Thermosensitive Hydrogel Mask Significantly Improves Skin Moisture and Skin Tone; Bilateral Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Product
2.2. Ethics
3. Study Design
3.1. Inclusion Criteria
- All ethnic background.
- Healthy subjects and those with no current or previous medical history.
- Subjects psychologically able to understand the study related information and to give a written informed consent.
- Subjects with healthy BMI (between 19 and 30).
3.2. Exclusion Criteria
- The use of a topical medication containing steroids to treat skin diseases more than once a month.
- Hypersensitive skin.
- Subjects with severe skin-related pathologies and abnormalities, such as severe acne, erythema, broken skin, eczema, cancer.
- Skin peeling, botox or other wrinkle removal procedure done within a month from the start of the current trial.
- Subject with specific allergies or hypersensitivity to any of the ingredients present in the products to be tested.
3.3. Restrictions
- The subjects could continue to use their usual moisturisers, cosmetics, wash products and toiletries. Any changes to the brand or use of new products were recorded.
- Subjects were advised to report any over-the-counter medication to study investigator.
- Subjects were advised to avoid the application of other face mask or the use of collagen-based supplements during the trial.
3.4. Inclusion Criteria
- Healthy female volunteers, aged 40 years+, with ageing skin (dehydrated or hyper pigmented skin).
- Subject has signed a written Informed Consent; consents to facial photography.
- Subject exhibits moderate to advanced photo-ageing according to the Glogau scale.
3.5. Exclusion Criteria
- Subject is pregnant, nursing, or planning to become pregnant.
- Heavy alcohol consumption in the opinion of the investigator.
- A fever in the last 12 h, prior to start of the study.
- Significant past medical history of hepatic, cancerous, multiple sclerosis, high blood pressure, renal, thrombosis/phlebitis, cardiac, pulmonary, digestive, haematological, neurological, locomotor or psychiatric disease, which in the opinion of the Investigator would compromise the safety of the subject.
- Insulin-dependent diabetes.
- Concurrent medication likely to affect the response to the test article or confuse the results of the study including anti-depressants, botox/collagen fillers and collagen-based food supplements in the last 1 to 3 months.
- Participation in an anti-ageing study in the last 28 days.
- Pacemaker.
- Photo Epilepsy for Light Therapy.
- People with chronic skin conditions such as psoriasis, eczema, melasma, etc.
3.6. Prohibitions and Restrictions
- Subject agrees to attend all visits with a clean face, free of makeup and hair tied back.
