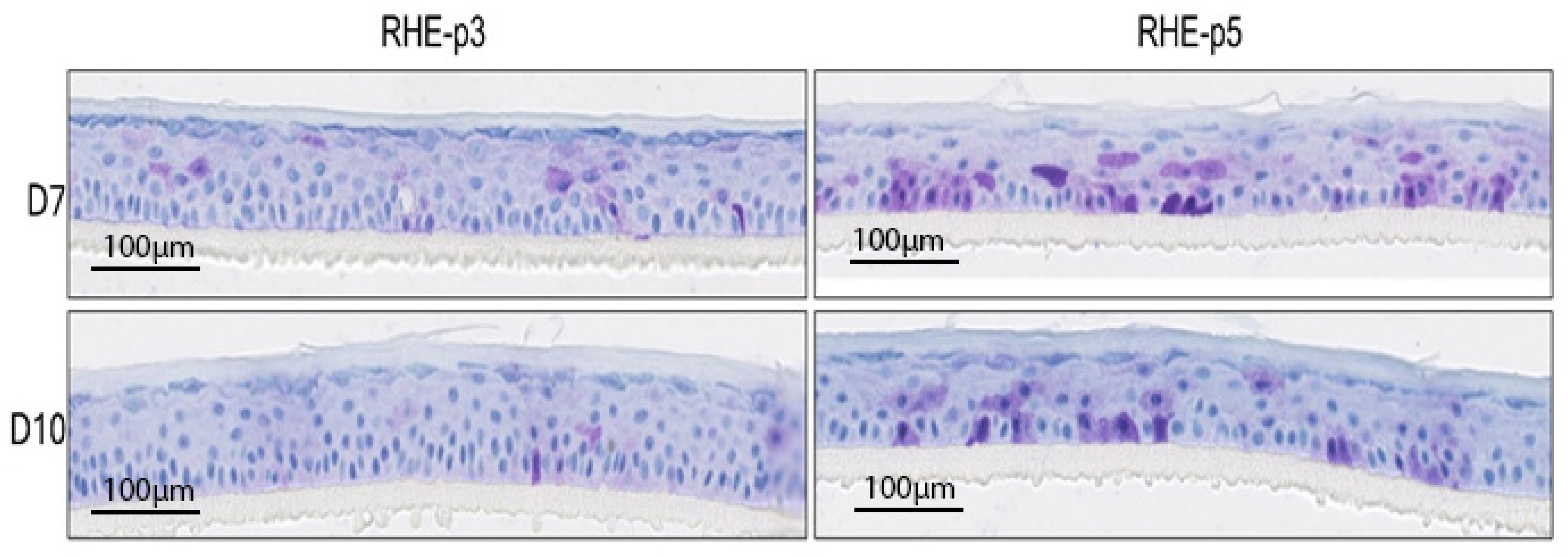
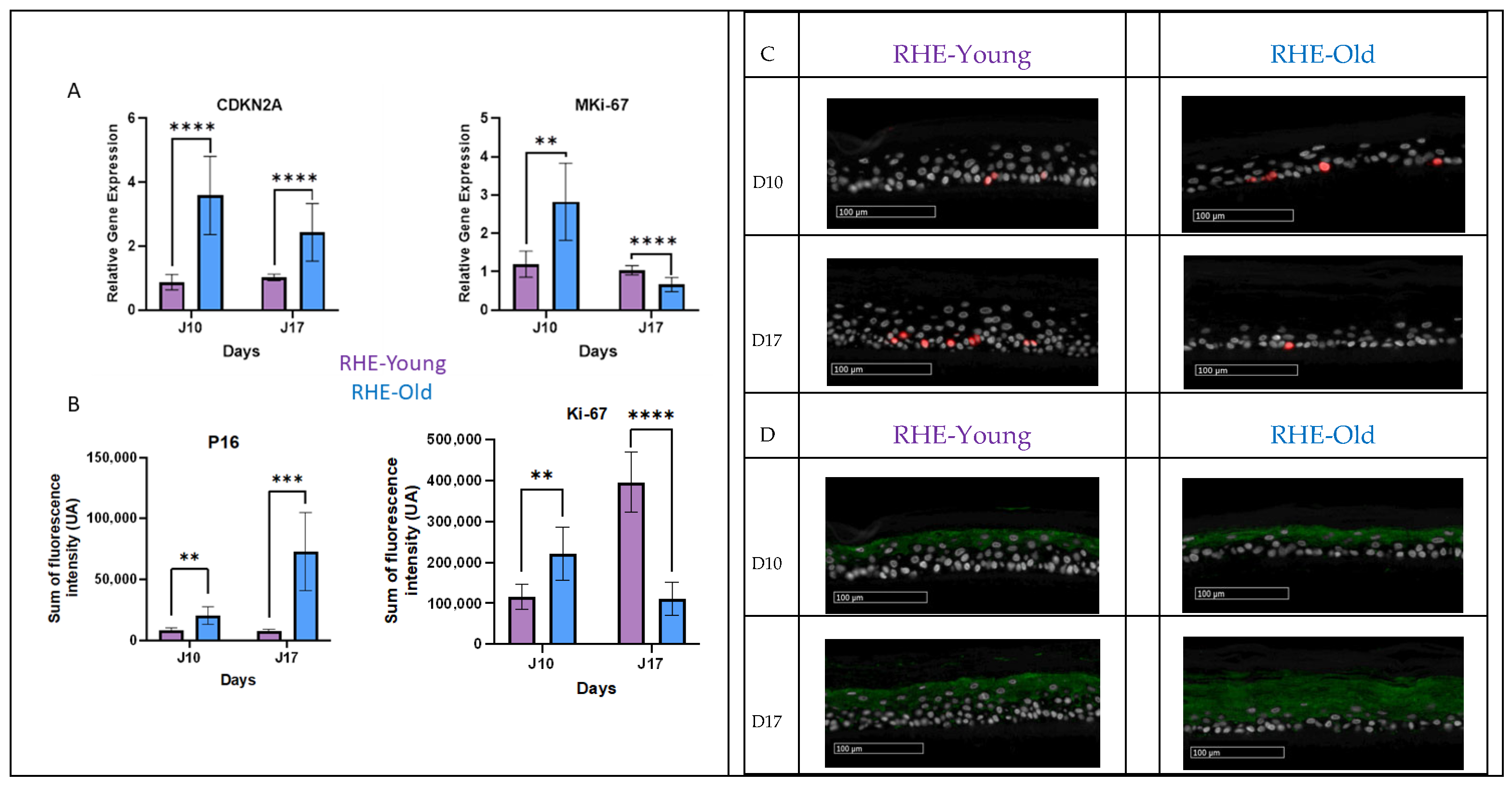
3.4. Analysis of Senescent Cell Accumulation in RHE-p3 vs. RHE-p5
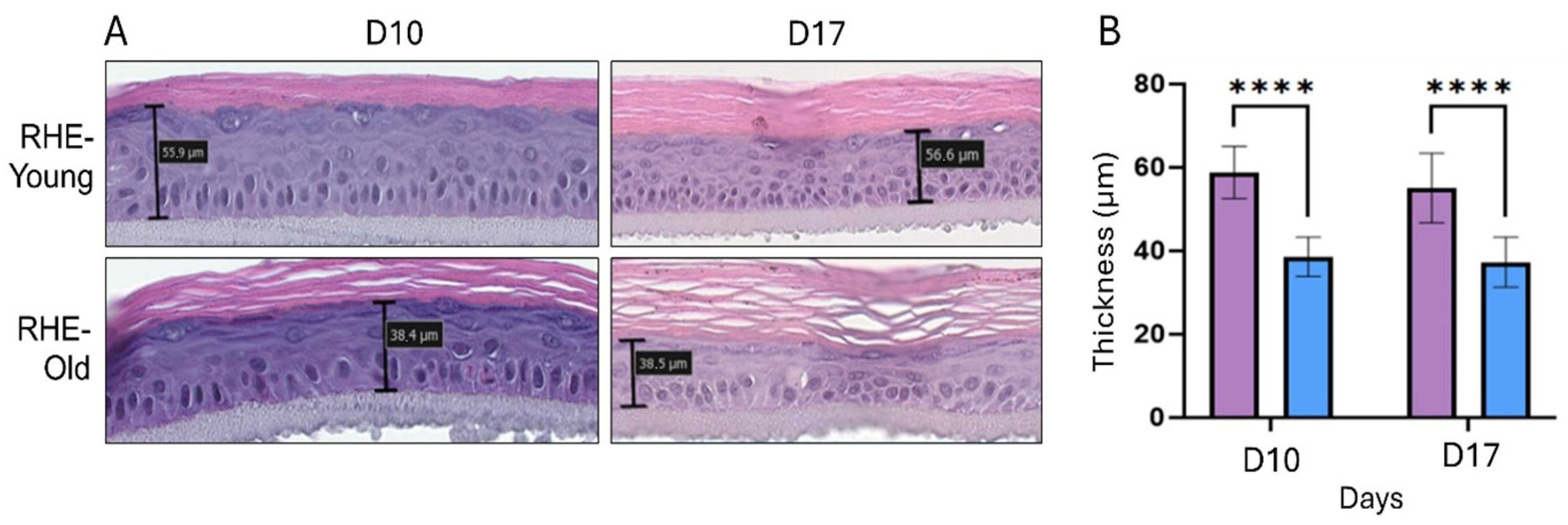
3.5. Morphological Analysis of RHEs Constructed from NHEK Derived from Young and Aged Donors
3.6. Analysis of Proliferation and Senescent Markers in RHE-Young vs. RHE-Old
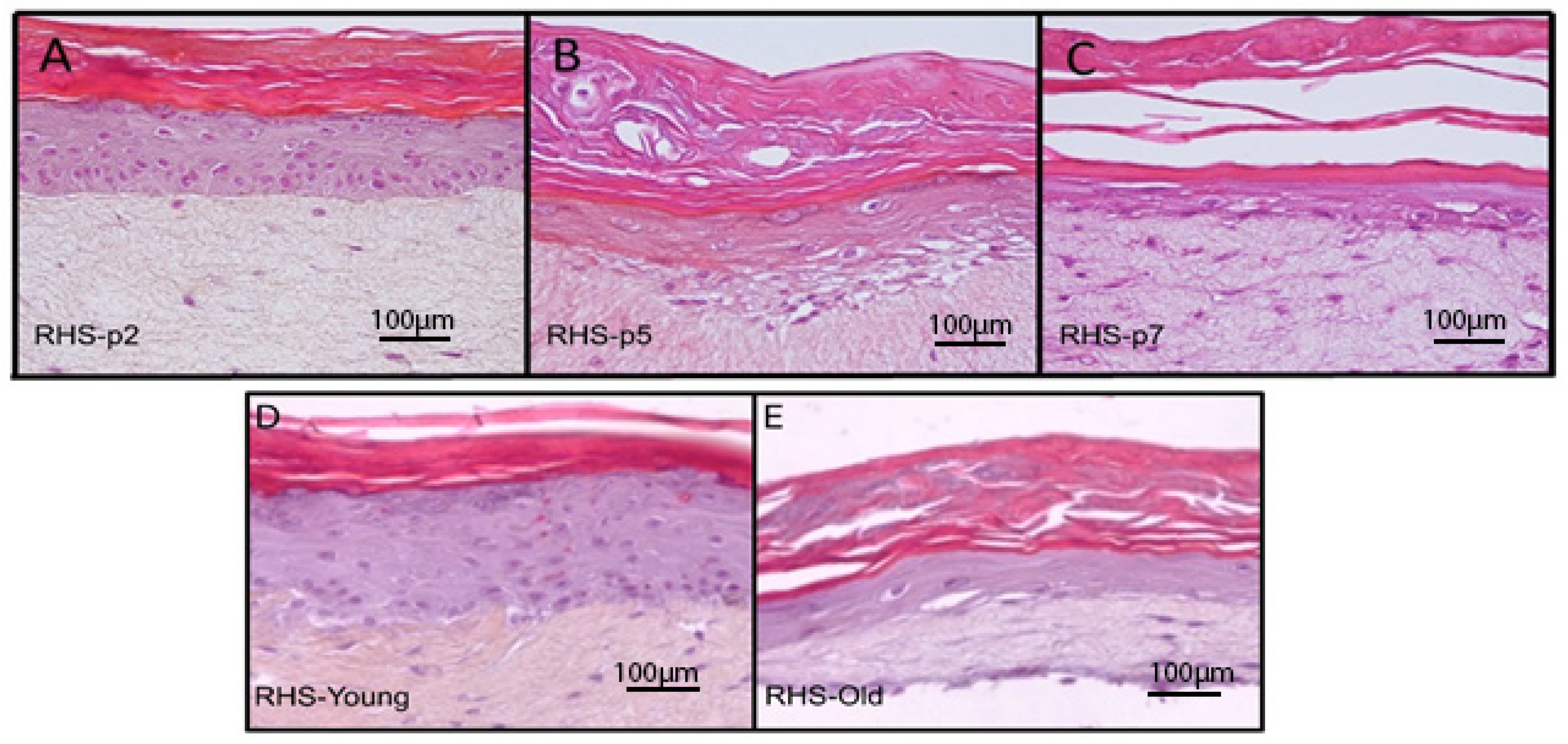
3.7. Morphological Analysis of RHS Constructed from NHEKs Subjected to Early to Late Passages
3.8. Morphological Analysis of RHS Constructed from NHEKs Derived from Young and Aged Donors
3.9. Differential Gene Expression in RHS-p5 Versus RHS-p2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyriazis, M. Aging as “Time-Related Dysfunction”: A Perspective. Front. Med. 2020, 7, 371. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.M.; Sun, M.; Gasek, N.S.; Hargis, G.R.; Sharafieh, R.; Xu, M. Role of Senescent Cells in Cutaneous Wound Healing. Biology 2022, 11, 1731. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Zhu, M.J.; Wang, X.; Shi, L.; Liang, L.Y.; Wang, Y. Senescence, oxidative stress and mitochondria dysfunction. Med. Res. Innov. 2018, 21, 24. [Google Scholar] [CrossRef]
- Kim, J.H.; Brown, S.L.; Gordon, M.N. Radiation-induced senescence: Therapeutic opportunities. Radiat. Oncol. 2023, 18, 10. [Google Scholar] [CrossRef]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef]
- Victorelli, S.; Lagnado, A.; Halim, J.; Moore, W.; Talbot, D.; Barrett, K.; Chapman, J.; Birch, J.; Ogrodnik, M.; Meves, A.; et al. Senescent human melanocytes drive skin ageing via paracrine telomere dysfunction. EMBO J. 2019, 38, e101982. [Google Scholar] [CrossRef]
