Abstract
Depigmentation and skin lightening products, which have been in use for ages in Asian countries where skin whiteness is a major esthetic criterion, are now also highly valued by Western populations, who expose themselves excessively to the sun and develop skin spots as a consequence. After discussing the various possible mechanisms of depigmentation, the different molecules that can be used as well as the status of the products containing them will now be presented. Hydroquinone and derivatives thereof, retinoids, alpha- and beta-hydroxy acids, ascorbic acid, divalent ion chelators, kojic acid, azelaic acid, as well as diverse herbal extracts are described in terms of their efficacy and safety. Since a genuine effect (without toxic effects) is difficult to obtain, prevention by using sunscreen products is always preferable.
1. Introduction
The allure of a pale complexion is nothing new and many doctors have been looking into this subject for some time, proposing diverse and varied recipes for eliminating all unsightly marks (freckles and liver spots were clearly targeted). Pliny the Elder (Naturalis Historia), Dioscoride (De Universa medicina), Castore Durante (Herbario nuove), and other authors from other time periods have addressed this issue. In the 16th century, Durante proposed diverse plant-based preparations: herbal teas prepared from Erythreae centaurium, Chamaeleon root, powdered Gentiana verna root mixed with honey, lemon juice, to name a few [1].
In sub-Saharan Africa where the practice is common, herbal teas are still being prepared from local plants (Tephrosia vogelii, Mirabilis jalapa, Phytolacca dodecandra). In Rwanda, about thirty plants are traditionally used to lighten the complexion of girls for certain ceremonies, such as marriage, for instance. The use of preparations to lighten skin tone is referred to as “voluntary depigmentation”. Depending on the African country in question, the procedure may be called “xessal” (Senegal), “tcha-tcho” (Mali), “ambi” (Gabon), “maquillage” (Congo), “kwitukuza”, which literally means “making one’s skin red” (Rwanda) [2]. Illegal cosmetics or preparations, in other words ones not regulated by any drug laws or cosmetics laws, may be used to achieve the desired results. Steroids, hydroquinone and its derivatives, kojic acid, and mercury derivatives are the most commonly used active ingredients. They are far from being harmless. Undesirable effects (hypercorticism) may be observed in subjects using dermocorticoids. Applying high-dosage preparations on large areas of skin can lead to an overall change in the state of health (skin atrophy, arterial hypertension, osteoporosis, diabetes, etc.) of the patient or of a family member; children may become poisoned by proxy [3]. Similar cases can also be seen with mercury. Anorexia, emaciation, arterial hypertension, hyperhidrosis, or intense pruritus manifesting in a child whose mother uses depigmenting preparations should alert the attending doctor [4]. It is estimated that 25% to 67% of urban-dwelling women in Africa and 20% of women in metropolitan France are affected by this practice [5].
Nor are Asian women immune to the allure of a pale complexion. A study sponsored by Chanel and conducted on around forty Chinese women in 2014 revealed that the “purity of the complexion” had an influence on the presumed age of a person. The Chinese women on the panel saw the lightening of skin spots with photograph retouching software as a means of giving the impression that one is four years younger than one’s actual age, versus just two years for wrinkle smoothing [6]. Considering these results, the interest of Japanese and Chinese women in depigmentation products becomes more understandable, as they are viewed as veritable anti-aging products.
The elite of Caucasian populations were preoccupied with the search for a pale complexion for centuries, until the discovery of tanning in the 1930s reversed the situation. However, overexposure to the sun still fuels the demand of Europeans for effective anti-dark spot preparations. Lastly, melasma, a type of skin hyperpigmentation linked to pregnancy, to thyroid disorders, and to certain drug treatments (contraceptives), is yet another reason for using skin lightening cosmetics [7].
2. Different Mechanisms of Action for Achieving a Depigmentation Effect
The different mechanisms of action of depigmentation agents are summarized in Table 1.

Table 1.
Different means of depigmentation.
A distinction should be made between cosmetics and medications. It is important to realize that in the pigment spot lightening field, there is a very distinct link between efficacy and irritating effect. A given active ingredient may be effective, but not necessarily non-toxic. It is necessary to bear in mind that for a good many molecules, efficacy is correlated with undesirable effects.
2.1. Skin Treatments
2.1.1. Hydroquinone and Its Derivatives
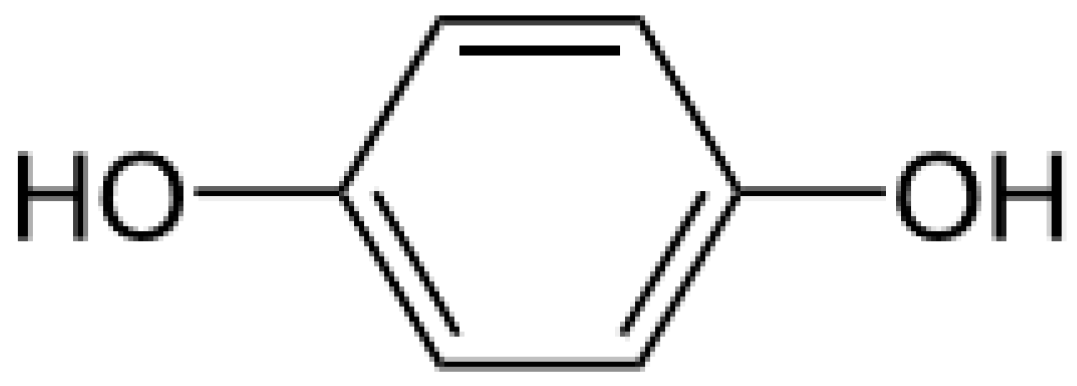
Hydroquinone (Figure 1) is a ubiquitous molecule. It is the oxidation product of certain aromatic compounds.
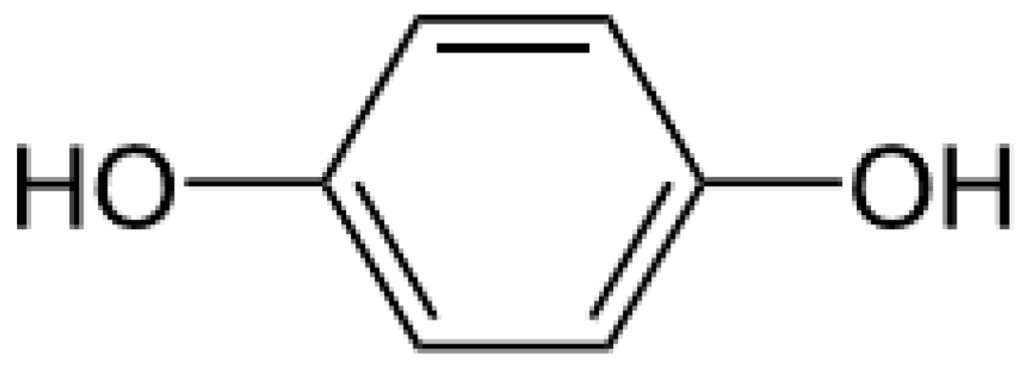
Figure 1.
Hydroquinone formula.
It is found in cigarette smoke, in diesel engine lubricants, etc. Frequently used in industry, this molecule constitutes an air pollutant that needs to be considered [8]. It has been used for depigmentation purposes since the 1960s. Its use is highly controversial. A steady increase in the percentages of hydroquinone incorporated in commercial preparations was noted between 1960 and 1966 [9].
As to its mode of action, various mechanisms have been suggested. The inhibition of tyrosinase (key enzyme responsible for melanin production) synthesis, the inhibitory effect on this enzyme, the destruction of melanocytes by the production of free radicals and interference with melanin-containing organelles (melanosomes) are instrumental in making this ingredient an effective depigmentation agent [10].
As for the toxicological profile of this molecule, it should be noted that its use in diverse industrial sectors (photograph development, anti-oxidant for protecting oils and fats, polymerization inhibitor in processes involving monomers such as vinyl acetate or acrylic monomers, paint stabilizers) is reflected in a certain number of deaths in the populations concerned [11]. A situational analysis focusing on a period of 48 years (1942–1990) demonstrates that this molecule is implicated in 879 deaths in the context of work-related illnesses [12]. With topical use it is known to cause ochronosis, and its metabolite has been implicated in bone marrow toxicity. At concentrations ranging from 2% to 5%, it can cause various undesirable effects such as irritative dermatitis, contact dermatitis, post-inflammatory pigmentation, ochronosis, and discoloration of the nails [13]. The observed cutaneous effects (ochronosis, colloid milium) have been known since the 1970s. The latency period between 1960 and 1970, during which nothing remarkable was reported, can be explained by the fact that these dermatoses are of gradual onset. Initially, the subject feels that the depigmentation treatment is satisfactory. Signs of skin blemishes do not appear until a few years later. In reaction to this situation, the user feels “compelled” to use “stronger and stronger” products. These so-called “extra strong” products most often comprise hydroquinone. The very same undesirable effects that one wished to avoid are thus intensified [14]. Findlay was one of the first to mention the appearance of papules on the cheekbones of African women who practice depigmentation. He compared the lesions that he observed to caviar [15]. Lastly, hydroquinone is suspected of having a carcinogenic effect. However, no mutagenic effect has been demonstrated in rats [16]. A competitive inhibitory effect on tyrosinase is a possible explanation of its action, but this has not been confirmed. Its cytotoxicity to melanocytes via the production of free radicals is undeniable [16]. Hydroquinone has been banned in cosmetic products for skin use in Europe since 01 March 2000. This does not stop certain companies from contravening the regulations and incorporating hydroquinone in topical preparations without marketing authorization. Depigmentation products are considered as over-the-counter (OTC) drugs in the United States. The dose “Generally Recognized As Safe and Effective” (GRASE) has been set between 1.5% and 2.0% since 1982. However, a large number of preparations containing levels of hydroquinone higher than these can still be found on the American market [17].