- Subject agrees to keep to their usual facial skin cleansing and moisturising products, and make up for the duration of the study.
- Subject agrees to only use the test article and no other facial mask products for the duration of the study.
- Avoid Area: metal pins/plates or silicone implants in face, open cuts and abrasions, skin and eye infections, severe sunburn, conjunctivitis, styes, and in flare eczema/psoriasis on face.
4. Instrumental Assessment
4.1. DermaView-PRO
4.2. Corneometer® Measurements for Skin Hydration
4.3. Chromameter CR300 Measurements for Skin Tone/Luminosity
5. Visual Assessment
5.1. Photography
5.2. Statistics
6. Results
6.1. Pilot Study
Study Population
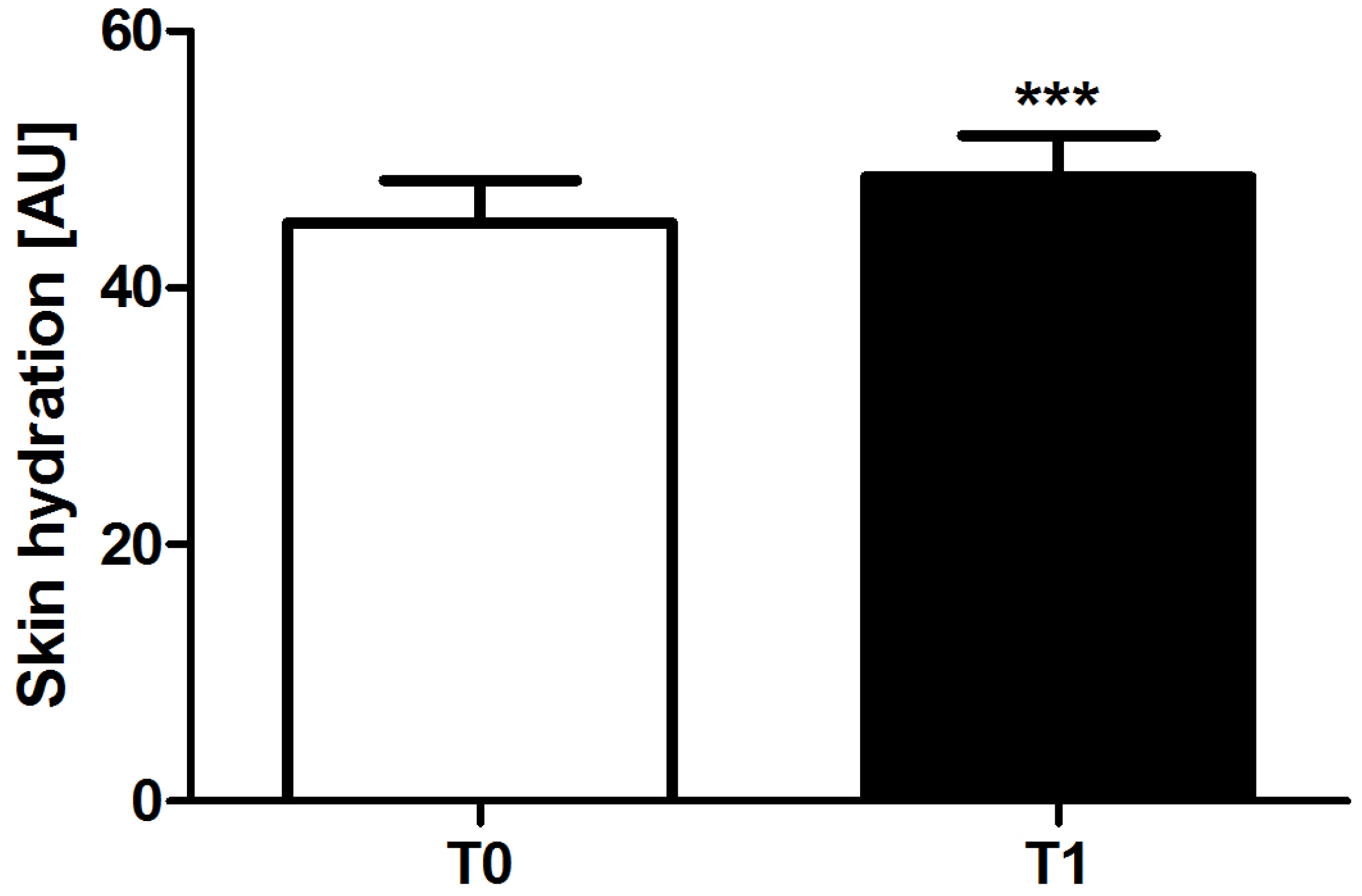
6.2. Hydration
6.3. Skin Tone (Erythema)
6.4. Self-Perception Questionnaires
6.5. Clinical Trial
6.5.1. Study Population
6.5.2. Corneometer®
6.5.3. Chromameter
6.6. Visual Assessment
6.6.1. Photography
6.6.2. Self-Perception Questionnaires
7. Discussion
8. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rush, A.; Muir, M. Maintaining skin integrity bariatric patients. Br. J. Community Nurs. 2012, 17, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Freinkel, R.K.; Woodley, D.T. The Biology of the Skin; Partheon Publishing Group Limited: Casterton, Carnforth, UK, 2001. [Google Scholar]
- Manfredini, M.; Mazzaglia, G.; Ciardo, S.; Simonazzi, S.; Farnetani, F.; Longo, C.; Pellacani, G. Does skin hydration influence keratinocyte biology? In vivo evaluation of microscopic skin changes induced by moisturizers by means of reflectance confocal microscopy. Skin Res. Technol. Off. J. Int. Soc. Bioeng. Skin 2013, 19, 299–307. [Google Scholar]
- Serup, J.; Blichmann, C. Epidermal hydration of psoriasis plaques and the relation to scaling. Measurement of electrical conductance and transepidermal water loss. Acta Derm. Venereol. 1987, 67, 357–359. [Google Scholar] [PubMed]
- Berardesca, E.; Fideli, D.; Borroni, G.; Rabbiosi, G.; Maibach, H. In vivo hydration and water-retention capacity of stratum corneum in clinically uninvolved skin in atopic and psoriatic patients. Acta Derm. Venereol. 1990, 70, 400–404. [Google Scholar] [PubMed]
- Sator, P.G.; Schmidt, J.B.; Honigsmann, H. Comparison of epidermal hydration and skin surface lipids in healthy individuals and in patients with atopic dermatitis. J. Am. Acad. Dermatol. 2003, 48, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Dal’Belo, S.E.; Gaspar, L.R.; Maia Campos, P.M. Moisturizing effect of cosmetic formulations containing aloe vera extract in different concentrations assessed by skin bioengineering techniques. Skin Res. Technol. Off. J. Int. Soc. Bioeng. Skin 2006, 12, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Flynn, T.C.; Petros, J.; Clark, R.E.; Viehman, G.E. Dry skin and moisturizers. Clin. Dermatol. 2001, 19, 387–392. [Google Scholar] [CrossRef]
