In Vitro DVS Approach to Evaluate Skin Reparation
Abstract
:1. Introduction
2. Methodology
2.1. Chemicals
2.2. Lipid Formulations
2.3. Isolation of SC
2.4. Lipid Extraction of SC
2.5. SC Treatments
2.6. Sorption Experiments
- Pre stabilization: temperature 25 °C, 0% relative humidity (RH) overnight. The sample remains in this step until its mass reaches equilibrium (arbitrarily defined by a change in mass of less than 0.02% per minute for 20 min).
- Absorption curve: initial absorption kinetics at 5% RH, then the sample previously stabilized at 5% RH is subjected to absorption tests, progressively increasing in steps from 30% up to 95%, and the sample being stabilized at 95% RH after the last step. The sample remains in each step until its mass reaches equilibrium (arbitrarily defined by a change in mass of less than 0.02% per minute for 20 min).
- Desorption curve: the sample that was stabilized at 95% RH after the absorption process kinetics is subjected to desorption tests progressively decreasing in steps from 30% down to 5%, with the sample stabilized at 5% RH after the last step. The sample remains in each stage until its mass reaches equilibrium (arbitrarily defined by a change in mass of less than 0.02% per minute for 20 min).
2.7. Freeze-Substitution Transmission Electron Microscopy Experiments (FSTEM)
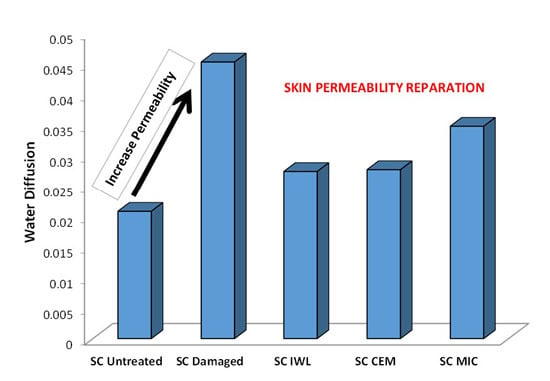
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jungersted, J.M.; Hellgren, L.I.; Jemec, G.B.; Agner, T. Lipids and skin barrier function—A clinical perspective. Contact Dermat. 2008, 58, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Wartewig, S.; Neubert, R.H. Properties of ceramides and their impact on the stratum corneum structure: A review. Part 1: Ceramides. Skin Pharmacol. Physiol. 2007, 20, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Coderch, L.; Lopez, O.; de la Maza, A.; Parra, J.L. Ceramides and skin function. Am. J. Clin. Dermatol. 2003, 4, 107–129. [Google Scholar] [CrossRef] [PubMed]
- Farwanah, H.; Raith, K.; Neubert, R.H.; Wohlrab, J. Ceramide profiles of the uninvolved skin in atopic dermatitis and psoriasis are comparable to those of healthy skin. Arch. Dermatol. Res. 2005, 296, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Scürer, N.Y. The biochemistry and function of stratum corneum lipids. Adv. Lipid Res. 1991, 24, 27–56. [Google Scholar]
- Elias, P.M. Lipids and the epidermal permeability barrier. Arch. Dermatol. Res. 1981, 270, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Friegber, S.; Kayali, I.; Rhein, L.; Simion, F.A.; Cagan, R.H. The importance of lipids for water uptake in stratum corneum. Biochim. Biophys. Acta 1987, 917, 108–111. [Google Scholar]
- Landmann, L. The epidermal permeability barrier. Anat. Embryol. 1988, 178, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Steven, A.C.; Steiner, P.M. Protein composition of cornified cell envelopes of epidermal keratinocytes. J. Cell Sci. 1994, 107, 693–700. [Google Scholar] [PubMed]
- Groen, D.; Poole, D.S.; Gooris, G.S.; Bouwstra, J.A. Is an orthorhombic lateral packing and a proper lamellar organization important for the skin barrier function? Biochim. Biophys. Acta 2011, 1808, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Janůšová, B.; Zbytovská, J.; Lorenc, P.; Vavrysová, H.; Palát, K.; Hrabálek, A.; Vávrová, K. Effect of ceramide acyl chain length on skin permeability and thermotropic phase behavior of model stratum corneum lipid membranes. Biochim. Biophys. Acta 2011, 1811, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Mojumdar, E.H.; Kariman, Z.; van Kerckhove, L.; Gooris, G.S.; Bouwstra, J.A. The role of ceramide chain length distribution on the barrier properties of the skin lipid membranes. Biochim. Biophys. Acta 2014, 1838, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Sahle, F.F.; Gebre-Mariam, T.; Dobner, B.; Wohlrab, J.; Neubert, R.H. Skin Diseases Associated with the Depletion of Stratum Corneum Lipids and Stratum Corneum Lipid Substitution Therapy. Skin Pharmacol. Physiol. 