In 1975, Albert Kligman proposed a formula based on tretinoin (0.1%), hydroquinone (5%) and dexamethasone (0.1%) for treating melasma, freckles, and post-inflammatory hyperpigmentations [18]. Other combinations have also been proposed, namely the preparations of Pathak (mixture of hydroquinone ether and tretinoin) and Westerhof (combination of phenol and vitamin C) [19].
Inspired by these preparations, about a decade ago Galderma Laboratories introduced a Triple Combination Cream (TCC) on the market, under the trade name Tri-Luma® cream (Galderma laboratories, Fortworth, TX, USA). This emulsion, which contains 4% hydroquinone, 0.05% tretinoin, and 0.01% fluocinolone acetonide, is the only Food and Drug Administration (FDA)-approved melasma treatment. Upon request by Galderma Laboratories, dermatologist Valérie Callender conducted the first clinical tests in 2004. The original idea was to compare the efficacy of the TCC to that of adapalene in subjects with mild to moderate acne or in subjects suffering post-inflammatory hyperpigmentation aftereffects of acne. The results of the tests, which were conducted on a small group of just ten patients, were not published [20]. Subsequently, Valérie Callender stated that this combination of active principles was effective and safe to use. One year later, it seemed that the volunteers included in the study were satisfied with the results obtained. However, in some cases long-term treatment was needed in order to prevent recurrences [21]. Based on the preliminary results obtained, Susan Taylor, a dermatologist at St. Luke’s—Roosevelt Hospital in Philadelphia, and also at the request of Galderma Laboratories, demonstrated the value of the TCC for treating barber’s itch (pseudofolliculitis barbae) [22]. Then came one study after another leading to the conclusion that the TCC was effective [23,24,25]. The undesirable effects observed were deemed moderate and similar to those observed with the standard molecules (e.g., retinoic acid). To compete with the TCC, in 2011 Neocutis Laboratories proposed a combination of hydroquinone (4%) with a complex of 4 active ingredients (leucine, disodium glycerophosphate, phenyl ethyl resorcinol, and undecylenoyl phenylalanine). Since a combination of irritating molecules would logically be more irritating than hydroquinone used alone, one may well question the absence of declared undesirable effects. Only one case of ochronosis has apparently arisen to date. This undesirable effect was attributed to a misuse on the part of the patient, who followed neither the dosage nor the treatment period recommendations (she applied her cream twice a day, whereas a single application is recommended, and for one year, whereas the maximum treatment period is 8 to 12 weeks) [26]. However, it should be noted that there are currently no specialty drugs in France containing hydroquinone.
Given that hydroquinone is one of the most effective depigmentation molecules, a codrug was obtained by esterifying hydroquinone with azelaic acid. The aim is threefold: obtain a synergistic effect, increase cutaneous permeation, and increase the stability of the active ingredients by masking the most labile functions. As for cutaneous penetration, the gamble paid off in that the tests performed in vitro showed that the permeation of the codrug was two times greater than that obtained with the parent compounds alone [27].
Hydroquinone mono methyl ether (MEHQ), also known as 4-hydroxyanisole or mequinol, is less irritating than hydroquinone and is not cytotoxic to human melanocytes. The combination of mequinol (2%) with retinoic acid (0.01%) turns out to be synergistic. A clinical trial performed on a group of 595 subjects (486 women and 109 men) with solar lentigines was able to demonstrate the value of this combination [28]. Other tests performed in vivo confirmed these results. However, it should be noted that this combination, though effective, did not produce any fewer undesirable effects. Erythema, burning, tingling, desquamation, and pruritus may be observed [29]. A number of specialty drugs (Any® 8% (Ostwald, France), Clairodermyl® 5% or 10% (Paris, France), Leucodinine B® 10% (Suresnes, France)) contain this active principle.
2.1.2. Retinoic Acid or Tretinoin or Vitamin A Acid
Initially used in combination with hydroquinone as a penetration factor, its inherent efficacy soon became evident. Tretinoin has several action mechanisms. It directly intervenes in the melanogenesis process by inhibiting tyrosinase induction and dispersing the pigments in the keratinocytes on the one hand, and is capable of accelerating epidermal turnover on the other hand [30]. The undesirable effects observed are erythema and desquamation [29]. Vitamin A acid, a molecule extensively metabolized in vertebrates, is thought to be the active metabolite. Fatty acid esterification, oxidation, dehydrogenation, and beta-glucuronosylation reactions are induced. The teratogenic nature of retinoic acid has been clearly demonstrated [31]. For this reason, this molecule is banned in the cosmetics industry.
At the present time, the purpose of tretinoin-based medications is to treat acne. Only one specialty drug (Rétinova® cream (Dijon, France)) was indicated for treating UV-induced premature aging, and its marketing authorization was cancelled in September 2006.
2.2. The Cosmetology Approach
2.2.1. Active Ingredients Having an Indirect Action on Already-Formed Melanins
Various exfoliating agents are grouped in this category. It should be noted that a given product will be placed in the cosmetics sector (superficial peel) or the dermatology sector (superficial, medium, or deep peel), according to the substances used (Table 2).

Table 2.
The different categories of peels.
We should specify that a very superficial peel only causes destruction of the stratum corneum, without affecting the underlying layers; a superficial or epidermal peel results in destruction of part or all of the epidermis (down to the granular or basal layers, depending on the particular case); a medium (or papillary dermal) peel destroys the epidermis and all or part of the papillary dermis; lastly, a deep peel causes the destruction of the epidermis and the dermis, down to the reticular layer [32].
Cosmetic peels would therefore be the most superficial ones.
In terms of regulation, there are numerous legal gaps in this area. Other than trichloroacetic acid, no exfoliating agent is regulated.
Also rather inappropriately called fruit acids, the alpha hydroxy acids (AHA) have been in use for a long time for alleviating the effects of photoaging. The main ones are glycolic acid (present in sugarcane), lactic acid (found in fermented milk, honey), malic acid (from apples, quinces, mountain ashes), citric acid (from citrus fruits), tartaric acid (from grapes), and mandelic acid (from bitter almonds).
Depending on their application rates, these acids are used for different purposes. At low rates (<1%), they are used as pH adjusters. At higher concentrations (around 10%), they act as hydrating agents capable of retaining moisture in the stratum corneum; above 50%, the reduction of inter-corneocyte cohesion is reflected in a keratolytic effect of importance in the anti-aging field. The peeling effect makes it possible to smooth wrinkles. The most commonly used concentrations in cosmetics are around 5% to 10% [33,34]. However, it is possible to find preparations with an alpha hydroxy acid content of 30%. Such is the case, for example, with Clairial Peel® (Le Plessis-Pâté, France) gel from SVR Laboratories, which contains 20% glycolic acid, 10% citric acid, and 5% vitamin C.
Glycolic acid is definitely the most commonly used alpha hydroxy acid of all. Studies recently conducted in vitro seem to indicate that the influx of H+ ions into the basal layer keratinocytes induces an increase in the proliferation of cells of this category [35]. It should also be noted that the production of collagen, elastin, and mucopolysaccharides is stimulated [33]. Glycolic acid can be used for superficial peels. Based on the percentage of glycolic acid in the preparation, a distinction is made between peels that can be done at home (5% to 20% glycolic acid, pH = 4–6) and peels that require a dermatologist (50% to 70% glycolic acid, pH = 1–2). The highest concentrations induce an epidermolysis down to the basal layer of the epidermis.
Pyruvic acid can be used at a concentration of 40%–50% by a dermatologist to reach the granular layer of the epidermis [36].
When one looks at cosmetic peels, one also notes that these ingredients are not regulated and hence there is a regulatory gap. It is, therefore, necessary to rely on the wisdom of the manufacturer who, depending on the percentage that he chooses, will position his product either with a cosmetic status or as a medical device.
Because alpha hydroxy acid-based preparations can increase the sensitivity of the skin to UV radiation, we would reiterate that applying such preparations and then exposing oneself to the sun is totally contraindicated. This leads to an increased rate of erythema development and sunburn cell formation [37].
Salicylic acid, a beta hydroxy acid, can also be used for superficial peels. It should be noted that the percentages used in the cosmetics field are restricted by law because of their potential toxicity. When it is used for purposes other than preservation, it can be used up to a limit of 3% in the case of products that are to be rinsed off and used for facial hair, or 2% in the case of other products. It must be borne in mind that using preparations with high concentrations of salicylic acid can lead to acute poisonings (salicylism). This can occur after using 6% preparations on an area corresponding to 40% of the body surface. The literature reports 25 cases of poisonings (including 4 deaths) from 1966 to the present day. In terms of its mode of action, it is known that salicylic acid is capable of reducing keratinocyte proliferation and solubilizing the intercellular cement responsible for the cohesion of the stratum corneum cells. It is thus traditionally used in dermatology to treat diseases of the epidermis. Preparations containing salicylic levels ranging from 10% to 40% are used for this purpose [38].
The actual efficacy of preparations containing only low percentages of salicylic acid is questionable. It could be demonstrated in vivo that the keratolytic effect of salicylic acid was observable with a low percentage of use (2%—preparation with a pH of 7) for a minimum exposure time of 6 h [39]. This active ingredient was chosen for Anti-blemish solutions® gel (Estée Lauder, New York, NY, USA) and Depiderm® cream (Uriage, Neuilly sur Seine, France).
