On the Key Role of Polymeric Rheology Modifiers in Emulsion-Based Cosmetics
Abstract
1. Introduction
- -
- Macroemulsions, where dispersed droplet size ranges between 0.1 and 100 [μm], allowing light scattering and thus giving the typical white creamy appearance to the system [30];
- -
- Microemulsions, characterized by droplets ranging from 10 to 100 nm and the presence of both emulsifiers and co-emulsifiers. Their peculiarity is to be thermodynamically stable (they form spontaneously) and transparent to the eye [31];
- -
- Nanoemulsions, presenting droplets with a mean characteristic dimension between 20 and 200 nm and transparent or translucent appearance [32] (they are formed spontaneously like microemulsions).
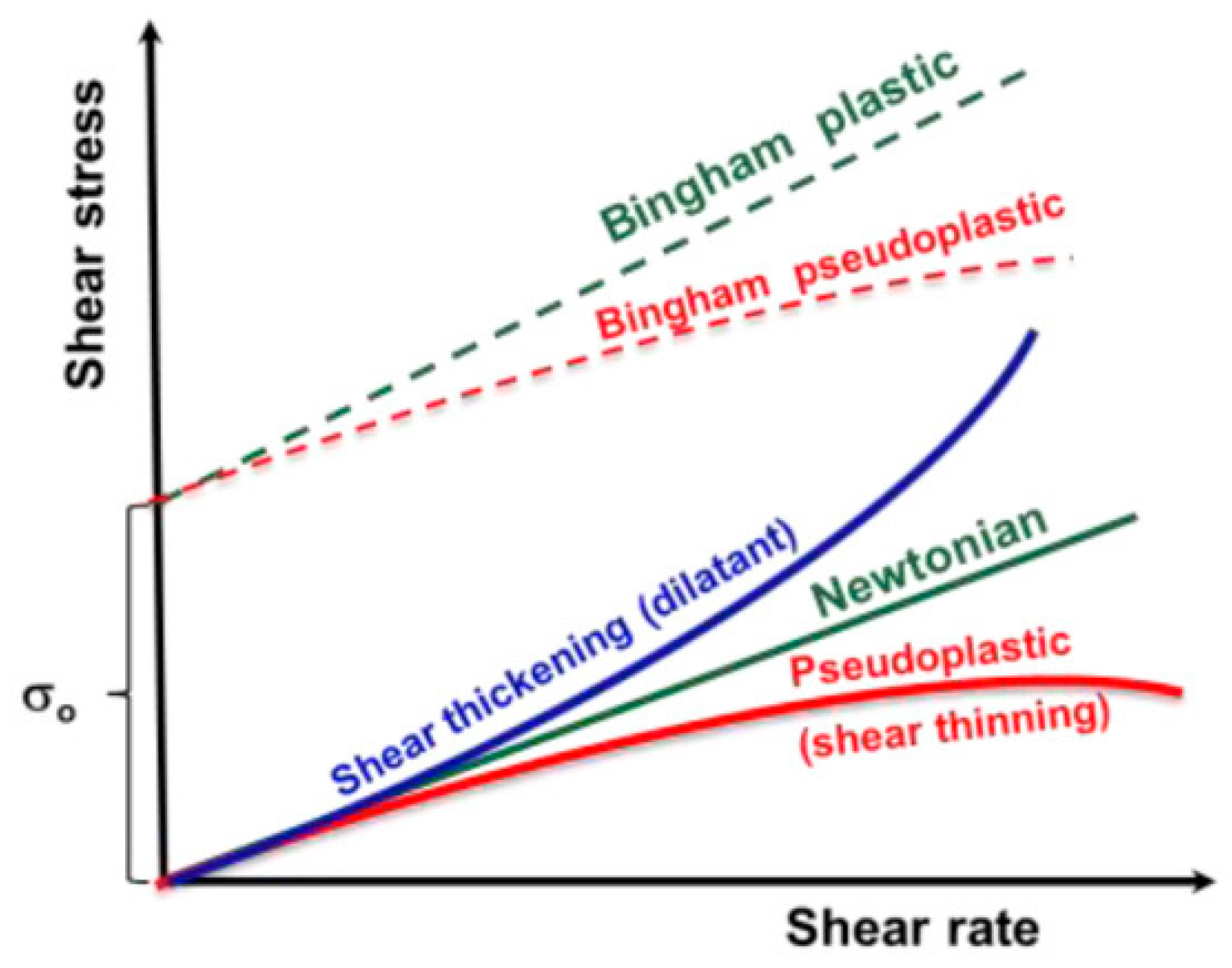
2. Rheology Modifiers in Cosmetics
- -
- capillary viscometers flow through a cylindrical tube caused either by application of gas pressure or by hydrostatic pressure from the fluid column;
- -
- rotation viscometers: the liquid is sheared between two surfaces on the stator–rotor principle using coaxial cylinders, cone and plate, or a disk and plate. Normally, the rotation rate is controlled, and the torsional is moment measured. However, there are also rheometers in which the applied force is controlled, which are used to determine initial yield stress;
- -
- falling ball viscosimeter correlates the speed of motion of a ball through a fluid with its viscosity;
- -
- bubble viscometer correlates the speed of motion of a bubble through a fluid with its viscosity;
- -
- efflux viscometer measures the viscosity using the time taken for fluid to flow through a hole;
- -
- forced oscillatory shearing: dynamic techniques that, using vibrations, permit the simultaneous investigation of both the dynamic viscous behavior and the elastic properties of fluid systems.
