A Novel Cassia fistula (L.)-Based Emulsion Elicits Skin Anti-Aging Benefits in Humans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Extracts
2.2. Instrumental Measurements
2.3. Creams Preparation, Composition and Characteristics
| Phase | No. | Composition | Placebo | Active Formulation |
|---|---|---|---|---|
| Oily Phase | 01 | Liquid Paraffin | 24 | 24 |
| 02 | Stearic Acid | 5 | 5 | |
| 03 | Span 20 ™ | 2 | 2 | |
| 04 | Bees wax | 7 | 7 | |
| 05 | Cetomacrogol ™ | 5 | 5 | |
| Aqueous Phase | 01 | Tween 80 ™ | 6 | 6 |
| 02 | Plant Extract | Nil | 5 | |
| 03 | Preservative | 1 | 1 | |
| 04 | D/W | 50 | 45 |
2.4. Study Protocol
2.5. Ethics Approval
2.6. Mathematical Analysis
- D0 = Baseline values (time 0 value).
- Dx = Value obtained at week 2, 4, 6, 8, 10 and 12 (for SH and TEWL) or at month 1, 2 and 3 (for SELS).
2.7. Statistical Analysis
3. Results
3.1. Cream Characterization
| Parameters | Placebo | Active Formulation |
|---|---|---|
| Type | Macro-emulsion; O/W | Macro-emulsion; O/W |
| Appearance | Milky; Pale; Yellow | Milky; Pale; Yellow |
| Organoleptic property | Odorless | Odorless |
| Thermo-Stability | 8 °C or 40 °C, with 75% RH | 8 °C or 40 °C, with 75% RH |
| pH | 5.5 | 5.5 |
| Electrical conductivity | 49 μS/cm | 55 μS/cm |
| Rheological stability | – | – |
| Flow index | 0.53 | 0.50 |
| % Confidence | 99.45 | 99.59 |
| Shear stress | 95–125 D/cm2 | 140–225 D/cm2 |
| Viscosity | 126.36 cP | 145.35 cP |
3.2. Patch Test (Skin Compatibility Test)
| Emulsion Type | Score | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| No. of Volunteers Indicating Itching/Irritation | ||||
| Placebo | 8 | 4 | 1 | 0 |
| Active formulation | 7 | 4 | 2 | 0 |
3.3. Skin Hydratation (SH) and Transepidermal Water Loss (TEWL)


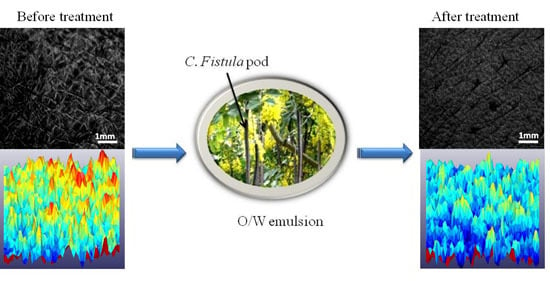
3.4. Surface Evaluation of Living Skin (SELS)


4. Discussion
5. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Acronyms
| C. fistula | Cassia fistula | SEw | surface evaluation of wrinkling |
| SH | skin hydratation | UV | ultraviolet |
| TEWL | transepidermal water loss | UVR | ultraviolet radiation |
| SELS | surface evaluation of living skin | DPPH | 2,2-Diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl |
| SEsc | surface evaluation of scaliness | SEsm | surface evaluation of smoothness |
| SEr | surface evaluation of roughness |
References
- Menaa, F.; Menaa, A.; Tréton, J. Polyphenols against skin aging. In Polyphenols in Human Health and Disease, 1st ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: New York, NY, USA, 2013; pp. 819–829. [Google Scholar]
- Menaa, F.; Badole, S.L.; Menaa, B.; Menaa, A. Promising Plant Extracts with in Vivo Anti-Melanoma Potential. In Bioactive Dietary Factors and Plant Extracts in Dermatology, 1st ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Humana Press Inc.: New York, NY, USA, 2013; pp. 283–290. [Google Scholar]
- Menaa, F.; Menaa, A. Skin Photoprotection by Polyphenols in Animal Models and Humans. In Polyphenols in Human Health and Disease, 1st ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: New York, NY, USA, 2013; pp. 831–838. [Google Scholar]