Dermatologists on the other hand use more concentrated (20% to 25%) preparations. They use 20% to 30% ethanol solutions or 50% salves such as the well-known Pâte d’Unna [40]. At these percentages, it is possible to reach the granular layer of the epidermis [36]. Rare cases of poisoning or salicylism have been reported [40]. Clinical tests conducted on around twenty Latin American women refute the efficacy of this type of peel. In women with melasma who were treated on half of their faces with 4% hydroquinone preparations (2 applications per day) with or without a 20% to 30% salicylic acid peel (1 peel every 2 weeks for an 8 week study period), no significant differences were observed between the two treated sides [41].
An element that must be taken into account is the incorporation of other ingredients in the formulation. Past studies have shown a synergistic effect when salicylic acid and propylene glycol are combined [42]. This moisturizer, which is found in a great many commercial formulations, helps improve the results obtained.
2.2.2. Active Ingredients Acting on the Melanin Formation Process
Ascorbic Acid and Its Derivatives
Ascorbic acid or vitamin C is readily degraded by oxidation, especially in aqueous media. For this reason, it is preferable to use more stable derivatives such as ascorbyl palmitate and magnesium-l-ascorbyl-2-phosphate (MAP) [43]. Vitamin C and its derivatives act as reducers and block the chain of oxidations transforming tyrosine into melanin at different points [19]. Furthermore, the interaction of ascorbic acid with copper, an essential cofactor in tyrosinase activity, explains the tyrosinase inhibitor effect observed in vitro. Although less effective than hydroquinone, ascorbic acid does not have the harmful effects of the latter [44]. This active ingredient is not used alone. It is always used in combination with another ingredient. Its use is not regulated and vitamin C is found in cosmetics in concentrations ranging from 4% to 20%.
Divalent Ion Chelators
Diethyl trioxopimelate and EDTA are metal ion chelators that can be used in the complexion lightening field. By chelating Cu2+ ions, these sequestering agents block the tyrosinase cofactor and thus interfere with the melanogenesis process. Thus far they have not undergone any objectively documented testing.
Retinol and Retinaldehyde
In the 1990s, retinol was widely used in anti-aging cosmetics. The concentration used generally ranged from 0.04% to 0.07% retinol equivalent [45]. In keratinocytes, retinol is oxidized to retinaldehyde and then to retinoic acid [46], a molecule that is banned in cosmetics in Europe. It should also be pointed out that studies conducted using radio-labeled molecules showed that 7% of the dose applied to the skin could be detected at the systemic level [47]. Pregnant women must therefore exercise caution when using depigmentation products containing ingredients of this kind. This point needs to be emphasized because pregnancy mask arising in pregnant women with dark complexions is a compelling reason for them to resort to depigmentation products. We feel that this measure is justified in spite of the fact that a consortium of industrialists including L’Oreal, Unilever, Johnson & Johnson, BASF, Henkel, DSM nutritional products, Beiersdorf and Shiseido showed in 2006 that the daily application of topical preparations (at a dose of 30,000 IU/day for 21 days) on an area equivalent to 1/6 of the body surface did not lead to a significant increase in endogenous plasma levels of vitamin A and its metabolites [48].
Furthermore, retinoids are known to induce the production of pro-inflammatory cytokines and are therefore irritating molecules [49]. Lastly, the “photomutagenicity” of retinol requires that certain precautions be taken in case of exposure to the sun [50]. A possible solution would be to combine retinol with a mixture of UV filters. Johnson & Johnson laboratories came up with this idea in 2008. It was thus possible to test a retinol-based cosmetic labeled with a Sun Protect Factor (SPF) of 30 on a group of 30 volunteers. Even though the results obtained in terms of reduction of photoaging signs may be positive [51], the message that this type of product conveys seems less than ideal. An optimum level of photo-protection will not be ensured for the entire day unless skin care cosmetics are applied at the rate of 2 mg/cm2 and re-applied every 2 h [52]. Skin care cosmetics displaying an SPF value pose the risk of clouding the public health message clearly indicating that a sunscreen product must be applied liberally and on a regular basis during the period of exposure.
Tyrosinase Inhibitors
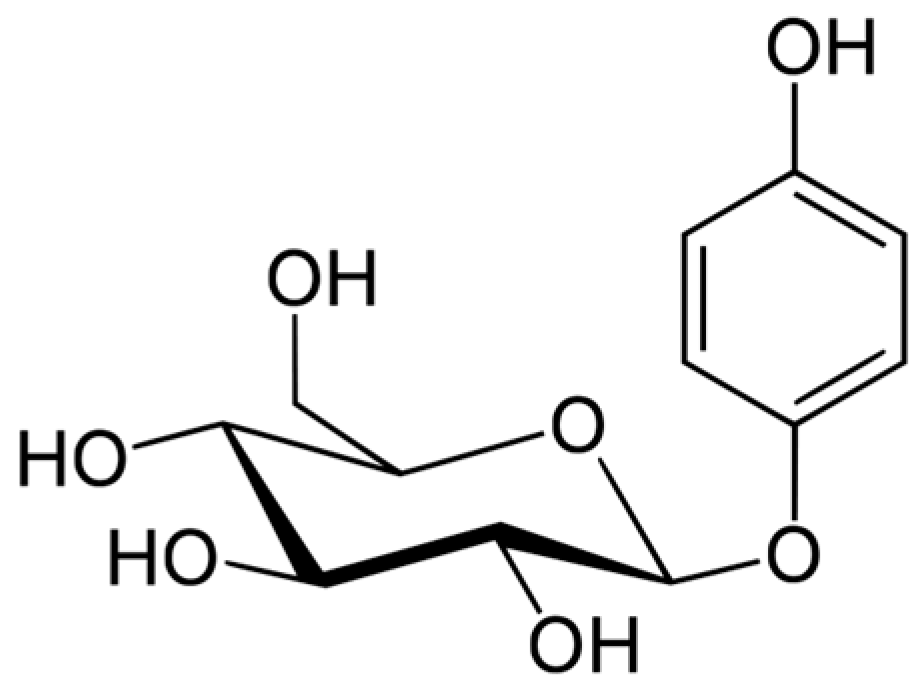
Arbutin (Figure 2) is a hydroquinone glycoside with 2 isoforms, 4-hydroxyphenyl-α-glucopyranoside and 4-hydroxyphenyl-β-glucopyranoside. It is obtained from various plants in the Ericaceae (bearberry, strawberry tree, huckleberry, heather), Saxifragaceae, Asteraceae, Rosaceae, Lamiaceae, and Apiaceae families.
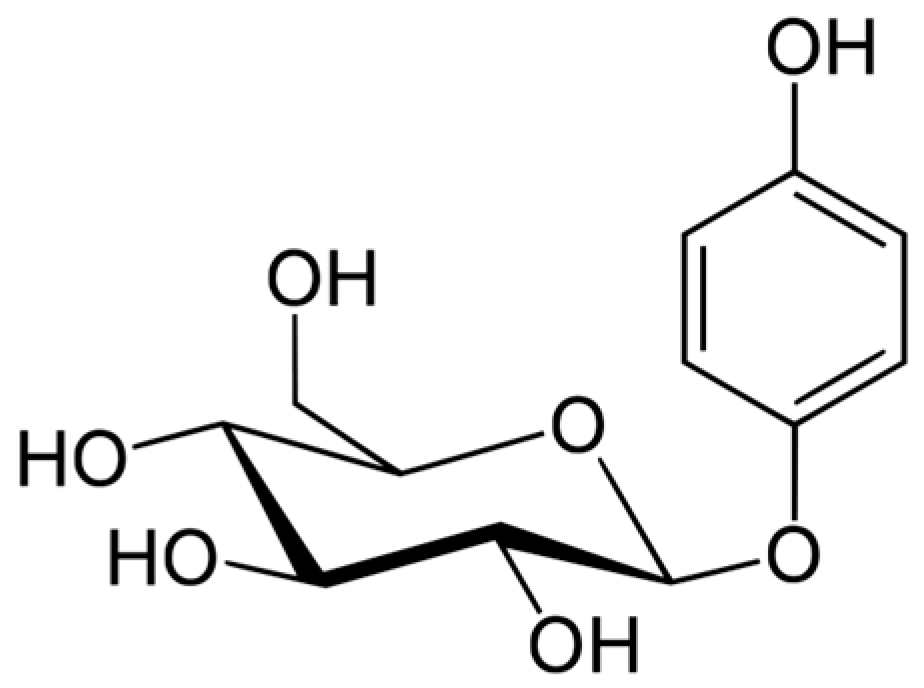
Figure 2.
Arbutin formula.
Bearberry (Arctostaphyllos uva ursi), traditionally used in herbal therapy to treat urinary tract disorders, is without doubt the plant most often used in the cosmetics industry for formulating skin lightening products. The percentage of arbutin present in a plant varies considerably according to the species (17% in the leaves of Arctostaphyllos uva ursi, 5% in the leaves of Origanum majorama) [53], the time of collection, and the growing conditions. Certain authors have shown that supplying hydroquinone in the growing medium induces an increase in the biomass of the plant [54]. The small South African shrub Myrothamnus flabellifolia is characterized by the richness of its leaves in arbutin (27% of the dry weight) and is therefore also of interest in this field [55].
Numerous studies show that arbutin is just as effective as hydroquinone, but less toxic [56]. The alpha isomer has the greatest inhibitory activity against mammalian tyrosinases [57]. This agent, which is photostable yet readily degraded by heat, would need to be incorporated at cold temperatures into the chosen excipient [58].