- -
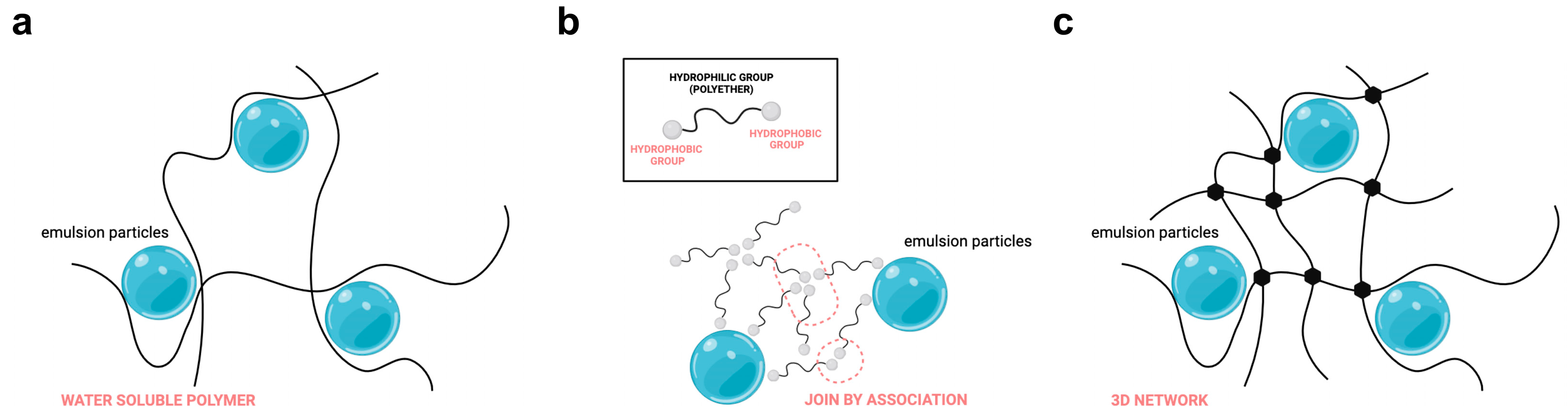
- chain entanglement (also referred to as physical cross-linking): a simple thickening mechanism in which polymer chains are dissolved into the solvent (usually water), providing soft entanglement that increases with increasing concentration of the polymer, since more chains are occupied in less space. As the concentration of the polymer continues to increase, it becomes more and more difficult to individually separate the entangled chains for the shear forces acting on the formulation [46] (Figure 2a);
- -
- associative mechanism: characterized by the formation of association network structures leading to viscosity increase as a result of the bridge connections and winding offered by the polymeric agent [46]. This is possible due to the presence of chemically attached hydrophobic groups able to interact with hydrophobic associations similar to those typical of conventional surfactants [47] (Figure 2b):
- -
- covalent cross-linking is realized when two polymeric chains attach to each other due to the interaction of a bifunctional monomer that forms a covalent bond linking them. The result is a tridimensional network of polymer chains throughout each particle. Cross-linking radically modifies the properties of the original polymer chains involved and represents one of the most important ways to achieve thickening at an industrial level (Figure 2c).
- -
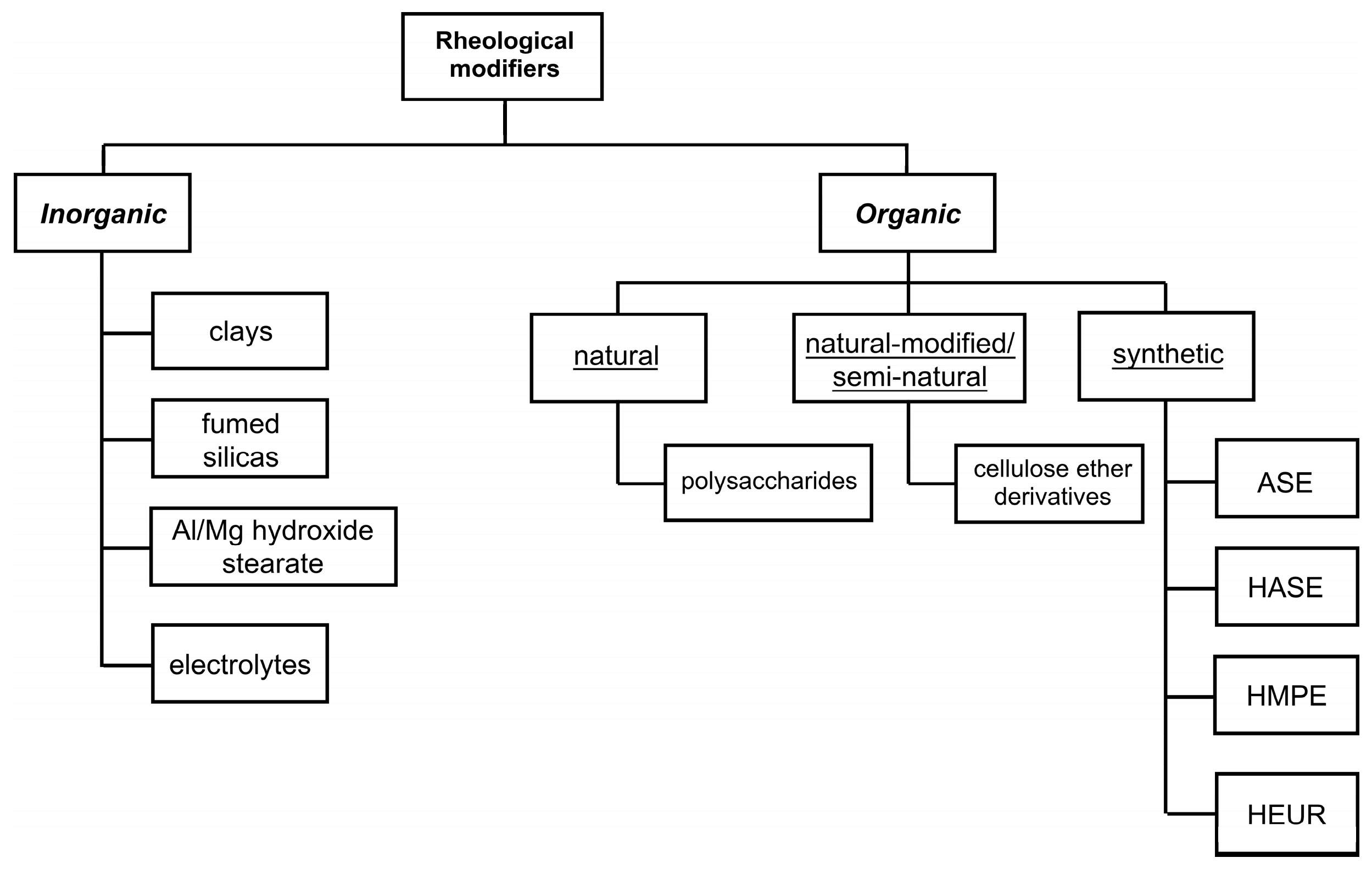
- organic rheological modifiers are further sorted into three categories depending on the substance origin:
- -
- natural modifiers are derived from plant, animal, or microbial origin and represented by large polymers chemically based on proteins or polysaccharides [51], like gums;
- -
- naturally modified modifiers are natural polymers treated with specific chemical modifications to enhance their performances in the final product;
- -
- synthetic modifiers are derived starting from oil-based polymers;
- -
- inorganic rheological modifiers comprise only mineral-based substances like clays and pyrogenic (or fumed) silica [43].
3. Synthetic Polymers
3.1. Acrylic Polymers
3.1.1. Carbomer
3.1.2. Sodium Polyacrylate
3.1.3. Considerations on Synthetic Polymers
4. Natural Polymers
4.1. Marine Polysaccharides
- -
- Carrageenans: a group of sulphated galactans extracted from different red seaweed (known as Rhodophyceae) species, mainly Eucheuma cottonii, Eucheuma spinosum, Chondrus crispus, and Gigartina. They are, in turn, split into three different types according to their ester sulphate content, which depends on the specific weed source: the lower the ester sulphate content, the higher the gelling inclination of the considered carrageenan. Fontes-Candia and coworkers prepared emulsions with sunflower oil using three different concentrations of carrageenan (from 0.5 to 2% w/v). The key role of carrageenan and salt was evaluated considering mechanical properties and rheology.