- Zanwar, A.A.; Badole, L.S.; Menaa, F. Curcuma Longa: Use for Skin Disease Care. In Bioactive Dietary Factors and Plant Extracts in Dermatology; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Humana Press Inc.: New York, NY, USA, 2013; pp. 391–396. [Google Scholar]
- Khan, B.A.; Akhtar, N.; Hussain, I.; Abbas, K.A.; Rasul, A. Whitening efficacy of plant extracts including Hippophae rhamnoides and Cassia fistula extracts on the skin of Asian patients with melasma. Postepy Dermatol. Alergol. 2013, 30, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.A.; Akhtar, N.; Braga, V.A. Anti-Aging Effects of Hippophae rhamnoides Emulsion on Human Skin. Trop. J. Pharm. Res. 2012, 11, 955–962. [Google Scholar] [CrossRef]
- Khan, B.A.; Akhtar, N.; Khan, H.M.S.; Waseem, K.; Mahmood, T.; Rasul, A.; Iqbal, M.; Khan, H. Basics of pharmaceutical emulsions: A review. Afr. J. Pharm. Pharmacol. 2011, 5, 2715–2725. [Google Scholar]
- Rasul, A.; Akhtar, N.; Khan, B.A.; Mahmood, T.; Zaman, S.U.; Ali, A.; Khan, H.M.S.; Parveen, R. Evaluation for antierythmic and depigmenting effects of a newly formulated emulsion containing basil extract. J. Med. Plant Res. 2011, 5, 6249–6253. [Google Scholar]
- Cuéllar, M.J.; Giner, R.M.; Recio, M.C.; Máñez, S.; Ríos, J.L. Topical anti-inflammatory activity of some Asian medicinal plants used in dermatological disorders. Fitoterapia 2001, 72, 221–229. [Google Scholar] [CrossRef]
- Gali, H.; Perchellet, E.; Makkar, H.; Perchellet, J. Ability of tannins extracted from the leaves of various trees and shrubs to inhibit the biomarkers of tumor promotion in mouse skin in vivo. Int. J. Oncol. 1996, 9, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Menaa, F.; Menaa, B. Polyphenols nanoformulations for topical dermal delivery and skin tissue engineering. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: New York, NY, USA, 2013; pp. 839–848. [Google Scholar]
- Menaa, F.; Menaa, A.; Treton, J.; Menaa, B. Nanoencapsulations of Dietary Polyphenols for Oncology and Gerontology: Resveratrol as a Good Example—Resveratrol Nano-Formulations: Suitable for Cancer Patients and the Elderly? In Introduction to Functional Food Science; Martirosyan, D.M., Ed.; Food Science Publisher: Dallas, TX, USA, 2013; pp. 383–404. [Google Scholar]
- Menaa, F.; Menaa, A.; Tréton, J.; Menaa, B. Dietary Intake of (-)-Epigallocatechin-3-gallate against Aging and Cancers: Nanoencapsulation of Multi-Rings Still Requires New Rounds! J. Nanomater. Mol. Nanotechnol. 2013, 2. [Google Scholar] [CrossRef]
- Mahmood, T.; Akhtar, N.; Khan, B.A.; Rasul, A.; Khan, H.M.S. Fabrication, physicochemical characterization and preliminary efficacy evaluation of a W/O/W multiple emulsion loaded with 5% green tea extract. Braz. J. Pharm. Sci. 2013, 49, 341–349. [Google Scholar] [CrossRef]
- Akhtar, N.; Hisham, J.; Khan, B.A.; Shoaib Khan, H.M.; Mahmood, T.; Rasul, A.; Iqbal, M.; Qayum, M. Cosmetic application of phenolic cream from mulberry bark extract. Asian J. Chem. 2012, 24, 1805–1808. [Google Scholar]
- Akhtar, N.; Anwar, M.; Khan, B.A.; Mahmood, T.; Zaman, S.U. Formulation development and pharmaceutical evaluation of a w/o emulsion of Coleus extract. Indian J. Pharm. Educ. Res. 2011, 45, 236–241. [Google Scholar]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, structure, and physical properties. J. Phys. Condens. Matt. 2006, 18, R635–R666. [Google Scholar] [CrossRef]