The Scientific Committee on Consumer Safety (SCCS) assessed the use safety of alpha arbutin (for concentrations greater than 2% in facial creams and greater than 0.5% for body lotions) and of beta arbutin (for concentrations greater than 7% in facial creams) [59,60]. A study conducted on 10 women with melasma revealed a significant reduction in melanin levels after a one-month treatment with a 1% preparation. However, it must be pointed out that cutaneous hydrolysis of the glycoside takes place, in which hydroquinone is released [61].
A synthetic derivative, deoxyarbutin (4-[(tetrahydro-2H-pyran-2-yl)oxy] phenol) would have the advantage over botanical arbutin of being more effective and less cytotoxic [62]. This is not the opinion of the SCCS, which decided in 2016 that this ingredient is not safe to use in finished products at a use rate greater than 3%. The amount of hydroquinone released as the finished products age means that they cannot be marketed with absolute safety [59].
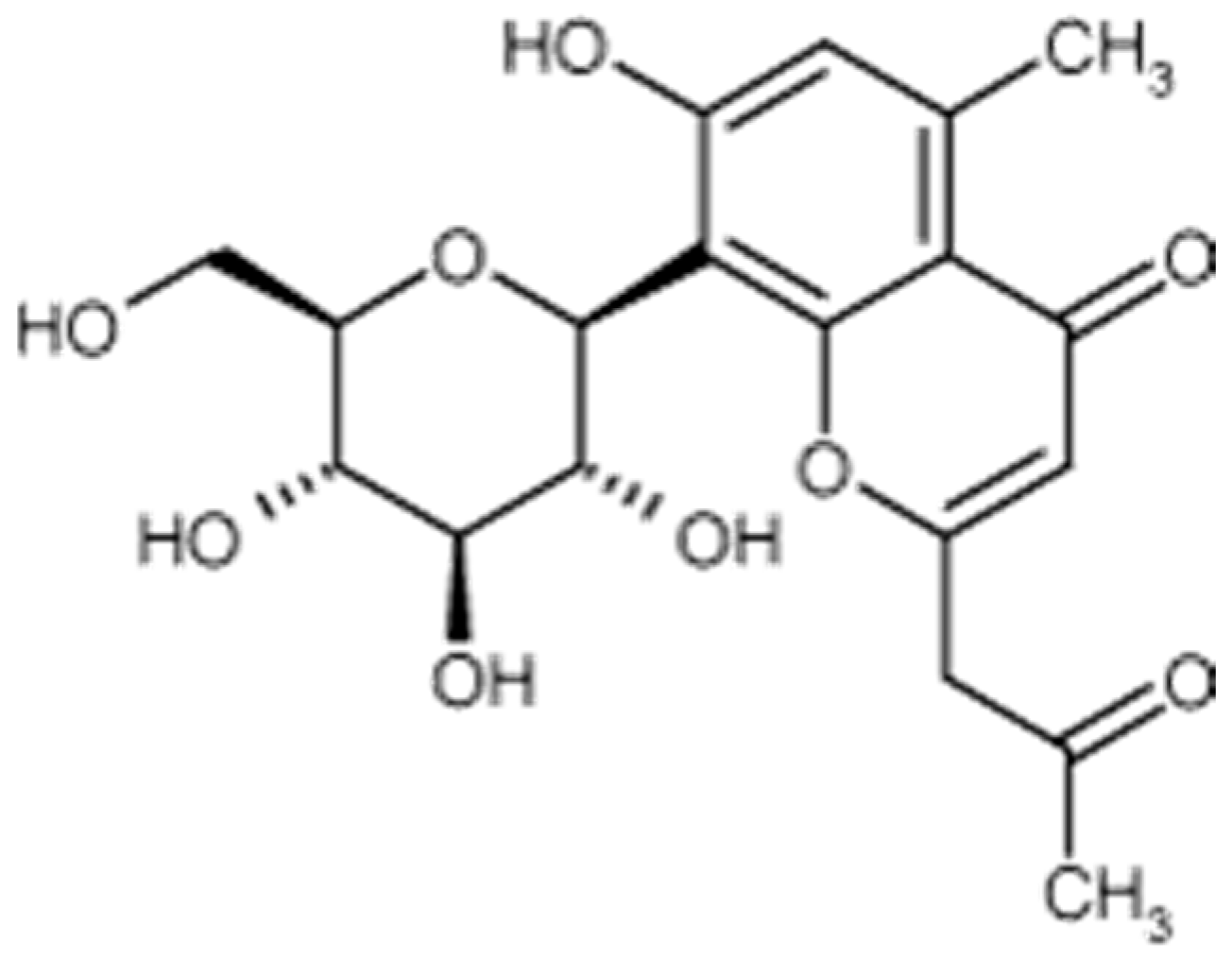
Aloesin is a low molecular weight glycoprotein (Figure 3) extracted from various species of plants in the genus Aloe, in which it can be found in significant amounts. Such is the case with Aloe ferrox. A dry extract of Aloe ferrox leaves may contain up to 25% aloesin [63].
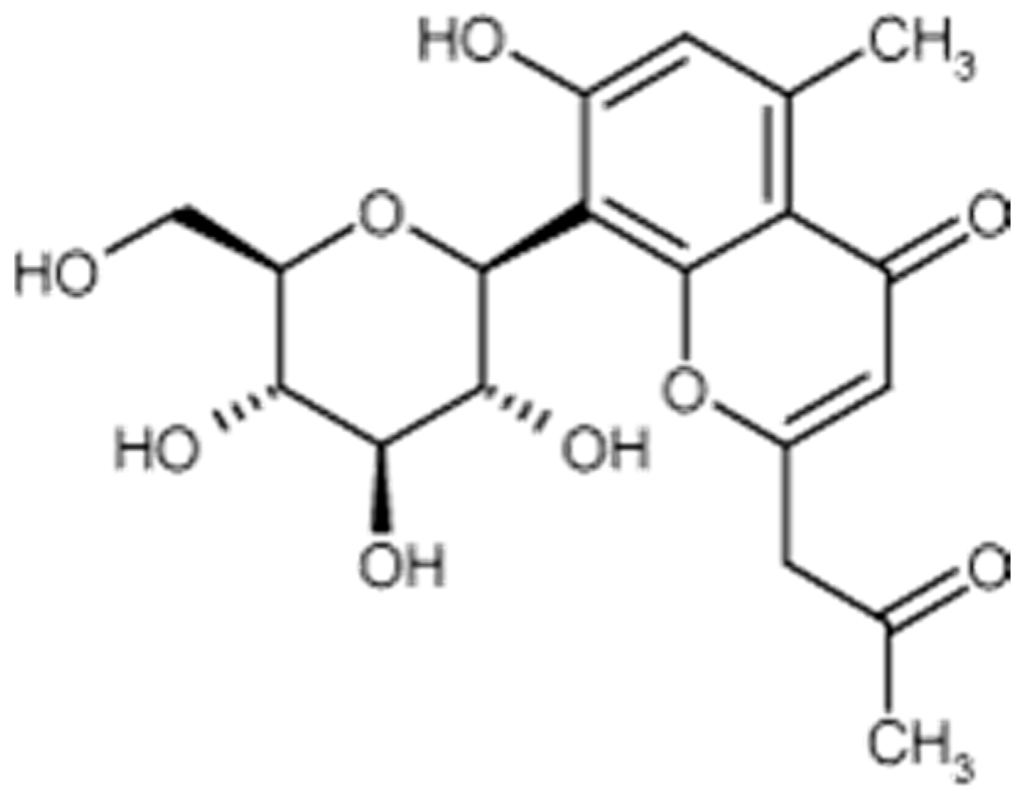
Figure 3.
Aloesin formula.
This molecule of human, animal, or fungal origin acts as a competitive inhibitor of tyrosinase [64]. Efficacy is proportional to the concentration used [65]. From the standpoint of its toxicology profile, aloesin is evidently safe to use. This molecule, which is non-genotoxic and has a high No Observable Adverse Effect Level (NOAEL) (2000 mg/Kg/day via the oral route) [66], is also a good candidate for making foods or food supplements with the aim of preventing diabetes [63].
Glabridin is present in the hydrophobic fraction of licorice root extract (licorice) and is capable of reducing the activity of tyrosinase on melanocytes in culture, and of inhibiting the induction of pigmentation by UVB and erythema formation in guinea pigs [67]. Its estrogenic effect is a drawback. Even though this effect is less than that of physiological estrogens (for example, doses 10 times greater than that of 17 beta-estradiol are required to bring about an observable increase in creatine kinase activity in female rats), this aspect must be considered [68].
Other flavonoids present in licorice root extract such as glabrene, isoliquiritigenin, licuraside, isoliquiritin, and licochalcone A are also of interest due to their tyrosinase inhibiting nature [65]. Lastly, we would point out that liquiritin may turn out to be of value in treating melasma. Although it does not inhibit tyrosinase, this molecule makes it possible to lighten skin tone by melanin dispersal [69]. Tests performed on around twenty women suffering from melasma demonstrated the positive effect of liquiritin at a substantial concentration of 20%, with reduction of pigmentation intensity (result observed in 70% of the women after 4 weeks of treatment) and reduction of the size of the lesions (result observed in 60% of the women after 4 weeks of treatment) [68,70].