- -
- Alginates: block copolymers composed of mannuronic (M) and guluronic (G) acid extracted from brown seaweed (Phaeophyceae) species like Macrocystis pyrifera, Laminaria hyperborea, and Ascophylum nodosum. The M to G ratio depends on the specific type of alginate and governs its final properties in terms of thickening and gelling performance. They are usually commercialized as water-soluble sodium salts, which require the addition of calcium to obtain the desired thickening effect.In this direction, Russo and coworkers [99] studied the effect of Pluronic (synthetic polymer) and alginate in producing stable emulsions. Pluronic was used as amphiphilic molecules to reduce interfacial tension, while alginate was used as a rheology modifier to slow down the droplet coalescence. By optimizing both alginate and Pluronic concentrations, emulsions stable up to 90 days were obtained.
- -
- Agar: a general term used to identify a complex mixture of polysaccharides extracted from the Gelidium and Gracilaria species of red seaweed. The major fractions are represented by agarose, a neutral polymer, and agaropectin, a charged sulphated polymer. When applied as a thickener, agar results in the formation of firm and brittle gels.
4.2. Botanical Polysaccharides
- -
- Galactomannans (or botanical gums): composed of a C1 to C4 linked mannose backbone with single galactose substituents; they include guar gum, locust bean gum, tara gum, and cassia gum, which differ in the degree of galactose substitution going from one galactose per every two mannoses (guar gum) to one every four (locust bean gum). They can all be used as thickeners in cosmetic formulations. Indeed, their use was compared with xanthan gum by Niknam and coworkers [101]. The emulsions not treated with microwave or ultrasound present viscous-like behavior, while treated samples showed weak gel behavior. Rheological parameters (storage modulus, loss modulus, and apparent viscosity) indicated that galactomannan had higher impact on the rheological aspects of emulsions compared with xanthan gum. In addition, the synergistic interaction between the two biopolymers resulted in better rheological aspects. By treating the samples with ultrasound and microwaves, the emulsion stability values of the samples increased, connected with various parameters, especially viscosity.
- -
- Pectins: extracted from different vegetal sources like apples and citrus fruits and composed of galacturonic acid residues with occasional rhamnose interruptions. They are usually classified depending on their degree of methyl esterification and not widely applied in the cosmetic field, whereas they are extremely diffused in the food industry. Four different low-methoxyl pectins (0.015–0.02 w/w) were prepared to be used as the dispersing phase in cosmetic emulsion gels [102]. The obtained formulated products were used, together with a common non-ionic surfactant (Tween 60), to prepare olive oil emulsion gels suitable to design new cosmetic products.
- -
- Tara gum, namely, Caesalpinia Spinosa Gum, represents one of the most promising thickening alternatives to polyacrylates in cosmetic formulations. It is obtained by grinding the endosperm of Caesalpinia Spinosa (also known as Peruvian carob) seeds, a plant belonging to the Leguminosae family and native to the Peruvian and Bolivian Andes. It is completely odorless and white to ivory colored, even if the resulting gel is usually brownish and translucent.
4.3. Microbial Polysaccharides
5. Natural-Modified Polymers
- -
- Modified cellulose products enhanced by chemical modifications to render the basic cellulose backbone soluble. This family of cellulose-based products offers a wide range of functions, from the thickening effect ensured by carboxymethyl cellulose (CMC) to the thermogelation in hydroxyethyl cellulose (HEC). Formulations based on CMC with bacterial cellulose are not only able to form high stable emulsions but also to reduce the amount of tensides needed due to their synergic effect through a Pickering effect and by structuring the continuous phase [113,114].
- -
- Modified native starches consist of starches enhanced by chemical transformations to improve their heat and acid resistance and increase processability while reducing the tendency for retrogradation. This category includes hydroxyethyl and hydroxypropyl modified starch. The synthesized starch [115] was obtained using microwave irradiation with less energy, solvent, and time needed. The final viscosity increases as molar substitution increases up to 250 cP. Its thickener effect was investigated with very promising results;
- -
- Modified alginates are obtained by esterification with propylene glycol to avoid precipitation at a pH lower than 4.0, a phenomenon typical of alginates. Self-supporting foams produced with functionalized alginate demonstrated high stability with proper mechanical properties and density with promising application in cosmetics [116];
- -
- Modified guar gums are enhanced by carboxymethylation to improve alkali compatibility, hydroxyalkylation to improve solubility, or phosphatization to enable cross-linking. At a low concentration (10–30 mg/mL), the functionalized polymer exhibited a gel-like nature and visco-elastic properties with a storage modulus (G’) higher than loss one (G”) at all polymer concentrations investigated. The role of tenside and salt is fundamental for the proper rheological properties of the formulated product and showed promising use as a rheology modifier [117]. Due to their enormous versatility, such modified natural polymers are used in several industrial fields, including cosmetics, food, oil drilling, pesticides, textiles, and many others [87,118]; even if some of them show a partial overlap of their properties, each modified hydrocolloid tends to excel in a few specific areas. However, despite the interesting results, only a few authors applied bacterial cellulose to cosmetics due to the high costs and challenge to scale up its production.
5.1. Modified Cellulose Products
5.2. Modified Starches
- -
- Cross-linking, the most frequent chemical transformation applied on starch, consists of the replacement of the hydrogen bonding between starch chains with stronger and more permanent interactions, namely, covalent bonds. Such a modification avoids the swelling of the starch granule, preventing disintegration by chemical attack or imposed shear [130];
- -
- Stabilization, usually applied in conjunction with cross-linking, is another fundamental chemical modification process applied on starch. Its aim is to prevent retrogradation by introducing some bulky groups able to create steric hindrance against chain re-alignment [131];
- -
- Conversions, comprising all those transformations occurring by chain-cleavage reactions of starch, like oxidation, acidic hydrolysis, dextrinization, and enzymatic hydrolysis, are able to guarantee various effects on the final properties of starch [132];
- -
- Lipophilic substitution is characterized by the addition of long hydrophobic chains to the starch macromolecules, resulting in higher lipophilicity and increased ability to stabilize interactions between oil and water. An example is given by starch octenylsuccinates, widely applied additives able to rapidly migrate towards an oil–water interface and stabilize it [133].