2015, 28, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Lew, B.L.; Cho, Y.; Kim, J.; Sim, W.Y.; Kim, N.I. Ceramides and cell signaling molecules in psoriatic epidermis: reduced levels of ceramides, PKC-α, and JNK. J. Korean Med. Sci. 2006, 21, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Farwanah, H.; Neubert, R.; Zellmer, S.; Raith, K. Improved procedure for the separation of major stratum corneum lipids by means of automated multiple development thin-layer chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 780, 443–450. [Google Scholar] [CrossRef]
- Shikawa, J.; Narita, H.; Kondo, N.; Hotta, M.; Takagi, Y.; Masukawa, Y.; Kitahara, T.; Takema, Y.; Koyano, S.; Yamazaki, S.; et al. Changes in the ceramide profile of atopic dermatitis patients. J. Investig. Dermatol. 2010, 130, 2511–2514. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Lew, B.L.; Seong, K.; Kim, N.I. An inverse relationship between ceramide synthesis and clinical severity in patients with psoriasis. J. Korean Med. Sci. 2004, 19, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.; Watkinson, A.; Rogers, J.; Mayo, A.M.; Hope, J.; Scott, I.R. Abnormalities in stratum corneum structure, lipid composition, and desmosome degradation in soap-induced winter xerosis. J. Soc. Cosmet. Chem. 1994, 45, 203–220. [Google Scholar]
- Rogers, J.; Harding, C.; Mayo, A.; Banks, J.; Rawlings, A. Stratum corneum lipids: The effect of ageing and the seasons. Arch. Dermatol. Res. 1996, 288, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Macheleidt, O.; Kaiser, H.W.; Sandhoff, K. Deficiency of epidermal protein-bound ω-hydroxyceramides in atopic dermatitis. J. Investig. Dermatol. 2002, 119, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Denda, M.; Koyama, J.; Hori, J.; Horii, I.; Takahashi, M.; Hara, M.; Tagari, H. Age- and sex-dependent change in stratum corneum sphingolipids. Arch. Dermatol. Res. 1993, 285, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Abe, A.; Jin, K.; Higaki, Y.; Kawashima, M.; Hidano, A. Decreased level of ceramides in stratum corneum of atopic dermatitis: An etiologic factor in atopic dry skin? J. Investig. Dermatol. 1991, 96, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Man, M.M.; Feingold, K.R.; Thornfeldt, C.R.; Elias, P.M. Optimization of physiological lipid mixtures for barrier repair. J. Investig. Dermatol. 1996, 106, 1096–1101. [Google Scholar]
- Yang, L.; Mao-Qiang, M.; Taljebini, M.; Elias, P.M.; Feingold, K.R. Topical stratum corneum lipids accelerate barrier repair after tape stripping, solvent treatment and some but not all types of detergent treatment. Br. J. Dermatol. 1995, 133, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Coderch, L.; de Pera, M.; Fonollosa, J.; de La Maza, A.; Parra, J.L. Efficacy of stratum corneum lipid supplementation on human skin. Contact Dermat. 2002, 47, 139–146. [Google Scholar] [CrossRef]
- Ramirez, R.; Marti, M.; Barba, C.; Mendez, S.; Parra, J.L.; Coderch, L. Skin efficacy of liposomes composed of internal wool lipids rich in ceramides. J. Cosmet. Sci. 2010, 61, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Sugarman, J.L.; Parish, L.C. Efficacy of a lipid-based barrier repair formulation in moderate-to-severe pediatric atopic dermatitis. J. Drugs Dermatol. 2009, 8, 1106–1111. [Google Scholar] [PubMed]