The leaves of the white mulberry (Morus alba, Moraceae) have been used for many years in traditional medicine in China, Korea, Japan, and Thailand. Fever-reducing, liver-protecting, and blood pressure-lowering properties are attributed to them. The polyphenols contained in the leaves have depigmentation properties that have been demonstrated in vitro [71]. In 2006, Taiwanese investigators tested a number of Chinese herbs used in folk medicine. An inhibitory effect on human tyrosinase was demonstrated for a number of extracts extracted by alcohol at 95°. This inhibitory effect was compared to the one obtained with a standard molecule, arbutin. With an inhibitory effect comparable to that of arbutin, the extract of white mulberry turned out to be the one of greatest value. It takes 100 μg/mL of Morus alba extract versus 138 μg/mL of arbutin to achieve a ca. 70% inhibition of tyrosinase [72].
Kojic acid is a molecule present in a number of fermented foods or beverages of Japanese origin (miso, shoyu, sake). It is formed by fermentation by various species of fungi such as Aspergillus, Penicillium, and others.
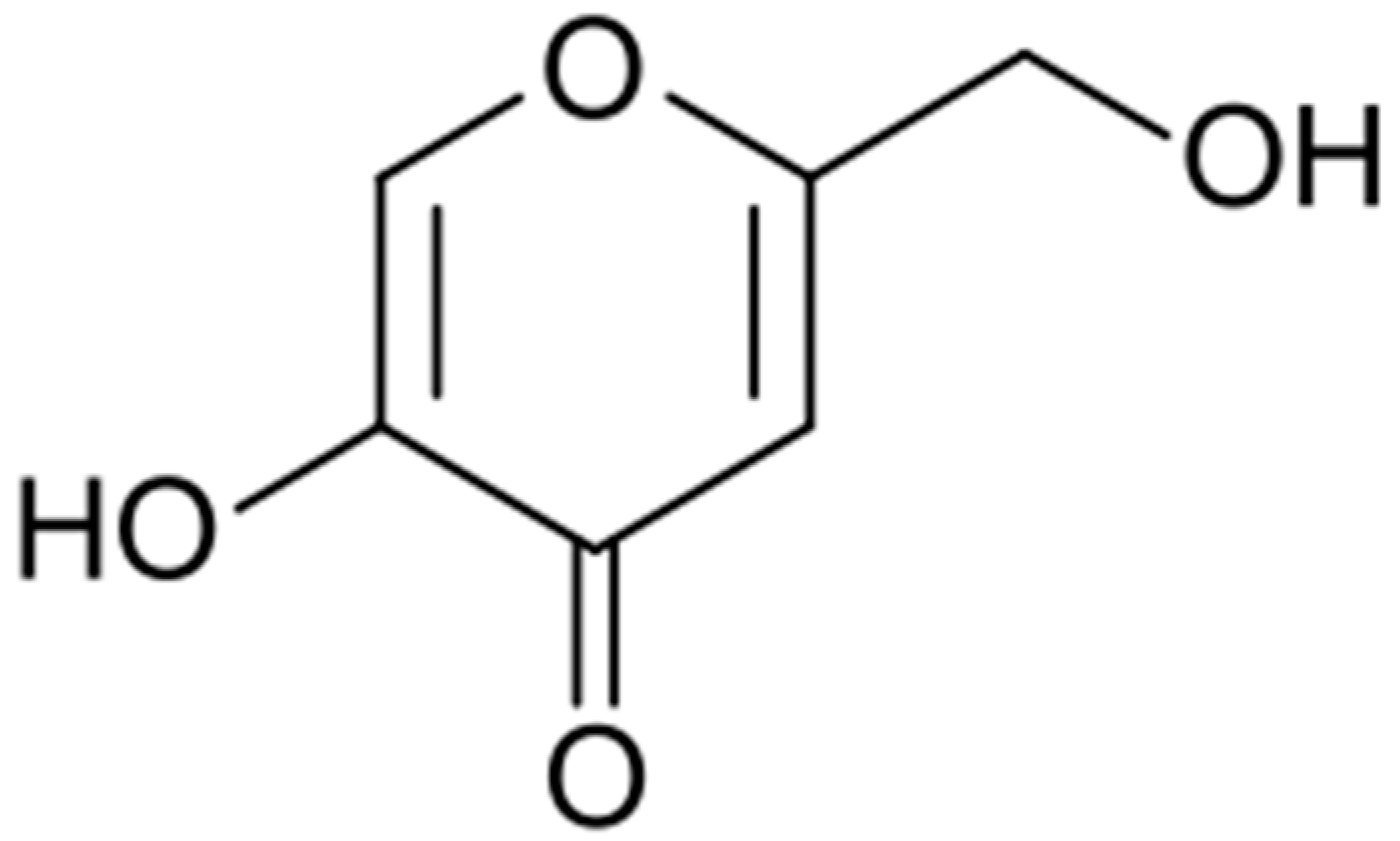
Discovered in 1907 by Saito in cultures of the fungus Aspergillus orizae, this acid (Figure 4) was soon found to have many practical applications.
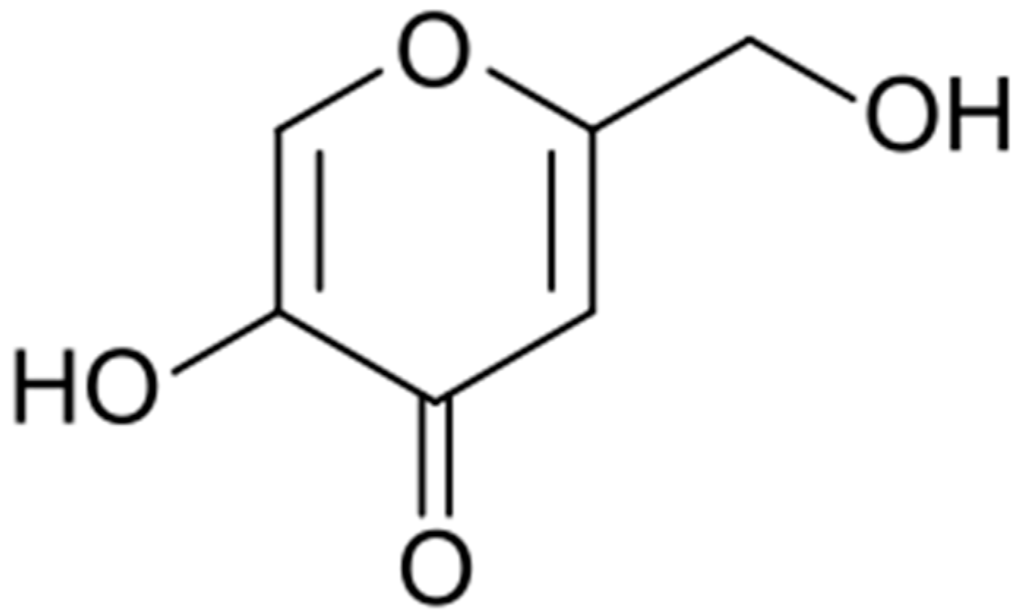
Figure 4.
Kojic acid formula.
Used as a food additive to preserve the natural color of foods such as fresh fruits and vegetables, crustaceans, etc., kojic acid is a common ingredient in depigmentation preparations [73].
It intervenes in the depigmentation process via various mechanisms. It acts as a chelator of divalent ions, as a free radical trapper, and as a tyrosinase inhibitor. Its cytotoxic effect and instability over time have led certain teams to work on improving it. For example, it has been conjugated with phenylalanine [74]. Because it acts as an iron chelator, anti-wrinkle properties have been attributed to it as well [75]. Depending on the periods considered, Japanese and Europeans have expressed conflicting opinions. In 2008, the Scientific Committee on Consumer Products (SCCP) pleaded in favor of the use safety of preparations containing 1% kojic acid, even though risks of sensitization are conceivable. Subsequent genotoxicity tests have lent support to this opinion. A literature review conducted in 2010 also concluded that it was safe to use this agent at concentrations of less than 1% [76].
As far as its efficacy is concerned, it is less in comparison to the standard molecule, hydroquinone. Tests on volunteers have shown that a 4% hydroquinone-based preparation is 5 times more effective than a combined preparation of kojic acid (0.75%) and vitamin C (2.5%) [61].
In 1978, the tyrosinase-inhibiting activity of certain lipid fractions, mainly C9–C11 dicarboxylic acids, was demonstrated for the first time in vitro. The interest in azelaic acid, which is a C9-dicarboxylic acid, for treating pigmented lesions thus ensued [77]. This acid is produced naturally by a yeast, Malassezia furfur. Its inhibitory activity against tyrosinase is reflected in the appearance of depigmented maculae on the skin of subjects suffering from a mycosis, Pityriasis versicolor [78]. This fungus produces lipoxygenases that are capable of acting on the unsaturated fatty acids present on the skin surface. In culture, this fungus is capable of oxidizing oleic acid into azelaic acid [77]. There is unanimous agreement regarding its efficacy and absence of undesirable effects of note. A placebo-controlled clinical study conducted on 52 women with dark or pigmented skin (phototypes IV to VI) suffering from melasma demonstrated the superiority of a cream containing 20% azelaic acid. The women found that their skin was smoother and were thus satisfied overall. However, some undesirable effects (burning, tingling) were reported [79]. It seems that the preparations containing 20% azelaic acid and 4% hydroquinone are equivalent in vivo [77]. The anti-inflammatory, anti-keratinizing and bacteriostatic activity of azelaic acid justifies its use in treating diseases such as rosacea or acne [80,81]. Azelaic acid apparently behaves differently, depending on the characteristics of the cells concerned. A cytotoxic effect on human melanocytes and a much higher capacity to penetrate abnormal cells than normal cells indicated possibilities for use in treating melanoma at one time. However, these hopes were dashed [82,83]. From a practical standpoint, very few cosmetics use this acid. However, mention can be made of the product Melascreen® from Ducray laboratories (Boulogne Billancourt, France).