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guzmán, E.; Ortega, F.; Rubio, R.G. Pickering Emulsions: A Novel Tool for Cosmetic Formulators. Cosmetics 2022, 9, 68. [Google Scholar] [CrossRef]
- Venkataramani, D.; Tsulaia, A.; Amin, S. Fundamentals and applications of particle stabilized emulsions in cosmetic formulations. Adv. Colloid Interface Sci. 2020, 283, 102234. [Google Scholar]
- Perrin, L.; Gillet, G.; Gressin, L.; Desobry, S. Interest of Pickering Emulsions for Sustainable Micro/Nanocellulose in Food and Cosmetic Applications. Polymers 2020, 12, 2385. [Google Scholar] [CrossRef]
- Durgut, E.; Claeyssens, F. Pickering polymerized high internal phase emulsions: Fundamentals to advanced applications. Adv. Colloid Interface Sci. 2025, 336, 103375. [Google Scholar] [PubMed]
- Xu, Y.; Wang, S.; Xin, L.; Zhang, L.; Liu, H. Interfacial mechanisms, environmental influences, and applications of polysaccharide-based emulsions: A review. Int. J. Biol. Macromol. 2025, 293, 139420. [Google Scholar]
- Heidari-Dalfard, F.; Tavasoli, S.; Assadpour, E.; Miller, R.; Jafari, S.M. Surface modification of particles/nanoparticles to improve the stability of Pickering emulsions; a critical review. Adv. Colloid Interface Sci. 2025, 336, 103378. [Google Scholar]
- Narciso, J.O.; Soliva-Fortuny, R.; Salvìa-Trujillo, L.; Martìn-Belloso, O. Pickering Emulsions as Catalytic Systems in Food Applications. ACS Food Sci. Technol. 2025, 5, 29–35. [Google Scholar]
- Funami, T.; Ishihara, S.; Maeda, K.; Nakauma, M. Review paper: Recent development in Pickering emulsion gel technology for food and beverage applications. Food Hydrocoll. 2025, 162, 110901. [Google Scholar]
- Rajoo, A.; Siva, A.P.; Siew Sia, C.; Chan, E.S.; Ti Tey, B.; Ee Low, L. Transitioning from Pickering emulsions to Pickering emulsion hydrogels: A potential advancement in cosmeceuticals. Eur. J. Pharm. Biopharm. 2024, 205, 114572. [Google Scholar]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielinska, A.; Silva, A.M. Microemulsions and Nanoemulsions in Skin Drug Delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef]
- Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.K.; Costa, F.F. Innovations and stability challenges in food emulsions. Sustain. Food Technol. 2025, 3, 96–122. [Google Scholar] [CrossRef]
- Elaine, E.; Bhandari, B.; Tan, C.P.; Nyam, K.L. Recent Advances in the Formation, Stability, and Emerging Food Application of Water-in-Oil-in-Water Double Emulsion Carriers. Food Bioprocess Technol. 2024, 17, 3440–3460. [Google Scholar] [CrossRef]
- Singh, I.R.; Pulikkal, A.K. Nano emulsions stabilized by natural emulsifiers: A comprehensive review on feasibility, stability and bio-applicability. J. Drug Deliv. Sci. Technol. 2024, 92, 105303. [Google Scholar] [CrossRef]
- Cen, S.; Li, S.; Meng, Z. Advances of protein-based emulsion gels as fat analogues: Systematic classification, formation mechanism, and food application. Food Res. Int. 2024, 191, 114703. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Ni, Y.; Yu, Q.; Li, J.; Fan, L.; Eskin, N.A.M. Deep learning in food science: An insight in evaluating Pickering emulsion properties by droplets classification and quantification via object detection algorithm. Adv. Colloid Interface Sci. 2022, 304, 102663. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef]
- Wong, S.F.; Lim, J.S.; Dol, S.S. Crude oil emulsion: A review on formation, classification and stability of water-in-oil emulsions. J. Petrol. Sci. Eng. 2015, 135, 498–504. [Google Scholar] [CrossRef]
- Klojdovà, I.; Stathopoulos, C. W/o/w multiple emulsions: A novel trend in functional ice cream preparations? Food Chem. X 2022, 16, 100451. [Google Scholar] [CrossRef]
- Klojdovà, I.; Stathopoulos, C. The Potential Application of Pickering Multiple Emulsions in Food. Foods 2022, 11, 1558. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Y.Q.; Chen, Y.; Li, J.; Wang, L.; Li, C. A review of multiple Pickering emulsions: Solid stabilization, preparation, particle effect, and application. Chem. Eng. Sci. 2022, 248, 117085. [Google Scholar] [CrossRef]
- Mahmood, T.; Akhtar, N. Stability of a Cosmetic Multiple Emulsion Loaded with Green Tea Extract. Sci. World 2013, 2013, 153695. [Google Scholar] [CrossRef]
- Khan, A.Y.; Talegaonkar, S.; Iqbal, Z.; Jalees, F. Multiple Emulsions: An Overview. Curr. Drug Deliv. 2006, 3, 429–443. [Google Scholar] [CrossRef]
- Boostani, S.; Sarabandi, K.; Tarhan, O.; Rezaei, A.; Assadpour, E.; Rostamabadi, H.; Falsafi, S.R.; Tan, C.; Zhang, F.; Jafari, S.M. Multiple Pickering emulsions stabilized by food-grade particles as innovative delivery systems for bioactive compounds. Adv. Colloid Interface Sci. 2024, 328, 103174. [Google Scholar] [CrossRef]
- Kim, J.W.; Han, S.H.; Choi, Y.H.; Hamonangan, W.M.; Oh, Y.; Kim, S.H. Recent advances in the microfluidic production of functional microcapsules by multiple-emulsion templating. Lab Chip 2022, 22, 2259–2291. [Google Scholar] [CrossRef]
- Clegg, P.S.; Tavacoli, J.W.; Wilde, P.J. One-step production of multiple emulsions: Microfluidic, polymer-stabilized and particle-stabilized approaches. Soft Matter 2016, 12, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Sheraz, M.A.; Ahmed, S.; Kazi, S.H.; Shanhnavi, I.A. Emulsion Separation, Classification and Stability Assessment. J. Pharm. Pharm. Sci. 2014, 2, 56–62. [Google Scholar]
- Nagar, L.; Saini, A.; Gandhi, L.; Awasthi, R.; Dua, K.; Dureja, H. Nanoemulsion-Based Nystatin Delivery: Formulation and Characterization Studies. BioNanoScience 2025, 15, 121. [Google Scholar] [CrossRef]
- Chen, K.; Yang, H.; Xu, G.; Hu, Y.; Tian, X.; Qin, S.; Jiang, T. Enhanced skin penetration of curcumin by a nanoemulsion-embedded oligopeptide hydrogel for psoriasis topical therapy. RSC Med. Chem. 2025, 16, 961–969. [Google Scholar] [CrossRef]