- Ressler, S.; Bartkova, J.; Niederegger, H.; Bartek, J.; Scharffetter-Kochanek, K.; Jansen-Dürr, P.; Wlaschek, M. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell 2006, 5, 379–389. [Google Scholar] [CrossRef]
- Ogrodnik, M.; Acosta, J.C.; Adams, P.D.; d’Adda di Fagagna, F.; Baker, D.J.; Bishop, C.L.; Chandra, T.; Collado, M.; Gil, J.; Gorgoulis, V.; et al. Guidelines for minimal information on cellular senescence experimentation in vivo. Cell 2024, 187, 4150–4175. [Google Scholar] [CrossRef]
- Ho, C.Y.; Dreesen, O. Faces of cellular senescence in skin aging. Mech. Ageing Dev. 2021, 198, 111525. [Google Scholar] [CrossRef]
- Rübe, C.E.; Bäumert, C.; Schuler, N.; Isermann, A.; Schmal, Z.; Glanemann, M.; Mann, C.; Scherthan, H. Human skin aging is associated with increased expression of the histone variant H2A.J in the epidermis. NPJ Aging Mech. Dis. 2021, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Capell, B.C. The Senescence-Associated Secretory Phenotype: Critical Effector in Skin Cancer and Aging. J. Investig. Dermatol. 2016, 136, 2133–2139. [Google Scholar] [CrossRef]
- Coppé, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.-Y.; Campisi, J. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef]
- Waldera Lupa, D.M.; Kalfalah, F.; Safferling, K.; Boukamp, P.; Poschmann, G.; Volpi, E.; Götz-Rösch, C.; Bernerd, F.; Haag, L.; Huebenthal, U.; et al. Characterization of Skin Aging–Associated Secreted Proteins (SAASP) Produced by Dermal Fibroblasts Isolated from Intrinsically Aged Human Skin. J. Investig. Dermatol. 2015, 135, 1954–1968. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; An, J.; Zou, M.H. Immune Clearance of Senescent Cells to Combat Ageing and Chronic Diseases. Cells 2020, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.M.; Gonçalves, M.C.; Marques, M.S.; Veiga, F.; Paiva-Santos, A.C.; Pires, P.C. In Vitro Models for Anti-Aging Efficacy Assessment: A Critical Update in Dermocosmetic Research. Cosmetics 2023, 10, 66. [Google Scholar] [CrossRef]
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Guenou, H.; Nissan, X.; Larcher, F.; Feteira, J.; Lemaitre, G.; Saidani, M.; Del Rio, M.; Barrault, C.C.; Bernard, F.-X.; Peschanski, M.; et al. Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: A preclinical study. Lancet 2009, 374, 1745–1753. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Gkegkes, I.D.; Aroni, K.; Agrogiannis, G.; Patsouris, E.S.; Konstantinidou, A.E. Expression of caspase-14 and keratin-19 in the human epidermis and appendages during fetal skin development. Arch. Dermatol. Res. 2013, 305, 379–387. [Google Scholar] [CrossRef]
- Ekman, A.K.; Vegfors, J.; Eding, C.B.; Enerbäck, C. Overexpression of Psoriasin (S100A7) Contributes to Dysregulated Differentiation in Psoriasis. Acta Derm.-Venereol. 2017, 97, 441–448. [Google Scholar] [CrossRef]
- Lau, L.; Porciuncula, A.; Yu, A.; Iwakura, Y.; David, G. Uncoupling the Senescence-Associated Secretory Phenotype from Cell Cycle Exit via Interleukin-1 Inactivation Unveils Its Protumorigenic Role. Mol. Cell. Biol. 2019, 39, e00586-18. [Google Scholar] [CrossRef] [PubMed]
- Davalos, A.R.; Coppe, J.P.; Campisi, J.; Desprez, P.Y. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010, 29, 273. [Google Scholar] [CrossRef] [PubMed]
