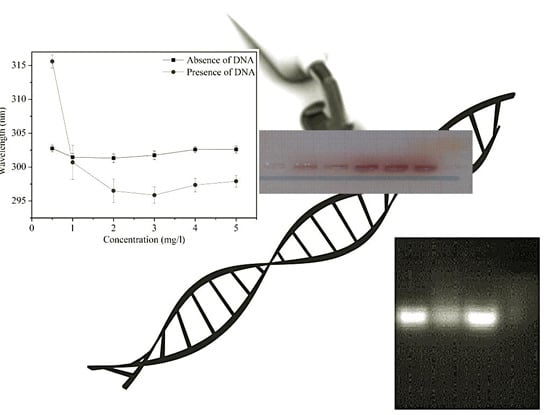
Hair Dye–DNA Interaction: Plausible Cause of Mutation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Spectroscopic Studies of Dye–DNA Interaction
2.2.1. Interaction of Dye with DNA
2.2.2. Dye–DNA Interaction in Sodium Dodecyl Sulfate (SDS) Micelles
2.2.3. UV-Vis Spectroscopic Studies on Autoxidation of Dye
2.3. Study of Dye–DNA Interaction Using Agarose Gel Electrophoresis
2.4. Statistical Analysis
3. Results and Discussions
3.1. Spectroscopic Study of Dye–DNA Interaction