- Draelos, Z.D. The effect of ceramide-containing skin care products on eczema resolution duration. Cutis 2008, 81, 87–91. [Google Scholar] [PubMed]
- Mojumdar, E.H.; Groen, D.; Gooris, G.S.; Barlow, D.J.; Lawrence, M.J.; Deme, B.; Bouwstra, J.A. Localization of cholesterol and fatty acid in a model lipid membrane: A neutron diffraction approach. Biophys. J. 2013, 105, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Jennemann, R.; Rabionet, M.; Gorgas, K.; Epstein, S.; Dalpke, A.; Rothermel, U.; Bayerle, A.; van der Hoeven, F.; Imgrund, S.; Kirsch, J.; et al. Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum. Mol. Genet. 2012, 21, 586–608. [Google Scholar] [CrossRef] [PubMed]
- Mojumdar, E.H.; Helder, R.W.J.; Gooris, G.S.; Bouwstra, J.A. Monounsaturated Fatty Acids Reduce the Barrier of Stratum Corneum Lipid Membranes by Enhancing the Formation of a Hexagonal Lateral Packing. Langmuir 2014, 30, 6534–6543. [Google Scholar] [CrossRef] [PubMed]
- Potts, R.O.; Francoeur, M.L. The influence of stratum corneum morphology on water permeability. J. Investig. Dermatol. 1991, 96, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Gournay, A.; Navarro, R.; Mathieu, J.; Riviere, M. Water retention of treated stratum corneum measured by a coupling method: thermal desorption-mass spectrometry. Int. J. Cosmet. Sci. 1995, 17, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Wortmann, F.; Hullmann, A.; Popescu, C. Water management of human hair. IFSCC Mag. 2007, 10, 317–320. [Google Scholar] [CrossRef]
- Manich, A.M.; Maldonado, F.; Carilla, J.; Catalina, M.; Marsal, A. Moisture Adsorption/Desorption Kinetics of Bovine Hide Powder. J. Soc. Leather Technol. Chem. 2010, 94, 15–20. [Google Scholar]
- Ramirez, R.; Marti, M.; Manich, A.; Parra, J.L.; Coderch, L. Ceramides Extracted from Wool: Pilot Plant Solvent Extraction. Text. Res. J. 2008, 78, 73–80. [Google Scholar] [CrossRef]
- Semenzato, A.; Caliceti, P.; Francescato, S.; Cortese, A.C.; Baratto, G. Epidermal lipid microparticles: A new ingredient for skin care products. In Proceedings of the 26th IFSCC Congress, Buenos Aire, Argentina, 20–23 September 2010.
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Lopez, O.; Cocera, M.; Wertz, P.M.; Lopez-Iglesias, C.; de la Maza, A. New arrangement of proteins and lipids in the stratum corneum cornified envelope. Biochim. Biophys. Acta 2007, 1768, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Barba, C.; Martí, M.; Semenzato, A.; Baratto, G.; Manich, A.M.; Coderch, L. Effect of lipid modification on stratum corneum permeability. J. Therm Anal. Calor. 2014. [Google Scholar] [CrossRef]
- Anderson, R.B.; Hall, W.K. Modifications of the Brunauer, Emmett and Teller Equation II1. J. Am. Chem. Soc. 1948, 70, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Arslan, N.; Togrul, H. The fitting of various models to water sorption isotherms of tea stored in a chamber under controlled temperature and humidity. J. Stored Prod. Res. 2006, 42, 112–135. [Google Scholar] [CrossRef]
- Vickerstaff, T. The Physical Chemistry of Dyeing; Oliver and Boid: London, UK, 1924. [Google Scholar]
- López, O.; Cócera, M.; López-Iglesias, C.; Walter, P.; Coderch, L.; Parra, J.L.; de la Maza, A. Reconstitution of Liposomes inside the Intercellular Lipid Domain of the Stratum Corneum. Langmuir 2002, 18, 7002–7008. [Google Scholar] [CrossRef]