- Hoar, T.P.; Schulman, J.H. Transparent water-in-oil dispersions: The oleopathic hydro-micelle. Nature 1943, 152, 102–103. [Google Scholar] [CrossRef]
- Aulton, M.E. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines; Aulton, M.E., Taylor, K.M.G., Eds.; Churchill Livingstone: London, UK, 2007. [Google Scholar]
- Troy, D.A.; Remington, J.P.; Beringer, P. Remington: The Science and Practice of Pharmacy. In University of the Sciences in Philadelphia, 21st ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 886–887. [Google Scholar]
- Ichihashi, M.; Ueda, M.; Budiyanto, A. UV-induced skin damage. Toxicology 2003, 189, 21–39. [Google Scholar] [CrossRef]
- Mukhtar, H.; Elmets, C.A. Photocarcinogenesis: Mechanisms, models and human health implications. Photochem. Photobiol. 1996, 63, 355–447. [Google Scholar]
- Alic, N.; Partridge, L. Death and dessert: Nutrient signalling pathways and ageing. Curr. Opin. Cell Biol. 2011, 23, 738–743. [Google Scholar] [PubMed]
- Kenyon, C.J. The genetics of ageing. Nature 2010, 464, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Haigis, M.C.; Yankner, B.A. The aging stress response. Mol. Cell 2010, 40, 333–344. [Google Scholar] [PubMed]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span-from yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, F.; Mitts, T.F.; Liu, K.; Wang, Y.; Hinek, A. Ellagic and tannic acids protect newly synthesized elastic fibers from premature enzymatic degradation in dermal fibroblast cultures. J. Invest. Dermatol. 2006, 126, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Masson, C.; Menaa, F.; Pinon-Lataillade, G.; Frobert, Y.; Chevillard, S.; Radicella, J.P.; Sarasin, A.; Angulo, J.F. Global genome repair is required to activate KIN17, a UVC-responsive gene involved in DNA replication. Proc. Natl. Acad. Sci. USA 2003, 100, 616–621. [Google Scholar] [PubMed]
- Masson, C.; Menaa, F.; Pinon-Lataillade, G.; Frobert, Y.; Radicella, J.P.; Angulo, J.F. Identification of KIN (KIN17), a human gene encoding a nuclear DNA-binding protein, as a novel component of the TP53-independent response to ionizing radiation. Radiat. Res. 2001, 156, 535–544. [Google Scholar] [CrossRef]
- Dong, Y.; Guha, S.; Sun, X.; Cao, M.; Wang, X.; Zou, S. Nutraceutical interventions for promoting healthy aging in invertebrate models. Oxid. Med. Cell Longev. 2012, 2012, 718491. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Parveen, R.; Khan, B.A.; Jamshaid, M.; Khan, H.M.S. Development of skin-friendly dermatological water-in-oil emulsion of pomegranate juice. Proc. Pakistan Acad. Sci. 2012, 49, 269–278. [Google Scholar]
- Akhtar, N.; Mahmood, T.; Khan, B.A.; Khan, H.M.S.; Saeed, T. Depigmenting and anti-erythematic effects of 3% green tea emulsion. HealthMED 2011, 5, 1165–1169. [Google Scholar]
- Peres, P.S.; Terra, V.A.; Guarnier, F.A.; Cecchini, R.; Cecchini, A.L. Photoaging and chronological aging profile: Understanding oxidation of the skin. J. Photochem. Photobiol. B 2011, 103, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Anthony, V.R.; Ian, R.S.; Clive, R.H.; Paul, A.B. Stratum Corneum Moisturization at the Molecular Level. J. Investig. Dermatol. 1994, 103, 731–740. [Google Scholar]