3.2. Spectroscopic Study of Dye in SDS Micelles

3.3. UV-vis Spectroscopic Studies on Autoxidation of Dye

3.4. Study of Dye–DNA Interaction Using Agarose Gel Electrophoresis

4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Bartman, E. Hair and the artifice of Roman female adornment. Am. J. Archaeol. 2001, 105, 1–25. [Google Scholar] [CrossRef]
- Corbett, J.F. An historical review of the use of dye precursors in the formulation of commercial oxidation hair dyes. Dyes Pigment. 1999, 41, 127–136. [Google Scholar] [CrossRef]
- Brown, K.C. Hair coloring. In Hair and Hair Care; Marcel Dekker: New York, NY, USA, 1997. [Google Scholar]
- Monnais, C. Hair color alterations agent. In Cosmetics-Development, Manufacture and Application of Cosmetics; Georg Thieme Verlag: Stuttgart, Germany, 1995. [Google Scholar]
- Ames, B.N.; Kammen, H.O.; Yamasaki, E. Hair dyes are mutagenic: Identification of a variety of mutagenic ingredients. Proc. Natl. Acad. Sci. 1975, 72, 2423–2427. [Google Scholar] [CrossRef] [PubMed]
- Garrigue, J.L.; Ballantyne, M.; Kumaravel, T.; Lloyd, M.; Nohynek, G.J.; Kirkland, D.; Toutain, H. In vitro genotoxicity of para-phenylenediamine and its N-monoacetyl or N,N′-diacetyl metabolites. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2006, 608, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Cosmetic Ingredient Review Expert Panel. Final report on the safety assessment of disperse Blue 7. Int. J. Toxicol. 2007, 26, 65–77. [Google Scholar]
- Murata, M.; Nishimura, T.; Chen, F.; Kawanishi, S. Oxidative DNA damage induced by hair dye components ortho-phenylenediamines and the enhancement by superoxide dismutase. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2006, 607, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Oikawa, S.; Ogawa, Y.; Miyakoshi, Y.; Ooida, M.; Asanuma, K.; Shimizu, H. Mutagenicity of p-aminophenol in E. coli WP2uvrA/pKM101 and its relevance to oxidative DNA damage. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1998, 415, 139–150. [Google Scholar] [CrossRef]
- Burnett, C.M.; Corbett, J.F. Failure of short-term in vitro mutagenicity tests to predict the animal carcinogenicity of hair dyes. Food Chem. Toxicol. 1987, 25, 703–707. [Google Scholar] [CrossRef]
- Haws, L.C.; Jackson, B.A.; Turnbull, D.; Dressler, W.E. Two approaches for assessing human safety of disperse Blue 1. Regul. Toxicol. Pharmacol. 1994, 19, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Genina, E.A.; Bashkatov, A.N.; Sinichkin, Y.P.; Kochubey, V.I.; Lakodina, N.A.; Altshuler, G.B.; Tuchin, V.V. In vitro and in vivo study of dye diffusion into the human skin and hair follicles. J. Biomed. Opt. 2002, 7, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Yourick, J.J.; Bronaugh, R.L. Percutaneous penetration and metabolism of 2-nitro-p-phenylenediamine in human and fuzzy rat skin. Toxicol. Appl. Pharmacol. 2000, 166, 13–21. [Google Scholar] [CrossRef]
- Steiling, W.; Kreutz, J.; Hofer, H. Percutaneous penetration/dermal absorption of hair dyes in vitro. Toxicol. in vitro 2001, 15, 565–570. [Google Scholar] [CrossRef]
- Burnett, C.; Fuchs, C.; Corbett, J.; Menkart, J. The effect of dimethylsulfoxide on the mutagenicity of the hair dye p-phenylenediamine. Mutat. Res. 1982, 103, 1–4. [Google Scholar] [PubMed]
- Bracher, M.; Faller, C.; Grotsch, W.; Marshall, R.; Spengler, J. Studies on the potential mutagenicity of p-phenylenediamine in oxidative hair dye mixtures. Mutati. Res. 1990, 241, 313–323. [Google Scholar] [CrossRef]
- Crebelli, R.; Conti, L.; Carere, A.; Zito, R. Mutagenicity of commercial p-phenylenediamine and of an oxidation mixture of p-phenylenediamine and resorcinol in salmonella typhimurium TA98. Food Chem. Toxicol. 1981, 19, 79–84. [Google Scholar] [CrossRef]
- Watanabe, T.; Kawata, A.; Koyanagi, T. Mutagenicity of hair dyes. J. Soc. Cosmet. Chem. Jpn. 1980, 41, 20–25. [Google Scholar]
- Spengler, J.; Bracher, M. Toxicological tests and health risk assessment of oxidative hair dye mixtures. Cosmet. Toilet. 1990, 105, 67–76. [Google Scholar]
- Maiti, S.; Sasmal, K.; Sinha, S.S.; Singh, M. Analysis of cytotoxicity and genotoxicity suggests a greater toxic potential of hair dye. Ecotox. Environ. Safe 2015. submitted. [Google Scholar]
- Mitra, R.K.; Sinha, S.S.; Maiti, S.; Pal, S.K. Sequence dependent ultrafast electron transfer of Nile Blue in oligonucleotides. J. Fluoresc. 2009, 19, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Maiti, P.; Sinha, S.S.; Mitra, R.K.; Pal, S.K. Molecular recognition of plant DNA: Does it differ from conventional animal DNA? Int. J. Biol. Macromol. 2009, 44, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Patist, A.; Kanicky, R.J.; Shukla, K.P.; Shah, O.D. Importance of micellar kinetics in relation to technological processes. J. Colloid Interface Sci. 2002, 245, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, R.J. Principles of Fluorescence Spectroscopy; Springer: Berlin, Germany, 2010. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiti, S.; Sinha, S.S.; Singh, M. Hair Dye–DNA Interaction: Plausible Cause of Mutation. Cosmetics 2015, 2, 313-321. https://doi.org/10.3390/cosmetics2040313
Maiti S, Sinha SS, Singh M. Hair Dye–DNA Interaction: Plausible Cause of Mutation. Cosmetics. 2015; 2(4):313-321. https://doi.org/10.3390/cosmetics2040313
Chicago/Turabian StyleMaiti, Swati, Sudarson Sekhar Sinha, and Mukesh Singh. 2015. "Hair Dye–DNA Interaction: Plausible Cause of Mutation" Cosmetics 2, no. 4: 313-321. https://doi.org/10.3390/cosmetics2040313
APA StyleMaiti, S., Sinha, S. S., & Singh, M. (2015). Hair Dye–DNA Interaction: Plausible Cause of Mutation. Cosmetics, 2(4), 313-321. https://doi.org/10.3390/cosmetics2040313