- Waaijer, M.E.C.; Gunn, D.A.; Adams, P.D.; Pawlikowski, J.S.; Griffiths, C.E.M.; van Heemst, D.; Slagboom, P.E.; Westendorp, R.G.J.; Maier, A.B. P16INK4a Positive Cells in Human Skin Are Indicative of Local Elastic Fiber Morphology, Facial Wrinkling, and Perceived Age. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2016, 71, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Wyles, S.P.; Carruthers, J.D.; Dashti, P.; Yu, G.; Yap, J.Q.; Gingery, A.; Tchkonia, T.; Kirkland, J. Cellular Senescence in Human Skin Aging: Leveraging Senotherapeutics. Gerontology 2023, 70, 7–14. [Google Scholar] [CrossRef]
- Ahn, M.; Cho, W.-W.; Park, W.; Lee, J.-S.; Choi, M.-J.; Gao, Q.; Gao, G.; Cho, D.-W.; Kim, B.S. 3D biofabrication of diseased human skin models in vitro. Biomater. Res. 2023, 27, 80. [Google Scholar] [CrossRef] [PubMed]
- Weinmüllner, R.; Zbiral, B.; Becirovic, A.; Stelzer, E.M.; Nagelreiter, F.; Schosserer, M.; Lämmermann, I.; Liendl, L.; Lang, M.; Terlecki-Zaniewicz, L.; et al. Organotypic human skin culture models constructed with senescent fibroblasts show hallmarks of skin aging. NPJ Aging Mech. Dis. 2020, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Galvis, D.; Walsh, D.; Harries, L.W.; Latorre, E.; Rankin, J. A dynamical systems model for the measurement of cellular senescence. J. R. Soc. Interface 2019, 16, 20190311. [Google Scholar] [CrossRef]
- Tigges, J.; Krutmann, J.; Fritsche, E.; Haendeler, J.; Schaal, H.; Fischer, J.W.; Kalfalah, F.; Reinke, H.; Reifenberger, G.; Stühler, K. The hallmarks of fibroblast ageing. Mech. Ageing Dev. 2014, 138, 26–44. [Google Scholar]
- Adamus, J.; Aho, S.; Meldrum, H.; Bosko, C.; Lee, J.M. p16INK4A Influences the Aging Phenotype in the Living Skin Equivalent. J. Invest. Dermatol. 2014, 134, 1131–1133. [Google Scholar] [CrossRef] [PubMed]