- Schramm, D.L. Emulsions, Foams and Suspensions–Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Clint, J.H. Microemulsions. In Surfactant Aggregation; Springer: Dordrecht, The Netherlands, 1992. [Google Scholar]
- Bouyer, E.; Mekhloufi, G.; Rosilio, V.; Grossiord, J.L.; Agnely, F. Proteins, polisaccarides and their complexes used as stabilizers for emulsions: Alternatives to synthetic surfactants in the pharmaceutical field. Int. J. Pharm. 2012, 436, 359–378. [Google Scholar] [CrossRef]
- Manga, M.S.; Higgins, L.; Kumar, A.A.; Lobel, B.T.; York, D.W.; Cayre, O.J. Exploring effects of polymeric stabiliser molecular weight and concentration on emulsion production via stirred cell membrane emulsification. Polym. Chem. 2023, 14, 5049–5059. [Google Scholar] [CrossRef]
- Barnes, H.A. Rheology of emulsions—A review. Coll. Surf. A 1994, 91, 89–95. [Google Scholar] [CrossRef]
- Derkach, S.R. Rheology of emulsions. Adv. Colloid Interface Sci. 2009, 151, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yang, C. Electrokinetics of non-Newtonian fluids: A review. Adv. Colloid Interface Sci. 2013, 201–202, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.; Yeneneh, A.M.; Sen, T.K.; Ang, H.M.; Kayaalp, A. A comprehensive review on rheological studies of sludge from various sections of municipal wastewater treatment plants for enhancement of process performance. Adv. Colloid Interface Sci. 2018, 257, 19–30. [Google Scholar] [CrossRef]
- Guo, L.P.; Han, X.; Lei, Y.; Wang, L.; Yu, P.F.; Shi, S. Study on the thixotropy and structural recovery characteristics of waxy crude oil emulsion. Pet. Sci. 2021, 18, 1195–1202. [Google Scholar] [CrossRef]
- Grecco Zanotti, M.A.; dos Santos, R.G. Thixotropic Behavior of Oil-in-Water Emulsions Stabilized with Ethoxylated Amines at Low Shear Rates. Chem. Eng. Technol. 2019, 42, 432–443. [Google Scholar] [CrossRef]
- Campanelli, J.R.; Cooper, D.G. Interfacial viscosity and the stability of emulsions. Can. J. Chem. Eng. 1989, 67, 851–855. [Google Scholar] [CrossRef]
- Pal, R. A novel method to correlate emulsion viscosity data. Coll. Surf. A 1998, 137, 275–286. [Google Scholar] [CrossRef]
- Bercea, M. Rheology as a Tool for Fine-Tuning the Properties of Printable Bioinspired Gels. Molecules 2023, 28, 2766. [Google Scholar] [CrossRef]
- Rigano, L.; Deola, M.; Zaccariotto, F.; Colleoni, T.; Lionetti, N. A new gelling agent and rheology modifier in cosmetics: Caesalpina Spinosa Gum. Cosmetics 2019, 6, 34. [Google Scholar]
- Abo-Shosha, M.H.; El-Zairy, M.R.; Ibrahim, N.A. Preparation and rheology of new synthetic thickeners based on polyacrylic acid. Dyes Pigm. 1994, 24, 249–257. [Google Scholar] [CrossRef]
- Fonnum, G.; Bakke, J.; Hansen, F.K. Associative thickeners. Part I: Synthesis, rheology and aggregation behavior. Colloid Polym. Sci. 1993, 271, 380–389. [Google Scholar]
- Nan, G.; Zhuo, Z.; Qingzhi, D. Preparation of Associative Polyurethane Thickener and Its Thickening Mechanism Research. J. Nanomater. 2015, 1, 137646. [Google Scholar] [CrossRef]
- Sakamoto, K.; Lochhead, R.Y.; Maibach, H.I.; Yamashita, Y. Cosmetic Science and Technology: Theoretical Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Cao, W.; Wei, B.; Pu, Y.C.; Wang, L.P.; Su, S.Q.; Bai, Y.P. Low-energy micron dispersion of entangled polymers based on phase inversion composition of emulsion. Polymer 2024, 311, 127519. [Google Scholar]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 50. [Google Scholar]
- Cortez, N.; Alves, I.A.; Aragon, D.M. Innovative Emulsifiers in Cosmetic Products: A Patent Review (2013–2023). ACS Omega 2024, 9, 48884–48898. [Google Scholar]
- Zheng, Y.J.; Loh, X.J. Natural rheological modifiers for personal care. Polym. Adv. Technol. 2016, 27, 1664–1679. [Google Scholar]
- Islam, M.R.; Beg, M.D.H.; Jamari, S.S. Development of vegetable-oil-based polymers. J. Appl. Polym. Sci. 2014, 131, 40787. [Google Scholar] [CrossRef]
- Gilbert, L.; Picard, C.; Savary, G.; Grisel, M. Rheological and textural characterization of cosmetic emulsions containing natural and synthetic polymers: Relationships between both data. Coll. Surf. A 2013, 421, 1501–1563. [Google Scholar]
- Savage, R.M. Effects of rheology modifiers on the flow curves of idealised and food suspensions. Food Hydrocoll. 2000, 14, 209–215. [Google Scholar] [CrossRef]
- Silva, A.C.Q.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Natural Polymers-Based Materials: A Contribution to a Greener Future. Molecules 2022, 27, 94. [Google Scholar] [CrossRef] [PubMed]
- Bekchanov, D.; Mukhamediev, M.; Yarmanov, S.; Lieberzeit, P.; Mujahid, A. Functionalizing natural polymers to develop green adsorbents for wastewater treatment applications. Carb. Polym. 2024, 323, 121397. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Zhao, X.; Zhao, H.; Qu, X. The Rheological and Skin Sensory Properties of Cosmetic Emulsions: Influence of Thickening Agents. J. Cosmet. Sci. 2018, 69, 67–75. [Google Scholar]
- Alves, T.F.R.; Morsink, M.; Batain, F.; Chaud, M.V.; Almeida, T.; Fernandes, D.A.; da Silva, C.F.; Souto, E.B.; Severino, P. Applications of Natural, Semi-Synthetic, and Synthetic Polymers in Cosmetic Formulations. Cosmetics 2020, 7, 75. [Google Scholar] [CrossRef]
- Nawalage, N.S.K.; Bellanthudawa, B.K.A. Synthetic polymers in personal care and cosmetics products (PCCPs) as a source of microplastic (MP) pollution. Mar. Pollut. Bull. 2022, 182, 113927. [Google Scholar] [CrossRef]
- Tafuro, G.; Costantini, A.; Baratto, G.; Francescato, S.; Semenzato, A. Evaluating Natural Alternatives to Synthetic Acrylic Polymers: Rheological and Texture Analyses of Polymeric Water Dispersions. ACS Omega 2020, 5, 15280–15289. [Google Scholar] [CrossRef]
- Vajihinejad, V.; Gumfekar, S.P.; Bazoubandi, B.; Najafabadi, Z.R.; Soares, J.B.P. Water Soluble Polymer Flocculants: Synthesis, Characterization, and Performance Assessment. Macromol. Mater. Eng. 2019, 304, 1800526. [Google Scholar] [CrossRef]