Tyrosinase Inhibitors Offering New Avenues in the Field of Skin Lightening
After reviewing the tyrosinase inhibitors that are well-known and very widely used in the cosmetics industry, we deemed it worthwhile to present various lesser known extracts or molecules.
Artocarpus heterophyllous (Moraceae) is a tree found in the tropical and subtropical regions of Asia. It is grown for its edible fruit in Vietnam. Its bark has numerous therapeutic properties: anti-inflammatory, anti-oxidant, and anti-aging. After a methanol extract demonstrated interesting anti-tyrosinase properties, a purification to detect the most effective molecules was deemed necessary and led to the isolation of artocarpanone. The IC50 of this molecule is indeed 22 times lower than that of the chosen standard molecule, kojic acid [84]. Another species, Artocarpus xanthocarpus, is also a source of depigmenting molecules (artoxanthol, alboctalol, steppogenin, norartocarpetin) worth exploiting. These molecules have IC50 values that are 50 times lower than that of kojic acid [85]. A dimeric stilbene with anti-tyrosinase activity could also be isolated from Artocarpus gomezianus [86]. The safety of such a molecule still needs to be demonstrated.
In 2010, an Italian team from Messina demonstrated the value of an ethanol extract of Betula pendula leaves. This extract shows a weak inhibitory activity, its efficacy being 50 times less than that of kojic acid. However, the value of this ingredient lies in its mechanism of action. It is not a competitive tyrosinase inhibitor, but a molecule capable of sequestering Cu2+ ions, which are essential cofactors for tyrosinase activity [87].
In 2016, Robertet laboratories (Grasse, France) started investigating the subject of depigmentation and demonstrated the valuable properties of an extract of Populus nigra buds in an ethanol/hexane mixture. A tyrosinase inhibitory effect two times lower than that of kojic acid was demonstrated under these conditions [88]. However, it must be pointed out that hexane is not a solvent of choice for a cosmetic use and that in order for any further developments to take place, the extraction solvent must be looked at again.
In 2015, Indian researchers in a division of Biotechnologies investigated the potential of 13 edible fruits from the northwestern Himalayans. Anti-aging and anti-tyrosinase activities were sought in particular. A dozen of the fruits tested exhibited a tyrosinase inhibitory effect of varying degrees. The acetone extract of Ziziphus nummularia that was retained caught our attention as well. The latter also has anti-collagenase and anti-elastase properties [89]. Nevertheless it must be pointed out that here as well, the extraction solvent is not compatible with a cosmetic use.
A collaboration among various research teams working in Indonesia, Japan, and Sudan, countries in which having a light complexion is of paramount importance, revealed the influence of the respective plant part on the level of efficacy attained. While the extraction solvent does indeed play a major role, the plant part used must also be taken into account. In the case of Curcuma xanthorrhiza, a plant widely used in traditional medicine, considerable differences will be observed depending on the part chosen. The rhizome extract in ethyl acetate is ten times less effective than the kojic acid standard, whereas the bract extract in the same solvent is 100 times less so, which clearly eliminates any possibility of skin lightening applications [90].
Phyla nodiflora herbal teas are used in Chinese folk medicine to treat inflammatory skin conditions. The active molecule, eupafolin, is a flavonoid. Its anti-inflammatory effect [91] and its skin lightening effect have been demonstrated. According to tests performed on keratinocytes and melanocytes, eupafolin is a molecule that is non-toxic to skin cells. Its lightening action lies in its property of being a tyrosine inhibitor (its efficacy is ca. 65% that of the standard molecule) and in its capacity to interfere with the synthesis of this enzyme [92].
2.2.3. Value of Combinations
As we have illustrated throughout this review, there are different ways to combat hyperpigmentation. It may thus prove useful to combine different active agents in the same formulation. This was demonstrated in a study conducted by Sophie Seité of L’Oréal laboratories. Accordingly, a preparation comprising 6 active agents with exfoliant, anti-tyrosinase, or soothing properties, namely PhE-Resorcinol, ferulic acid, Ginkgo biloba, lipohydroxy acid, niacinamide, and La Roche Posay thermal spring water brought about a visible reduction of solar lentigines on the hands in thirty volunteers [93]. Some commercial products are presented Table 3.

Table 3.
Some commercial lighting products.
3. Prevention, the Most Effective Means of Combat
It must be concluded that hydroquinone-based skin treatments are not totally satisfactory from a use safety standpoint. Effective sun screening is thus necessary when there is a risk of developing hyperpigmentation. However, the need for protecting one’s skin from exposure to the sun is not unanimously accepted. A study conducted in Germany in 2006 showed that a third of the persons in the study group of 2823 adults with a mean age of 49 years old do not use sunscreen products when they are exposed to the sun. Those who protect themselves prefer products with low indexes (the median SPF factor was 12). They are equally likely to use sunscreen products as well as cosmetics displaying an SPF [83]. An American study recently conducted on a mostly white, non-Hispanic population (67.4%) showed that compliance is gender-dependent, with women being much more alert to the consequences of the effects of UV radiation on the skin. 40% of the men in the study group admitted that they never use sunscreen products versus 30% of the women surveyed [94].
Sunscreen products that protect against UVA and UVB should be used. Products combining organic and inorganic filters should be preferred over those that just use inorganic filters. High levels of protection cannot be achieved with the latter. The product must also be reapplied frequently (every 2 h). A detrimental effect is observed in the event of failure to reapply. The anti-inflammatory nature of the formula associated with the presence of the filters themselves or the addition of anti-inflammatory molecules (bisabolol, allantoin, etc.) masks sunburn but does not provide protection from the deleterious effects of UV [95].
4. Conclusions
The field of complexion lightening is the subject of numerous studies because the cosmetics sector is on the lookout for effective and well-tolerated active ingredients. Polyphenols constitute a family of particular value in this type of application.
Author Contributions
Laurence Coiffard and Celine Couteau both conceived the idea and wrote the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Scarpa, A.; Guerci, A. Depigmenting procedures and drugs employed by melanoderm populations. J. Ethnopharmacol. 1987, 19, 17–66. [Google Scholar] [CrossRef]
- Kamagaju, L.; Bizuru, E.; Minani, V.; Morandini, R.; Stévigny, C.; Ghanem, G.; Duez, P. An ethnobotanical survey of medicinal plants used in Rwanda for voluntary depigmentation. J. Ethnopharmacol. 2013, 150, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Fiori, F.; Andrisano, V. LC–MS method for the simultaneous determination of six glucocorticoids in pharmaceutical formulations and counterfeit cosmetic products. J. Pharm. Biomed. Anal. 2014, 91, 185–192. [Google Scholar]
- Perry, A.; Petit, A.; Bagot, M.; Villa, A.; Bellaiche, M.; Bourrat, E. Intoxication au mercure par procuration chez un enfant: Une nouvelle complication de la dépigmentation volontaire. Arch. Pédiatr. 2014, 21, 22–24. (In French) [Google Scholar] [CrossRef]
- Sène, D.; Huong-Boutin, D.L.T.; Thiollet, M.; Barete, S.; Cacoub, P.; Piette, J.C. Insuffisance surrénalienne haute symptomatique compliquant l’usage de dermocorticoïdes pour dépigmentation volontaire. Rev. Méd. Int. 2008, 29, 1030–1033. (In French) [Google Scholar] [CrossRef] [PubMed]
- Porcheron, A.; Latreille, J.; Jdid, R.; Tschachler, E.; Morizot, F. Influence of skin ageing features on Chinese women’s perception of facial age and attractiveness. Int. J. Cosmet. Sci. 2014, 36, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.; Fitzpatrick, T.B.; Kraus, E.W. Usefulness of retinoic acid in the treatment of melasma. J. Am. Acad. Dermatol. 1986, 15, 894–899. [Google Scholar] [CrossRef]
- Akyol, A.; Can, O.T.; Bayramoglu, M. Treatment of hydroquinone by photochemical oxidation and electrocoagulation combined process. J. Water Process Eng. 2015, 8, 45–54. [Google Scholar] [CrossRef]
- Findlay, G.H. Ochronosis following skin bleaching with hydroquinone. J. Am. Acad. Dermatol. 1982, 6, 1092–1093. [Google Scholar] [CrossRef]
- Papaspyrides, C.D.; Protopapas, S.A. E.s.r. approach on hydroquinone-melanin possible interaction. Int. J. Biol. Macromol. 1988, 10, 62–63. [Google Scholar] [CrossRef]
- Devillers, J.; Boule, P.; Vasseur, P.; Prevot, P.; Steiman, R.; Seigle-Murandi, F.; Benoit-Guyod, J.L.; Nendza, M.; Grioni, C.; Dive, D.; et al. Environmental and health risks of hydroquinone. Ecotoxicol. Environ. Saf. 1990, 19, 327–354. [Google Scholar] [CrossRef]