| Genes | P2 | P3 | P4 | P5 | P6 | P7 | ||
|---|---|---|---|---|---|---|---|---|
| Fold Change Versus p1 | ||||||||
| Calmodulin-like 5 | CALML5 | 1.09 | 0.76 | 0.58 | 0.26 | 0.12 | 0.05 | |
| Keratinocyte differentiation | Caspase 14, apoptosis-related cysteine peptidase | CASP14 | 1.01 | 0.65 | 0.48 | 0.32 | 0.17 | 0.07 |
| Filaggrin | FLG | 1.01 | 0.93 | 0.84 | 0.38 | 0.16 | 0.08 | |
| Keratin 1 | KRT1 | 0.96 | 0.58 | 0.58 | 0.33 | 0.23 | 0.20 | |
| Keratin 19 | KRT19 | 1.16 | 1.19 | 2.22 | 4.85 | 9.39 | 16.14 | |
| Sulfotransferase family, cytosolic, 2B, member 1 | SULT2B1 | 0.97 | 0.68 | 0.89 | 0.45 | 0.23 | 0.28 | |
| Laminin, gamma 2 | LAMC2 | 0.85 | 0.51 | 1.87 | 3.39 | 5.94 | 7.81 | |
| Glycerol/water transport | Aquaporin 3 (Gill blood group) | AQP3 | 1.17 | 0.73 | 0.82 | 0.76 | 0.42 | 0.42 |
| Cytokines/Chemokines | Interleukin 1, alpha | IL1A | 0.95 | 1.22 | 1.73 | 1.26 | 1.84 | 3.07 |
| Anti-microbian peptides, innate immunity | Defensin, beta 4A | DEFB4A | 0.65 | 0.81 | 2.86 | 0.09 | 0.02 | 0.01 |
| S100 calcium-binding protein A7 | S100A7 | 0.87 | 1.10 | 6.04 | 3.76 | 0.75 | 1.25 | |
| Cell–cell interactions | Corneodesmosin | CDSN | 0.82 | 0.76 | 0.74 | 0.38 | 0.12 | 0.14 |
| Desmoglein 1 | DSG1 | 1.16 | 0.88 | 1.14 | 0.65 | 0.45 | 0.41 | |
 | ||||||||
| Gene Title | Gene Symbol | Fold Change | Function Related to Skin |
|---|---|---|---|
| Keratinocyte Differentiation | |||
| Keratin 13 | KRT13 | 11.07 | Structure of intermediate filaments in epithelial cells |
| Keratin 19 | KRT19 | 8.4 | |
| Keratin 77 | KRT77 | 7.82 | |
| Keratin 75 | KRT75 | 7.07 | |
| Keratin 4 | KRT4 | 4.87 | |
| Keratin 2 | KRT2 | 4.75 | |
| Small proline-rich protein 4 | SPRR4 | 11.63 | Epidermal barrier |