- Vanpoucke, D.E.P.; Delgove, M.A.F.; Stouten, J.; Noordijk, J.; De Vos, N.; Matthysen, K.; Deroover, G.G.P.; Mehrkanoon, S.; Bernaerts, K.V. A machine learning approach for the design of hyperbranched polymeric dispersing agents based on aliphatic polyesters for radiation-curable inks. Polym. Int. 2022, 71, 966–975. [Google Scholar] [CrossRef]
- Ulrich, K.; Centeno, S.A.; Arslanoglu, J.; Del Federico, E. Absorption and diffusion measurements of water in acrylic paint films by single-sided NMR. Prog. Org. Coat. 2011, 71, 283–289. [Google Scholar] [CrossRef]
- Altinkaya, S.A.; Topcuoglu, O.; Yurekli, Y.; Balkose, D. The influence of binder content on the water transport properties of waterborne acrylic paints. Prog. Org. Coat. 2010, 69, 417–425. [Google Scholar] [CrossRef]
- Loiseau, J.; Doerr, N.; Suau, J.M.; Egraz, J.B.; Flauro, M.F.; Ladavière, C.; Claverie, J. Synthesis and Characterization of Poly(acrylic acid) Produced by RAFT Polymerization. Application as a Very Efficient Dispersant of CaCO3, Kaolin, and TiO2. Macromolecules 2003, 9, 3066–3077. [Google Scholar] [CrossRef]
- Dusicka, E.; Nikitin, A.N.; Lacìk, I. Propagation rate coefficient for acrylic acid polymerization in bulk and in propionic acid by the PLP–SEC method: Experiment and 3D simulation. Polym. Chem. 2019, 10, 5870–5878. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.K.; Seyfoddin, A.; Fatihhi, S.J. Acrylic acid/acrylamide based hydrogels and its properties–A review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Khan, M.A.; Azad, A.K.; Safdar, M.; Nawaz, A.; Akhlaq, M.; Paul, P.; Hossain, M.K.; Habibur Rahman, M.; Baty, R.S.; El-kott, A.F.; et al. Synthesis and Characterization of Acrylamide/Acrylic Acid Co-Polymers and Glutaraldehyde Crosslinked pH-Sensitive Hydrogels. Gels 2022, 8, 47. [Google Scholar] [CrossRef]
- Zakzak, K.; Semenescu, A.D.; Moaca, E.A.; Predescu, I.; Draghici, G.; Vlaia, L.; Vlaila, V.; Borcan, F.; Dehelean, C.A. Comprehensive Biosafety Profile of Carbomer-Based Hydrogel Formulations Incorporating Phosphorus Derivatives. Gels 2024, 10, 477. [Google Scholar] [CrossRef] [PubMed]
- Caron, I.; Rossi, F.; Papa, S.; Aloe, R.; Sculco, M.; Mauri, E.; Sacchetti, A.; Erba, E.; Panini, N.; Parazzi, V.; et al. A new three dimensional biomimetic hydrogel to deliver factors secreted by human mesenchymal stem cells in spinal cord injury. Biomaterials 2016, 75, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Pizzetti, F.; Maspes, A.; Rossetti, A.; Rossi, F. The addition of hyaluronic acid in chemical hydrogels can tune the physical properties and degradability. Eur. Polym. J. 2021, 161, 110843. [Google Scholar] [CrossRef]
- Mauri, E.; Sacchetti, A.; Vicario, N.; Peruzzotti-Jametti, L.; Rossi, F.; Pluchino, S. Evaluation of RGD functionalization in hybrid hydrogels as 3D neural stem cell culture systems. Biomater. Sci. 2018, 8, 501–510. [Google Scholar] [CrossRef]
- Contreras, M.D.; Sànchez, R. Application of a factorial design to the study of specific parameters of a Carbopol ETD 2020 gel.: Part, I.: Viscoelastic parameters. Int. J. Pharm. 2002, 234, 139–147. [Google Scholar] [CrossRef]
- Contreras, M.D.; Sànchez, R. Application of a factorial design to the study of the flow behavior, spreadability and transparency of a Carbopol ETD 2020 gel. Part II. Int. J. Pharm. 2002, 234, 149–157. [Google Scholar] [PubMed]
- Rivera, A.J.; Garcia-Blanco, Y.J.; Quitian-Ardila, L.H.; Germer, E.M.; Franco, A.T. Visualizing flow dynamics and restart of Carbopol gel solutions in tube and parallel-plates geometries with wall slip. Soft Matter 2024, 20, 5983–6001. [Google Scholar]
- Masood, S.; Arshad, M.S.; Khan, H.M.S.; Begum, M.Y.; Khan, K.R. Encapsulation of Leptadenia pyrotechnica (Khip) Extract in Carbomer Based Emulgel for Its Enhanced Antioxidant Effects and Its In Vitro Evaluation. Gels 2023, 9, 977. [Google Scholar] [CrossRef]
- Vishnyakov, A.; Mao, R.; Kam, K.; Potanin, A.; Neimark, A.V. Interactions of Crosslinked Polyacrylic Acid Polyelectrolyte Gels with Nonionic and Ionic Surfactants. J. Phys. Chem. B 2021, 125, 13817–13828. [Google Scholar]
- Medina-Torres, L.; Calderas, F.; Sanchez-Olivares, G.; Nunez-Ramirez, D.M. Rheology of Sodium Polyacrylate as an Emulsifier Employed in Cosmetic Emulsions. Ind. Eng. Chem. Res. 2014, 53, 18346–18351. [Google Scholar]
- Wang, F.; Yang, H.; Li, M.; Kang, X.; Zhang, X.; Zhang, H.; Zhao, H.; Kang, W.; Sarsenbekuly, B.; Aidarova, S.; et al. Study on stabilization of emulsion formed by the supramolecular system of amphiphilic polymer and sodium polyacrylic acid. J. Mol. Liq. 2020, 314, 113644. [Google Scholar]
- Rodriduez-Torres, R.; Rist, S.; Almeda, R.; Nielsen, T.G.; Pedrotti, M.L.; Hartmann, N.B. Research trends in nano- and microplastic ingestion in marine planktonic food webs. Environ. Pollut. 2024, 363, 125136. [Google Scholar]
- Arnon, S. Making waves: Unraveling microplastic deposition in rivers through the lens of sedimentary processes. Water Res. 2025, 272, 122934. [Google Scholar]
- Dissanayake, P.D.; Kim, S.; Sarkar, B.; Oleszczuk, P.; Sang, M.K.; Hauqe, M.N.; Ahn, J.H.; Bank, M.S.; Ok, Y.S. Effects of microplastics on the terrestrial environment: A critical review. Environ. Res. 2022, 209, 112734. [Google Scholar]
- Li, Y.; Tao, L.; Wang, F.; Li, G.; Song, M. Potential Health Impact of Microplastics: A Review of Environmental Distribution, Human Exposure, and Toxic Effects. Environ. Health 2023, 1, 249–257. [Google Scholar]
- Gan, Q.; Cui, J.; Jin, B. Environmental microplastics: Classification, sources, fates, and effects on plants. Chemosphere 2023, 313, 137559. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; Hosny, M.; Eltaweil, A.S.; Omar, S.; Elgarahy, A.M.; Farghali, M.; Yap, P.S.; Wu, Y.S.; Nagandran, S.; Batumalaie, K.; et al. Microplastic sources, formation, toxicity and remediation: A review. Environ. Chem. Lett. 2023, 21, 2129–2169. [Google Scholar]