- Van den Bergh, B.A.; Bouwstra, J.A.; Junginger, H.E.; Wertz, P.W. Elasticity of vesicles affects hairless mouse skin structure and permeability. J. Control. Release 1999, 62, 367–379. [Google Scholar] [CrossRef]
- Tonon, R.; Baroni, A.; Brabet, C.; Guibert, O.; Pallet, D.; Hubinger, M. Effect of stirring on osmotic dehydration of yellow pitahaya (Selenicereus megalanthus s.). J. Food Eng. 2009, 34, 492–496. [Google Scholar]
- Jonquières, A.; Fane, A. Modified BET models for modeling water vapor sorption in hydrophilic glassy polymers and systems deviating strongly from ideality. J. Appl. Polym. Sci. 1998, 67, 1415–1430. [Google Scholar] [CrossRef]
- Zografi, G.; Kontny, M.J.; Yang, A.Y.S.; Brenner, G.S. Surface area and water vapor sorption of macrocrystalline cellulose. Int. J. Pharm. 1984, 18, 99–116. [Google Scholar] [CrossRef]
- Lewicki, P.P. The applicability of the GAB model to food water sorption isotherms. Int. J. Food Sci. Technol. 1997, 32, 553–537. [Google Scholar] [CrossRef]
- Monleon, M.; Salmeron, M.; Gallego, G.; Gomez, J.L. Thermodynamics and statistical mechanics of multilayer adsorption. J. Chem. Phys. 2004, 121, 8524–8531. [Google Scholar] [CrossRef] [PubMed]
- Barba, C.; Martí, M.; Manich, A.M.; Carilla, J.; Parra, J.L.; Coderch, L. Water absorption/desorption of human hair and nails. Thermochim. Acta 2010, 503–504, 33–39. [Google Scholar] [CrossRef]
- Primavera, G.; Berardesca, E. Dynamic Measurements: The plastic occlusion stress test, moisture accumulation test, and sorption-desorption test. In Bioengineering of the Skin; Dermatology: Clinical and Basic Sciences Series; CRC Press: Boca Raton, FL, USA, 2005; Chapter 19; pp. 237–245. [Google Scholar]
- Pilgram, G.S.; Vissers, D.C.; van der Meulen, H.; Pavel, S.; Lavrijsen, S.P.; Bouwstra, J.A.; Koerten, H.K. Aberrant lipid organization in stratum corneum of patients with atopic dermatitis and lamellar ichthyosis. J. Investig. Dermatol. 2001, 117, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Lopez, O.; Walther, P.; Cocera, M.; de La Maza, A.; Coderch, L.; Parra, J.L. Structural modifications in the stratum corneum by effect of different solubilizing agents: a study based on high-resolution low-temperature scanning electron microscopy. Skin Pharmacol. Appl. Skin Physiol. 2000, 13, 265–272. [Google Scholar] [PubMed]
- Hofland, H.E.; Bouwstra, J.A.; Bodde, H.E.; Spies, F.; Junginger, H.E. Interactions between liposomes and human stratum corneum in vitro: Freeze fracture electron microscopical visualization and small angle X-ray scattering studies. Br. J. Dermatol. 1995, 132, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Hofland, H.E.J.; Bouwstra, J.A.; Spies, F.; Boddé, F.E.; Nagelkerke, F.J.; Cullander, C.; Junginger, H.E. Interactions between non-ionic surfactant vesicles and human stratum corneum in vitro. J. Liposome Res. 1995, 5, 241–263. [Google Scholar] [CrossRef]
- Van den Bergh, B.A.I.; Salomons-de Vries, I.; Bouwstra, J.A. Interactions between liposomes and human stratum corneum studied by freeze-substitution electron microscopy. Int. J. Pharm. 1998, 167, 57–67. [Google Scholar] [CrossRef]
- López, O.; Cócera, M.; Walther, P.; Wehrli, E.; Coderch, L.; Parra, J.L.; de la Maza, A. Effect of liposomes on delipidized stratum corneum structure: An in vitro study based on high resolution low temperature scanning electron microscopy. Colloids Surf. A Physicochem. Eng. Asp. 2001, 182, 35–42. [Google Scholar] [CrossRef]
- Berardesca, E.; Barbareschi, M.; Veraldi, S.; Pimpinelli, N. Evaluation of efficacy of a skin lipid mixture in patients with irritant contact dermatitis, allergic contact dermatitis or atopic dermatitis: A multicenter study. Contact Dermat. 2001, 45, 280–285. [Google Scholar] [CrossRef]