- Korać, R.R.; Khambholja, K.M. Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn Rev. 2011, 5, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Kottner, J.; Schario, M.; Bartels, N.G.; Pantchechnikova, E.; Hillmann, K.; Blume-Peytavi, U. Comparison of two in vivo measurements for skin surface topography. Skin Res. Technol. 2013, 19, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermato-Endocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Danish, M.; Singh, P.; Mishra, G.; Srivastava, S.; Jha, K.K.; Khosa, R.L. Cassia Fistula Linn. (Amulthus)—An Important Medicinal Plant: A Review of Its Traditional Uses, Phytochemistry and Pharmacological Properties. J. Nat. Prod. Plant Resour. 2011, 1, 101–118. [Google Scholar]
- Bahorun, T.; Neergheen, V.S.; Aruoma, O.I. Phytochemical constituents of Cassia fistula. Afr. J. Biotechnol. 2005, 4, 1530–1540. [Google Scholar] [CrossRef]
- Sebastian, P. Ayurvedic Medicine: The Principles of Traditional Practice; Singing Dragon: London, UK, 2012. [Google Scholar]
- Manonmani, G.; Bhavapriya, V.; Kalpana, S.; Govindasamy, S.; Apparanantham, T. Antioxidant activity of Cassia fistula (Linn.) flowers in alloxan induced diabetic rats. J. Ethnopharmacol. 2005, 97, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Bhalodia, N.R.; Nariya, P.B.; Acharya, R.N.; Shukla, V.J. In vitro antioxidant activity of hydro alcoholic extract from the fruit pulp of Cassia fistula Linn. Ayu 2013, 34, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.O. Macro- and micro-emulsions: Theory and applications. In Proceedings of the 186th Meeting of the American Chemical Society, Washington, DC, USA, 28 August–2 September 1983.
- Chanchal, D.; Swarnlata, S. Novel approaches in herbal cosmetics. J. Cosmet. Dermatol. 2008, 7, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Zoe, D. The cosmeceutical realm. Clin. Dermatol. 2008, 26, 627–632. [Google Scholar]
- Zoe, D. The latest cosmeceutical approaches for anti-aging. J. Cosmet. Dermatol. 2007, 6, 2–6. [Google Scholar]
- Ozgen, O. Anti-Aging Cosmeceutics for Facial Skin Care in Aging. Turk. J. Med. Sci. 2009, 29, 40–43. [Google Scholar]
- Rasul, A.; Akhtar, N. Formulation and in vivo evaluation for anti-aging effects of an emulsion containing basil extract using non-invasive biophysical techniques. DARU 2011, 19, 344–350. [Google Scholar] [PubMed]
- Song, J.H.; Bae, E.Y.; Choi, G.; Hyun, J.W.; Lee, M.Y.; Lee, H.W.; Chae, S. Protective effect of mango (Mangifera indica L.) against UVB-induced skin aging in hairless mice. Photodermatol. Photoimmunol. Photomed. 2013, 29, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Ahshawat, M.S.; Saraf, S.; Saraf, S. Preparation and characterization of herbal creams for improvement of skin viscoelastic properties. Int. J. Cosmet. Sci. 2008, 30, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Menaa, F. When Pharma Meets Nano or the Emerging Era of Nano-Pharmaceuticals. Pharmaceut. Anal. Acta 2013, 4. [Google Scholar] [CrossRef]