- Sharma, S.; Sharma, V.; Chatterjee, S. Contribution of plastic and microplastic to global climate change and their conjoining impacts on the environment–A review. Sci. Total Environ. 2023, 875, 162627. [Google Scholar] [CrossRef]
- Mendoza-Munoz, N.; Leyva-Gomez, G.; Pinon-Segundo, E.; Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; Del Prado Audelo, M.L.; Urban-Morlan, Z. Trends in biopolymer science applied to cosmetics. Int. J. Cosmet. Sci. 2023, 45, 699–724. [Google Scholar] [CrossRef]
- Picken, C.A.R.; Buensoz, O.; Price, P.D.; Fidge, C.; Points, L.; Shaver, M.P. Sustainable formulation polymers for home, beauty and personal care: Challenges and opportunities. Chem. Sci. 2023, 14, 12926. [Google Scholar] [CrossRef] [PubMed]
- Duis, K.; Junker, T.; Coors, A. Environmental fate and effects of water-soluble synthetic organic polymers used in cosmetic product. Environ. Sci. Eur. 2021, 33, 21. [Google Scholar] [CrossRef]
- Himashree, P.; Sengar, A.S.; Sunil, C.K. Food thickening agents: Sources, chemistry, properties and applications–A review. Int. J. Gastron. Food Sci. 2022, 27, 100468. [Google Scholar] [CrossRef]
- Chattopadhyay, S.N.; Pan, N.C. Ecofriendly printing of jute fabric with natural dyes and thickener. J. Nat. Fibers 2018, 16, 1077–1088. [Google Scholar] [CrossRef]
- Wei, Y.; Guo, Y.; Li, R.; Ma, A.; Zhang, H. Rheological characterization of polysaccharide thickeners oriented for dysphagia management: Carboxymethylated curdlan, konjac glucomannan and their mixtures compared to xanthan gum. Food Hydrocoll. 2021, 110, 106198. [Google Scholar] [CrossRef]
- Leal, M.R.S.; Albuquerque, P.B.S.; Rodrigues, N.E.R.; dos Santos Silva, P.M.; de Oliveira, W.F.; dos Santos Correira, M.T.; Kennedy, J.F.; Coelho, L.C.B.B. A review on the use of polysaccharides as thickeners in yogurts. Carbohydr. Polym. Tech. 2024, 8, 100547. [Google Scholar] [CrossRef]
- Fonseca, S.; Amaral, M.N.; Reis, C.P.; Custodio, L. Marine Natural Products as Innovative Cosmetic Ingredients. Mar. Drugs 2023, 21, 170. [Google Scholar] [CrossRef] [PubMed]
- Simat, V.; Elabed, N.; Kulawik, P.; Ceylan, Z.; Jamroz, E.; Yazgan, H.; Cagalj, M.; Regenstein, J.M.; Ozogul, F. Recent Advances in Marine-Based Nutraceuticals and Their Health Benefits. Mar. Drugs 2020, 18, 627. [Google Scholar] [CrossRef] [PubMed]
- Fontes-Candia, C.; Strom, A.; Lopez-Sanchez, P.; Lopez-Rubio, A.; Martinez-Sanz, M. Rheological and structural characterization of carrageenan emulsion gels. Algal Res. 2020, 47, 101873. [Google Scholar] [CrossRef]
- Leandro, A.; Gonçalves, A.M.M.; Pereira, L. Diverse applications of marine macroalgae. Mar. Drugs 2019, 18, 17. [Google Scholar] [CrossRef]
- Purwaningsih, S.; Salamah, E.; Adnin, M.N. Photoprotective effect of sunscreen cream with addition of carrageenan and black mangrove fruit (Rhizopora mucronata lamk.). J. Ilm. Tekno Kel. Trop. 2015, 7, 9819. [Google Scholar]
- Russo, N.; Avallone, P.R.; Grizzuti, N.; Pasquino, R. Stable O/W emulsions by combining Pluronic L64 and Sodium Alginate. Coll. Surf. A 2024, 701, 134776. [Google Scholar] [CrossRef]
- Xiao, Q.; Chen, G.; Zhang, Y.H.; Chen, F.Q.; Weng, H.F.; Xiao, A.F. Agarose Stearate-Carbomer940 as Stabilizer and Rheology Modifier for Surfactant-Free Cosmetic Formulations. Mar. Drugs 2021, 19, 344. [Google Scholar] [CrossRef]
- Niknam, R.; Soudi, M.R.; Mousavi, M. Rheological and Stability Evaluation of Emulsions Containing Fenugreek Galactomannan—Xanthan Gum Mixtures: Effect of Microwave and Ultrasound Treatments. Macromol. 2022, 2, 361–373. [Google Scholar] [CrossRef]
- Lupi, F.R.; Gabriele, D.; Seta, L.; Baldino, N.; de Cindio, B.; Marino, R. Rheological investigation of pectin-based emulsion gels for pharmaceutical and cosmetic uses. Rheol. Acta 2015, 54, 41–52. [Google Scholar] [CrossRef]
- Yermagambetova, A.; Tazhibayeva, S.; Takhistov, P.; Tyussyupova, B.; Tapia-Hernandez, J.A.; Musabekov, K. Microbial Polysaccharides as Functional Components of Packaging and Drug Delivery Applications. Polymers 2024, 16, 2854. [Google Scholar] [CrossRef]
- Mahmoud, Y.A.G.; El-Naggar, M.E.; Abdel-Megeed, A.; El-Newehy, M. Recent Advancements in Microbial Polysaccharides: Synthesis and Applications. Polymers 2021, 13, 4136. [Google Scholar] [CrossRef] [PubMed]
- Furtado, I.F.S.P.C.; Sydney, E.B.; Rodrigues, S.A.; Sydney, A.C.N. Xanthan gum: Applications, challenges, and advantages of this asset of biotechnological origin. Biotechnol. Res. Innov. 2022, 6, e202204. [Google Scholar]
- Zheng, Z.; Sun, Z.; Li, M.; Yang, J.; Yang, Y.Q.; Liang, H.; Xiang, H.; Meng, J.; Zhou, X.; Liu, L.; et al. An update review on biopolymer Xanthan gum: Properties, modifications, nanoagrochemicals, and its versatile applications in sustainable agriculture. Int. J. Biol. Macromol. 2024, 281, 136562. [Google Scholar]
- Petri, D.F.S. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 132, 42035. [Google Scholar]
- Ribeiro, S.; Almeida, R.; Batista, L.; Lima, J.; Sarinho, A.; Nascimento, A.; Lisboa, H. Investigation of Guar Gum and Xanthan Gum Influence on Essential Thyme Oil Emulsion Properties and Encapsulation Release Using Modeling Tools. Foods 2024, 13, 816. [Google Scholar] [CrossRef] [PubMed]
- Krstonosic, V.; Dokic, L.; Nikolic, I.; Milanovic, M. Influence of xanthan gum on oil-in-water emulsion characteristics stabilized by OSA starch. Food Hydrocoll. 2015, 45, 9–17. [Google Scholar]
- Hadde, E.K.; Mossel, B.; Chen, J.; Prakash, S. The safety and efficacy of xanthan gum-based thickeners and their effect in modifying bolus rheology in the therapeutic medical management of dysphagia. Food Hydrocoll. Health 2021, 1, 100038. [Google Scholar]
- Gilbert, L.; Loisel, V.; Savary, G.; Grisel, M.; Picard, C. Stretching properties of xanthan, carob, modified guar and celluloses in cosmetic emulsions. Carb. Polym. 2013, 93, 644–650. [Google Scholar]