- O’Donaghue, J.L.; David, P.; Richardson, W.; Dyer, M. Hydroquinone and hepatitis. Lancet 1995, 346, 1427–1428. [Google Scholar] [CrossRef]
- Rendon, M.; Berneburg, M.; Arellano, I.; Picardo, M. Treatment of melasma. J. Am. Acad. Dermatol. 2006, 54, S272–S281. [Google Scholar] [CrossRef] [PubMed]
- Tidman, M.J.; Horton, J.J.; MacDonald, D.M. Hydroquinone-induced ochronosis—Light and electronmicroscopic features. Clin. Exp. Dermatol. 1986, 11, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Findlay, G.H.; Morrisson, J.G.L.; Simson, I.W. Exogenous ochronosis and pigmented colloid milium from hydroquinone bleaching creams. Br. J. Dermatol. 1975, 93, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Masumori, S.; Hirata-Koizumi, M.; Ono, A.; Honma, M.; Yokoyama, K.; Hirose, A. Evaluation of in vivo mutagenicity of hydroquinone in Muta™ mice. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 775–776, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.M.; Zhou, Q.; Lei, T.C.; Ding, S.F.; Xu, S.Z. Effects of hydroquinone and its glucoside derivatives on melanogenesis and antioxidation: Biosafety as skin whitening agents. J. Dermatol. Sci. 2009, 55, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Kligman, A.M.; Willis, I. A new formula for depigmenting human skin. Arch. Dermatol. 1975, 111, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Katsambas, A.D.; Stratigos, A.J. Depigmenting and bleaching agents: Coping with hyperpigmentation. Clin. Dermatol. 2001, 19, 483–488. [Google Scholar] [CrossRef]
- Callender, V.D. An open-label study of the use of adapalene cream and a triple-combination therapy for the treatment of mild-to-moderate acne and post-inflammatory hyperpigmentation. J. Am. Acad. Dermatol. 2004, 50. [Google Scholar] [CrossRef]
- Callender, V.D. Maintaining remission of melasma with triple-combination cream therapy. J. Am. Acad. Dermatol. 2005, 52. [Google Scholar] [CrossRef]
- Taylor, S. Open-label case study on triple-combination cream in patients with pseudofolliculitis barbae. J. Am. Acad. Dermatol. 2005, 52. [Google Scholar] [CrossRef]
- Grimes, P.E. Management of Hyperpigmentation in Darker Racial Ethnic Groups. Semin. Cutan. Med. Surg. 2009, 28, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.; Lori, A.; Luz, E.; Gold, M.; Gottschalk, R. Results from a split-face study comparing sequential treatment with triple combination cream and intense pulsed light versus a control cream and intense pulsed light in patients with moderate to severe melasma. J. Am. Acad. Dermatol. 2009, 60, AB161. [Google Scholar]
- Hexsel, D.; Sidou, F.; Kerrouche, N.; Cestari, T. Combination of a triple combination cream and tretinoin cream in subjects with mottled hyperpigmentation associated with photodamage. J. Am. Acad. Dermatol. 2010, 62. [Google Scholar] [CrossRef]
- Scissors, B.; Gathers, R.C. A case of exogenous ochronosis caused by a triple combination bleaching cream. J. Am. Acad. Dermatol. 2010, 62. [Google Scholar] [CrossRef]
- Hsieh, P.W.; Al-Suwayeh, S.A.; Fang, C.L.; Lin, H.F.; Chen, C.C.; Fang, J.Y. The co-drug of conjugated hydroquinone and azelaic acid to enhance topical skin targeting and decrease penetration through the skin. Eur. J. Pharm. Biopharm. 2012, 81, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, A.B., Jr.; Schwartzel, E.H.; Colby, S.I.; Altman, D.J. The combination of 2% 4-hydroxyanisole (Mequinol) and 0.01% tretinoin is effective in improving the appearance of solar lentigines and related hyperpigmented lesions in two double-blind multicenter clinical studies. J. Am. Acad. Dermatol. 2000, 42, 459–467. [Google Scholar] [CrossRef]
- Ortonne, J.P.; Pandya, A.G.; Lui, H.; Hexsel, D. Treatment of solar lentigines. J. Am. Acad. Dermatol. 2006, 54, S262–S271. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gover, M.D.; Nouri, K.; Taylor, S. The treatment of melasma: A review of clinical trials. J. Am. Acad. Dermatol. 2006, 55, 1048–1065. [Google Scholar] [CrossRef] [PubMed]
- Tzimas, G.; Collins, M.D.; Nau, H. Identification of 14-hydroxy-4,14-retro-retinol as an in vivo metabolite of vitamin A. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1996, 1301, 1–6. [Google Scholar] [CrossRef]
- Clark, E.; Scerri, L. Superficial and medium-depth chemical peels. Clin. Dermatol. 2008, 26, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Inan, S.; Oztukcan, S.; Vatansever, S.; Ermertcan, A.T.; Zeybek, D.; Oksal, A.; Giray, G.; Muftuoglu, S. Histopathological and ultrastructural effects of glycolic acid on rat skin. Acta Histochem. 2006, 108, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Okuda, M.; Donahue, D.A.; Kaufman, L.E.; Avalos, J.; Simion, F.A.; Story, D.C.; Sakaguchi, H.; Fautz, R.; Fuchs, A. Negligible penetration of incidental amounts of alpha-hydroxy acid from rinse-off personal care products in human skin using an in vitro static diffusion cell model. Toxicol. Vitr. 2011, 25, 2041–2047. [Google Scholar] [CrossRef] [PubMed]
- Denda, S.; Denda, M.; Inoue, K.; Hibino, T. Glycolic acid induces keratinocyte proliferation in a skin equivalent model via TRPV1 activation. J. Dermatol. Sci. 2010, 57, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Kempiak, S.J.; Uebelhoer, N. Superficial Chemical Peels and Microdermabrasion for Acne Vulgaris. Semin. Cutan. Med. Surg. 2008, 27, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Kornhauser, A.; Wie, R.R.; Yamaguchi, Y.; Coelho, S.G.; Kaidbey, K.; Barton, C.; Takahashi, K.; Beer, J.Z.; Miller, S.A.; Hearing, V.J. The effects of topically applied glycolic acid and salicylic acid on ultraviolet radiation-induced erythema, DNA damage and sunburn cell formation in human skin. J. Dermatol. Sci. 2009, 55, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Madan, R.K.; Jacob, L. A review of toxicity from topical salicylic acid preparations. J. Am. Acad. Dermatol. 2014, 70, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.J.; Dreher, F.; Chew, A.L.; Zhai, H.; Levin, C.; Stern, R.; Maibach, H.I. Cutaneous bioassay of salicylic acid as a keratolytic. Int. J. Pharm. 2005, 292, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Landau, M. Chemical peels. Clin. Dermatol. 2008, 26, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Kodali, S.; Guevara, I.L.; Carrigan, C.R.; Daulat, S.; Blanco, G.; Boker, A.; Hynan, L.S.; Pandya, A.G. A prospective, randomized, split-face, controlled trial of salicylic acid peels in the treatment of melasma in Latin American women. J. Am. Acad. Dermatol. 2010, 63, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Baden, H.P.; Alper, J.C. A Keratolytic Gel Containing Salicylic Acid in Propylene Glycol. J. Investig. Dermatol. 1973, 61, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Gallarate, M.; Carlotti, M.E.; Trotta, M.; Bovo, S. On the stability of ascorbic acid in emulsified systems for topical and cosmetic use. Int. J. Pharm. 1999, 188, 233–241. [Google Scholar] [CrossRef]
- Jutley, G.S.; Rajaratnam, R.; Halpern, J.; Salim, A.; Emmett, C. Systematic review of randomized controlled trials on interventions for melasma: An abridged Cochrane review. J. Am. Acad. Dermatol. 2014, 70, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Bowe, W.P.; Shalita, A.R. Effective over-the-counter acne treatments. Semin. Cutan. Med. Surg. 2008, 27, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Słoczyńska, K.; Gunia-Krzyżak, A.; Żelaszczyk, D.; Waszkielewicz, A.M.; Marona, H. Skin metabolism established with the use of MetaSite for selected retinoids employed in topical and systemic treatment of various skin disorders and found in cosmeceuticals. Acta Biochim. Pol. 2015, 62, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Yourick, J.; Jung, C.; Bronaugh, R. In vitro and in vivo percutaneous absorption of retinol from cosmetic formulations: Significance of the skin reservoir and prediction of systemic absorption. Toxicol. Appl. Pharmacol. 2008, 231, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, G.J.; Meuling, W.J.; Vaes, W.H.; Lawrence, R.S.; Shapiro, S.; Schulte, S.; Steiling, W.; Bausch, J.; Gerber, E.; Sasa, H.; et al. Repeated topical treatment, in contrast to single oral doses, with Vitamin A-containing preparations does not affect plasma concentrations of retinol, retinyl esters or retinoic acids in female subjects of child-bearing age. Toxicol. Lett. 2006, 163, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Bae-Hwan, K.; Yong-Soon, L.; Kyung-Sun, K. The mechanism of retinol-induced irritation and its application to anti-irritant development. Toxicol. Lett. 2003, 146, 65–73. [Google Scholar]
- Mélot, M.; Pudney, P.D.A.; Williamson, A.M.; Caspers, P.J.; Van Der Pol, A.; Puppels, G.J. Studying the effectiveness of penetration enhancers to deliver retinol through the stratum cornum by in vivo confocal Raman spectroscopy. J. Controll. Release 2009, 138, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Miller, D. Skin benefits of a retinol-containing daily moisturizer with photostable SPF-30. J. Am. Acad. Dermatol. 2008, 58, AB23. [Google Scholar]