| Small proline-rich protein 3 | SPRR3 | 5.17 | |
| Repetin | RPTN | 5.36 | |
| Late cornified envelope 5A | LCE5A | 6.18 | |
| Trichohyalin | TCHH | 15.65 | |
| Cornulin | CRNN | 8.58 | |
| E74-like factor 5 (ets domain transcription factor) | ELF5 | 9.72 | |
| Cell–Cell Interaction | |||
| Integrin, alpha M (complement component 3 receptor 3 subunit) | ITGAM | 8.73 | Cell adhesion and immune response |
| Cell Cycle Regulation | |||
| Cyclin-dependent kinase inhibitor 2A | CDKN2A | 3.36 | Regulation of cell cycle and cellular senescence |
| Cyclin D1 | CCND1 | 3.12 | Regulation of cell cycle |
| Cyclin B2 | CCNB2 | 0.33 | |
| Cyclin-dependent kinase 1 | CDK1 | 0.27 | |
| Cyclin-dependent kinase inhibitor 3 | CDKN3 | 0.2 | |
| Matrix Synthesis and Degradation | |||
| Matrix metallopeptidase 7 (matrilysin, uterine) | MMP7 | 0.1 | Extracellular matrix degradation and tissue remodeling |
| Inflammation | |||
| Chemokine (C-C motif) ligand 5 | CCL5 | 0.28 | Modulation of immune response |
| Vascular endothelial growth factor A | VEGFA | 0.4 | Regulation of angiogenesis and vascular permeability |
| Additional Key Processes (Senescence, Growth Arrest, Apoptosis Resistance) | |||
| Sestrin 3 | SESN3 | 2.9 | Regulation of oxidative stress and cellular signaling |
| Cellular retinoic acid binding protein 2 | CRABP2 | 3.04 | Regulation of retinoic acid activity |
| Protein phosphatase 1, regulatory (inhibitor) subunit 1C | PPP1R1C | 3.59 | Regulation of cellular signaling |
| Apolipoprotein E | APOE | 6.7 | Lipid transport and tissue repair |
| Kallikrein-related peptidase 6 | KLK6 | 6.31 | Proteolysis and modulation of skin desquamation |