- Kircik, L.H.; del Rosso, J.Q.; Aversa, D. Evaluating Clinical Use of a Ceramide-Dominant, Physiologic Lipid-Based Topical Emulsion for Atopic Dermatitis. J. Clin. Aesthet Dermatol. 2011, 4, 34–40. [Google Scholar] [PubMed]
- Na, J.I.; Hwang, J.S.; Park, H.J.; Kim, D.H.; Park, W.S.; Youn, S.W.; Huh, C.H.; Park, K.C. A new moisturizer containing physiologic lipid granules alleviates atopic dermatitis. J. Dermatol. Treat. 2010, 21, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Man, M.Q.; Feingold, K.R.; Elias, P.M. Exogenous lipids influence permeability barrier recovery in acetone-treated murine skin. Arch. Dermatol. 1993, 129, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Zettersten, E.M.; Ghadially, R.; Feingold, K.R.; Crumrine, D.; Elias, P.M. Optimal ratios of topical stratum corneum lipids improve barrier recovery in chronologically aged skin. J. Am. Acad. Dermatol. 1997, 37, 403–408. [Google Scholar] [CrossRef]
- Barba, C.; Parra, J.L.; Coderch, L.; Semenzato, A. In vivo and in vitro evaluation of topical formulations containign physiological lipid mixture for replacement of skin barrier function. G Ital. Venerol. 2014, 149, 347–353. [Google Scholar]






| Model | Mathematical Equation |
| GAB [ 43] | W = Wm · Cg · K · aw/[(1 − Kaw + Cg · K · aw)] |
| Parameter | Definition |
| aw | Water activity expressed as the relative vapor pressure, p/p0, where p0 is the saturated vapor pressure. |
| W | Equilibrium moisture content at aw in g sorbed/100 g of sorbent on a dry basis. |
| Wm | Monolayer moisture content in g sorbed/100 g of sorbent on a dry basis (d.b.) |
| Cg | Energy constant related to the difference between the free enthalpies of the water molecules in the pure liquid state and in the monolayer. This constant is proportional to the rate between both the attachment and the escape rate constants of the primary sites. |
| K | Ratio between of the standard vapor pressure of the liquid and the vapor pressure of the sorbate in the secondary (upper) layers. This ratio is proportional to the rate between the attachment and the escape rate constant for all higher layers. |
| SC Samples | Regain at 95%RH (%) | Wm | Cg | K | R2 | tT (min) | DA (min−1) | DAd (min−1) |
|---|---|---|---|---|---|---|---|---|
| NT SC | 37.88 | 0.048 | 3.581 | 0.902 | 0.999 | 3780.42 | 0.0214 ± 0.015 | 0.0208 ± 0.017 |
| LE SC | 29.12 | 0.073 | 3.937 | 0.741 | 0.999 | 2260.75 | 0.0396 ± 0.024 | 0.0453 ± 0.032 |
| LE SC IWL | 28.83 | 0.060 | 4.639 | 0.809 | 0.999 | 3480.82 | 0.0257 ± 0.017 | 0.0274 ± 0.012 |
| LE SC CEM | 29.16 | 0.062 | 3.972 | 0.801 | 0.999 | 3500.79 | 0.0250 ± 0.014 | 0.0277 ± 0.013 |
| LE SC MIC | 29.10 | 0.055 | 5.009 | 0.829 | 0.999 | 3160.78 | 0.0361 ± 0.021 | 0.0348 ± 0.014 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barba, C.; Alonso, C.; Semenzato, A.; Baratto, G.; Coderch, L. In Vitro DVS Approach to Evaluate Skin Reparation. Cosmetics 2016, 3, 15. https://doi.org/10.3390/cosmetics3020015
Barba C, Alonso C, Semenzato A, Baratto G, Coderch L. In Vitro DVS Approach to Evaluate Skin Reparation. Cosmetics. 2016; 3(2):15. https://doi.org/10.3390/cosmetics3020015
Chicago/Turabian StyleBarba, Clara, Cristina Alonso, Alessandra Semenzato, Giovanni Baratto, and Luisa Coderch. 2016. "In Vitro DVS Approach to Evaluate Skin Reparation" Cosmetics 3, no. 2: 15. https://doi.org/10.3390/cosmetics3020015
APA StyleBarba, C., Alonso, C., Semenzato, A., Baratto, G., & Coderch, L. (2016). In Vitro DVS Approach to Evaluate Skin Reparation. Cosmetics, 3(2), 15. https://doi.org/10.3390/cosmetics3020015