- Séhédic, D.; Hardy-Boismartel, A.; Couteau, C.; Coiffard, L.J. Are cosmetic products which include an SPF appropriate for daily use? Arch. Dermatol. Res. 2009, 301, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Lukas, B.; Schmiderer, C.; Mitteregger, U.; Novak, J. Arbutin in marjoram and oregano. Food Chem. 2010, 121, 185–190. [Google Scholar] [CrossRef]
- Migas, P.; Krauze-Baranowska, M. The significance of arbutin and its derivatives in therapy and cosmetics. Phytochem. Lett. 2015, 13, 35–40. [Google Scholar] [CrossRef]
- Suau, R.; Cuevas, A.; Valpuesta, V.; Reid, M.S. Arbutin and sucrose in the leaves of the resurrection plant Myrothamnus flabellifolia. Phytochemistry 1991, 30, 2555–2556. [Google Scholar] [CrossRef]
- Nordlund, J.J.; Grimes, P.E.; Ortonnes, J.P. The safety of hydroquinone. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Nomura, K.; Nishimura, T.; Kiso, T.; Sugimoto, K.; Kuriki, T. Syntheses of α-arbutin-α-glycosides and their inhibitory effects on human tyrosinase. J. Biosci. Bioeng. 2005, 99, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Coiffard, L.J.M. Photostability determination of arbutin, a vegetable whitening agent. II Farmaco 2000, 55, 410–413. [Google Scholar] [CrossRef]
- Degen, G.H. Opinion of the Scientific Committee on Consumer Safety (SCCS)—Opinion on the safety of the use of α-arbutin in cosmetic products. Regul. Toxicol. Pharmacol. 2016, 74, 75–76. [Google Scholar]
- Degen, G.H. Opinion of the Scientific Committee on Consumer Safety (SCCS)—Opinion on the safety of the use of β-arbutin in cosmetic products. Regul. Toxicol. Pharmacol. 2015, 73, 866–867. [Google Scholar] [PubMed]
- Shin, J.W.; Yoon, S.W.; Jeong, J.B.; Park, K.C. Different responses of the melanin index to ultraviolet irradiation in relation to skin color and body site. Photodermatol. Photoimmunol. Photomed. 2014, 30, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.H.; Sriwiriyanont, P.; deLong, M.A.; Visscher, M.O.; Wickett, R.R.; Boissy, R.E. Comparative efficacy and safety of deoxyarbutin, a new tyrosinase-inhibiting agent. J. Cosmet. Sci. 2006, 57, 291–308. [Google Scholar] [PubMed]
- Lynch, B.; Simon, R.; Roberts, A. In vitro and in vivo assessment of the genotoxic activity of aloesin. Regul. Toxicol. Pharmacol. 2011, 61, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. The cosmeceutical realm. Clin. Dermatol. 2008, 26, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Gao, J. The Use of Botanical Extracts as Topical Skin-Lightening Agents for the Improvement of Skin Pigmentation Disorders. J. Investig. Dermatol. Symp. Proc. 2008, 13, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Yimam, M.; Brownell, L.; Jia, Q. In vivo safety evaluation of UP780, a standardized composition of aloe chromone aloesin formulated with an Aloe vera inner leaf fillet. Regul. Toxicol. Pharmacol. 2014, 69, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Park, K.C.; Huh, S.Y.; Choi, H.R.; Kim, D.S. Biology of melanogenesis and the search for hypopigmenting agents. Dermatol. Sin. 2010, 28, 53–57. [Google Scholar] [CrossRef]
- Somjen, D.; Katzburg, S.; Vaya, J.; Kaye, A.M.; Hendel, D.; Posner, G.H.; Tamir, S. Estrogenic activity of glabridin and glabrene from licorice roots on human osteoblasts and prepubertal rat skeletal tissues. J. Steroid Biochem. Mol. Biol. 2004, 91, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Li, H.; Wang, X.; Lee, F.S.; Cui, S. Isolation and identification of flavonoids in licorice and a study of their inhibitory effects on tyrosinase. J. Agric. Food Chem. 2005, 53, 7408–7414. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.; Metwalli, M. Topical liquiritin improves melasma. Int. J. Dermatol. 2000, 39, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Y.; Wang, Y.; Zhang, Y. Bioassay-guided screening and isolation of α-glucosidase and tyrosinase inhibitors from leaves of Morus alba. Food Chem. 2012, 131, 617–622. [Google Scholar] [CrossRef]
- Wang, K.H.; Lin, R.D.; Hsu, F.L.; Huang, Y.H.; Chang, H.C.; Huang, C.Y.; Lee, M.H. Cosmetic applications of selected traditional Chinese herbal medicines. J. Ethnopharmacol. 2006, 106, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, G.J.; Kirkland, D.; Marzin, D.; Toutain, H.; Leclerc-Ribaud, C.; Jinnai, H. An assessment of the genotoxicity and human health risk of topical use of kojic acid. Food Chem. Toxicol. 2004, 42, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.N.; Kwak, S.Y.; Seo, H.S.; Seo, J.H.; Kim, B.G.; Lee, Y.S. Kojic acid-amino acid conjugates as tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 5586–5589. [Google Scholar] [CrossRef] [PubMed]
- Mitani, H.; Koshiishi, I.; Sumita, T.; Imanari, T. Prevention of the photodamage in the hairless mouse dorsal skin by kojic acid as an iron chelator. Eur. J. Pharmacol. 2001, 411, 169–174. [Google Scholar] [CrossRef]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Final report of the safety assessment of Kojic acid as used in cosmetics. Int. J. Toxicol. 2010, 29, 244S–273S. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Park, K.C. Current clinical use of depigmenting agents. Dermatol Sin. 2014, 32, 205–210. [Google Scholar] [CrossRef]
- Nazzaro-Porro, M. Azelaic acid. J. Am. Acad. Dermatol. 1987, 17, 1033–1041. [Google Scholar] [CrossRef]
- Lowe, N.J.; Rizk, D.; Grimes, P.; Billips, M.; Pincus, S. Azelaic acid 20% cream in the treatment of facial hyperpigmentation in darker-skinned patients. Clin. Ther. 1998, 20, 945–959. [Google Scholar] [CrossRef]
- Webster, G. Combination azelaic acid therapy for acne vulgaris. J. Am. Acad. Dermatol. 2000, 43, S47–S50. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z. Multiple mechanisms of action of azelaic acid: New findings. J. Am. Acad. Dermatol. 2009, 60, AB86. [Google Scholar]
- Mingrone, G.; Greco, A.V.; Ciardiello, A.; Passo, S.; Nazzaro-Porro, M. Distribution of radiolabelled azelaic acid in eye membranes and fluids of rabbits. Exp. Pathol. 1984, 25, 85–88. [Google Scholar] [CrossRef]
- Schäfer, T.; Merkl, J.; Klemm, E.; Wichmann, H.E.; Ring, J.; KORA Study Group. The Epidemiology of Nevi and Signs of Skin Aging in the Adult General Population: Results of the KORA-Survey 2000. J. Investig. Dermatol. 2006, 126, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Nguyen, N.T.; Nguyen, M.H.; Le, T.H.; Van Do, T.N.; Hung, T.M.; Nguyen, M.T. Tyrosinase inhibitory activity of flavonoids from Artocarpus heterophyllous. Chem. Cent. J. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.J.; Lin, C.C.; Lu, T.M.; Li, J.H.; Chen, I.S.; Kuo, Y.H.; Ko, H.H. Chemical constituents derived from Artocarpus xanthocarpus as inhibitors of melanin biosynthesis. Phytochemistry 2015, 117, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, U.B.; Bapat, V.A. Artocarpus: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2010, 129, 142–166. [Google Scholar] [CrossRef] [PubMed]
- Germanò, M.P.; Cacciola, F.; Donato, P.; Dugo, P.; Certo, G.; D’Angelo, V.; Mondello, L.; Rapisarda, A. Betula pendula leaves: Polyphenolic characterization and potential innovative use in skin whitening products. Fitoterapia 2012, 83, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Maack, A.; Pegard, A. Populus nigra (Salicaceae) absolute rich in phenolic acids, phenylpropanoïds and flavonoids as a new potent tyrosinase inhibitor. Fitoterapia 2016, 111, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Himani, S.; Madhuri, K.L.; Koushalya, D. Evaluation and comparison of polyphenols and bioactivities of wild edible fruits of North-West Himalaya, India. Asian Pac. J. Trop. Dis. 2015, 5, 888–893. [Google Scholar]
- Batubara, I.; Julita, I.; Latifah, K.; Darusman, A.M.M.; Mitsunaga, T. Flower Bracts of Temulawak (Curcuma Xanthorrhiza) for Skin Care: Anti-acne and Whitening Agents. Procedia Chem. 2015, 14, 216–224. [Google Scholar] [CrossRef]
- Sung, H.C.; Liang, C.J.; Lee, C.W.; Yen, F.L.; Hsiao, C.Y.; Wang, S.H.; Jiang-Shieh, Y.F.; Tsai, J.S.; Chen, Y.L. The protective effect of eupafolin against TNF-α-induced lung inflammation via the reduction of intercellular cell adhesion molecule-1 expression. J. Ethnopharmacol. 2015, 170, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.H.; Chiang, Y.C.; Tsai, M.H.; Liang, C.J.; Hsu, L.F.; Li, S.Y.; Wang, M.C.; Yen, F.L.; Lee, C.W. Eupafolin, a skin whitening flavonoid isolated from Phyla nodiflora, downregulated melanogenesis: Role of MAPK and Akt pathways. J. Ethnopharmacol. 2014, 151, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Seité, S.; Oresajo, C. A double-blind, placebo controlled clinical trial to evaluate the efficacy and safety of a new skin whitening combination formula in patients with solar lentigines. J. Am. Acad. Dermatol. 2016, 74, AB10. [Google Scholar]
- Holman, D.M.; Berkowitz, Z.; Gery, G.P., Jr.; Hawkins, N.A.; Saraiya, M.; Watson, M. Patterns of sunscreen use on the face and other exposed skin among US adults. J. Am. Acad. Dermatol. 2015, 73, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Chauvet, C.; Paparis, E.; Coiffard, L.J. Influence of certain ingredients on the SPF determined in vivo. Arch. Dermatol. Res. 2012, 304, 817–821. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).