| Kallikrein 1 | KLK1 | 4.47 | |
| Triggering receptor expressed on myeloid cells 2 | TREM2 | 6.85 | Modulation of immune response |
| Chloride channel accessory 4 | CLCA4 | 11.67 | Regulation of chloride ion transport and ionic homeostasis |
| Parathyroid hormone-like hormone | PTHLH | 10.18 | Regulation of cell growth and development |
| Alcohol dehydrogenase 7 (class IV), mu or sigma polypeptide | ADH7 | 10.8 | Metabolism of retinoids and alcohol |
| Fetuin B | FETUB | 10.51 | Inhibition of pathological calcification and regulation of cell proliferation |
| Galanin prepropeptide | GAL | 3.22 | Regulation of inflammation and cellular stress response |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pentecouteau, L.; Laperdrix, C.; Rouxel, E.; Folligné, A.; Morvan, P.-Y.; Vallée, R. Development and Characterization of 3D Senescent Models Mimicking Skin Aging. Cosmetics 2025, 12, 71. https://doi.org/10.3390/cosmetics12020071
Pentecouteau L, Laperdrix C, Rouxel E, Folligné A, Morvan P-Y, Vallée R. Development and Characterization of 3D Senescent Models Mimicking Skin Aging. Cosmetics. 2025; 12(2):71. https://doi.org/10.3390/cosmetics12020071
Chicago/Turabian StylePentecouteau, Loïc, Céline Laperdrix, Emeline Rouxel, Annick Folligné, Pierre-Yves Morvan, and Romuald Vallée. 2025. "Development and Characterization of 3D Senescent Models Mimicking Skin Aging" Cosmetics 12, no. 2: 71. https://doi.org/10.3390/cosmetics12020071
APA StylePentecouteau, L., Laperdrix, C., Rouxel, E., Folligné, A., Morvan, P.-Y., & Vallée, R. (2025). Development and Characterization of 3D Senescent Models Mimicking Skin Aging. Cosmetics, 12(2), 71. https://doi.org/10.3390/cosmetics12020071