- Chou, S.T.; Chang, W.L.; Chang, C.T.; Hsu, S.L.; Lin, Y.C.; Shih, Y. Cinnamomum cassia essential oil inhibits α-MSH-induced melanin production and oxidative stress in murine B16 melanoma cells. Int. J. Mol. Sci. 2013, 14, 19186–19201. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Yoon, T.; Jang, J.Y.; Park, S.J.; Jeong, G.H.; Kim, H.K. Inhibitory effects of Cinnamomum cassia extract on atopic dermatitis-like skin lesions induced by mite antigen in NC/Nga mice. J. Ethnopharmacol. 2011, 133, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Singhal, M.; Kansara, N. Cassia tora linn cream inhibits ultraviolet-B-induced psoriasis in rats. ISRN Dermatol. 2012, 2012, 346510. [Google Scholar] [CrossRef] [PubMed]
- Babitha, S.; Shin, J.H.; Nguyen, D.H.; Park, S.J.; Reyes, G.A.; Caburian, A.; Kim, E.K. A stimulatory effect of Cassia occidentalis on melanoblast differentiation and migration. Arch. Dermatol. Res. 2011, 303, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Koyama, J.; Morita, I.; Tagahara, K.; Nobukuni, Y.; Mukainaka, T.; Kuchide, M.; Tokuda, H.; Nishino, H. Chemopreventive effects of emodin and cassiamin B in mouse skin carcinogenesis. Cancer Lett. 2002, 182, 135–139. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions, Principles, Practices and Techniques, 2nd ed.; McClements, D.J., Ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Waqas, M.K.; Akhtar, N.; Ahmad, M.; Murtaza, G.; Khan, H.M.S.; Iqbal, M.; Rasul, A.; Bhatti, N.S. Formulation and characterization of a cream containing extract of fenugreek seeds. Acta Pol. Pharm. Drug Res. 2010, 67, 173–178. [Google Scholar]
- Akhtar, R.N.; Akhtar, B.A.; Khan, T.M.; Uz Zaman, S.; Shoaib Khan, H.M. Formulation development of a cream containing fennel extract: In vivo evaluation for anti-aging effects. Int. J. Pharm. Sci. 2012, 67, 54–58. [Google Scholar]
- Heinrich, U.; Tronnier, H.; Stahl, W.; Béjot, M.; Maurette, J.M. Antioxidant supplements improve parameters related to skin structure in humans. Skin Pharmacol. Physiol. 2006, 19, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Sakamoto, W.; Odanaka, W.; Yoshida, K.; Urishibata, O. Clinical effects of dietary hyaluronic acid on dry, rough skin. Aesthetic. Dermatol. 2002, 12, 109–120. [Google Scholar]
- Menaa, F.; Menaa, B.; Sharts, O. Development of carbon-fluorine spectroscopy for pharmaceutical and biomedical applications. Faraday Discuss. 2011, 149, 269–278. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, B.A.; Akhtar, N.; Menaa, A.; Menaa, F. A Novel Cassia fistula (L.)-Based Emulsion Elicits Skin Anti-Aging Benefits in Humans. Cosmetics 2015, 2, 368-383. https://doi.org/10.3390/cosmetics2040368
Khan BA, Akhtar N, Menaa A, Menaa F. A Novel Cassia fistula (L.)-Based Emulsion Elicits Skin Anti-Aging Benefits in Humans. Cosmetics. 2015; 2(4):368-383. https://doi.org/10.3390/cosmetics2040368
Chicago/Turabian StyleKhan, Barkat Ali, Naveed Akhtar, Abder Menaa, and Farid Menaa. 2015. "A Novel Cassia fistula (L.)-Based Emulsion Elicits Skin Anti-Aging Benefits in Humans" Cosmetics 2, no. 4: 368-383. https://doi.org/10.3390/cosmetics2040368
APA StyleKhan, B. A., Akhtar, N., Menaa, A., & Menaa, F. (2015). A Novel Cassia fistula (L.)-Based Emulsion Elicits Skin Anti-Aging Benefits in Humans. Cosmetics, 2(4), 368-383. https://doi.org/10.3390/cosmetics2040368