- Cui, R.; Zhu, F. Ultrasound modified polysaccharides: A review of structure, physicochemical properties, biological activities and food applications. Trends Food Sci. 2021, 107, 491–508. [Google Scholar]
- Pacheco Guilherme, G.P.; de Mello, C.V.; Chiari-Andréo, B.G.; Isaac, V.L.B.; Ribeiro, S.J.L.; Pecoraro, E.; Trovatti, E. Bacterial cellulose skin masks–Properties and sensory tests. J. Cosmet. Dermatol. 2017, 1, 840–847. [Google Scholar]
- Martins, D.; Rocha, F.; Dourado, F.; Gama, M. Bacterial Cellulose-Carboxymethyl Cellulose (BC:CMC) dry formulation as stabilizer and texturizing agent for surfactant-free cosmetic formulations. Coll. Surf. A 2021, 617, 126380. [Google Scholar] [CrossRef]
- Ozturk, Y.S.; Dolaz, M. Synthesis and Characterization of Hydroxyethyl Starch from Chips Wastes Under Microwave Irradiation. J. Environ. Polym. Degrad. 2021, 29, 948–957. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Hattrem, M.N.; Draget, K.I. Propylene glycol alginate (PGA) gelled foams: A systematic study of surface activity and gelling properties as a function of degree of esterification. Food Hydrocoll. 2016, 57, 80–91. [Google Scholar] [CrossRef]
- Gupta, N.R.; Torris, A.T.A.; Wadgaonkar, P.P.; Rajamohanan, P.R.; Ducouret, G.; Hourdet, D.; Creton, C.; Badiger, M.V. Synthesis and characterization of PEPO grafted carboxymethyl guar and carboxymethyl tamarind as new thermo-associating polymers. Carb. Polym. 2015, 117, 331–338. [Google Scholar] [CrossRef]
- Fan, C.; Dang, M.; Liang, Y.; Feng, P.; Wei, F.; Fu, L.; Xu, C.; Lin, B. Polysaccharides synergistic boosting drug loading for reduction pesticide dosage and improve its efficiency. Carb. Polym. 2022, 297, 120041. [Google Scholar] [CrossRef]
- Bianchet, R.T.; Cubas, A.L.V.; Machado, M.M.; Moecke, E.H.S. Applicability of bacterial cellulose in cosmetics–Bibliometric review. Biotechnol. Rep. 2020, 27, e00502. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.Z.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Kim, J.T.; Roy, S.; Jayakumar, A. Recent advances in carboxymethyl cellulose-based active and intelligent packaging materials: A comprehensive review. Int. J. Biol. Macromol. 2024, 259, 129194. [Google Scholar] [CrossRef]
- Buyukurganci, B.; Basu, S.K.; Neuner, M.; Guck, J.; Wierschem, A.; Reichel, F. Shear rheology of methyl cellulose based solutions for cell mechanical measurements at high shear rates. Soft Matter 2023, 19, 1739–1748. [Google Scholar] [CrossRef]
- Wustenberg, T. Sodium Carboxymethylcellulose. In Cellulose and Cellulose Derivatives in the Food Industry; Wustenberg, T., Ed.; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Lopez, C.G.; Colby, R.H.; Cabral, J.T. Electrostatic and Hydrophobic Interactions in NaCMC Aqueous Solutions: Effect of Degree of Substitution. Macromolecules 2018, 51, 3165–3175. [Google Scholar] [CrossRef]
- Rahman, S.; Hassan, S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, S.; Shiddiky, M.J.A.; Ahmed, M.S. Recent Developments of Carboxymethyl Cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef]
- Wei, Q.; Zheng, H.; Han, X.; Zheng, C.; Huang, C.; Jin, Z.; Li, Y.; Zhou, J. Octenyl succinic anhydride modified starch with excellent emulsifying properties prepared by selective hydrolysis of supramolecular immobilized enzyme. Int. J. Biol. Macromol. 2023, 232, 123383. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.J.; Baik, M.Y. Characteristics of physically modified starches. Food Sci. Biotechnol. 2023, 32, 875–883. [Google Scholar] [CrossRef]
- Wen, H.; Hu, J.; Zou, S.; Xiao, Y.; Li, Y.; Feng, H.; Fan, L. Preparation and characterization of aminoethyl hydroxypropyl starch modified with collagen peptide. Int. J. Biol. Macromol. 2017, 101, 996–1003. [Google Scholar] [CrossRef]
- Sionkowska, A.; Michalska-Sionkowska, M.; Walczak, M. Preparation and characterization of collagen/hyaluronic acid/chitosan film crosslinked with dialdehyde starch. Int. J. Biol. Macromol. 2020, 149, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gu, Z.; Cheng, L.; Li, Z.; Li, C.; Ban, X.; Hong, Y. Preparation and stability mechanisms of double emulsions stabilized by gelatinized native starch. Carb. Polym. 2021, 262, 117926. [Google Scholar] [CrossRef]
- Haghayegh, G.; Schonlechner, R. Physically modified starches: A review. J Food Agric. Environ. 2011, 9, 27–29. [Google Scholar]
- Wang, S.; Hu, X.; Wang, Z.; Bao, Q.; Zhou, B.; Li, T.; Li, S. Preparation and characterization of highly lipophilic modified potato starch by ultrasound and freeze-thaw treatments. Ultrason. Sonochem. 2020, 64, 105054. [Google Scholar] [CrossRef]
| Marine | Botanical | Microbial |
|---|---|---|
| Carrageenans | Guar gum | Xanthan gum |
| Agar-agar | Locust bean gum | Gellan gum |
| Alginates | Gum tragacanth | Pullulan |
| Konjac glucomannan | Curdlan | |
| Tara gum | Dextran | |
| Cassia gum | Welan gum | |
| Gum Arabic | Rhamsan | |
| Pectin | Succinoglycan | |
| Starches |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franceschini, M.; Pizzetti, F.; Rossi, F. On the Key Role of Polymeric Rheology Modifiers in Emulsion-Based Cosmetics. Cosmetics 2025, 12, 76. https://doi.org/10.3390/cosmetics12020076
Franceschini M, Pizzetti F, Rossi F. On the Key Role of Polymeric Rheology Modifiers in Emulsion-Based Cosmetics. Cosmetics. 2025; 12(2):76. https://doi.org/10.3390/cosmetics12020076
Chicago/Turabian StyleFranceschini, Matteo, Fabio Pizzetti, and Filippo Rossi. 2025. "On the Key Role of Polymeric Rheology Modifiers in Emulsion-Based Cosmetics" Cosmetics 12, no. 2: 76. https://doi.org/10.3390/cosmetics12020076
APA StyleFranceschini, M., Pizzetti, F., & Rossi, F. (2025). On the Key Role of Polymeric Rheology Modifiers in Emulsion-Based Cosmetics. Cosmetics, 12(2), 76. https://doi.org/10.3390/cosmetics12020076