- Shimada, E.; Matsumura, G. Viscosity and molecular weight of hyaluronic acids. J. Biochem. 1975, 78, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Tammi, R.; Saamanen, A.M.; Maibach, H.I.; Tammi, M. Degradation of newly synthesized high molecular mass hyaluronan in the epidermal and dermal compartments of human skin in organ culture. J. Investig. Dermatol. 1991, 97, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.E. Secondary structures in hyaluronan solutions: Chemical and biological implications. In Ciba Foundation Symposium 143—The Biology of Hyaluronan; Evered, D., Whelan, J., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2007; pp. 281–285. [Google Scholar]
- Laurent, T.C.; Fraser, J.R. Hyaluronan. FASEB J. 1992, 6, 2397–2404. [Google Scholar] [PubMed]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its nature, distribution, functions and turnover. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef]
- Naylor, E.C.; Watson, R.E.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Thorleifsson, A.; Wulf, H.C. Emollients and the response of facial skin to a cold environment. Br. J. Dermatol. 2003, 148, 1149–1152. [Google Scholar] [CrossRef] [PubMed]
- Egawa, M.; Oguri, M.; Kuwahara, T.; Takahashi, M. Effect of exposure of human skin to a dry environment. Skin Res. Technol. Off. J. Int. Soc. Bioeng. Skin 2002, 8, 212–218. [Google Scholar] [CrossRef]
- Kligman, L.H. Photoaging. Manifestations, prevention, and treatment. Dermatol. Clin. 1986, 4, 517–528. [Google Scholar] [PubMed]
- Guercio-Hauer, C.; Macfarlane, M.D.; Deleo, V.A. Photodamage, photoaging and photoprotection of the skin. Am. Fam. Physician 1994, 50, 327–332. [Google Scholar] [PubMed]
- Kohl, E.; Steinbauer, J.; Landthaler, M.; Szeimies, R.M. Skin ageing. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Altmayer, P.; Hoffmann, H.K.; Stucker, M. Skin Cancer and UV Radiation; Springer: New York, NY, USA, 1997. [Google Scholar]
- Moyal, D.; Fourtanier, A. Photoaging; Rigel, D.S., Weiss, R.A., Lim, H.W., Dover, J.S., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004; Chapter 2; p. 15. [Google Scholar]
- Zastrow, L.; Ferrero, L.; Herrling, T.; Groth, N. Integrated sun protection factor: A new sun protection factor based on free radicals generated by UV irradiation. Skin Pharmacol. Physiol. 2004, 17, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, V.; Piva, T.J. The UV response of the skin: A review of the MAPK, NFkappaB and TNFalpha signal transduction pathways. Arch. Dermatol. Res. 2010, 302, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Rittie, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Averbeck, M.; Beilharz, S.; Bauer, M.; Gebhardt, C.; Hartmann, A.; Hochleitner, K.; Kauer, F.; Voith, U.; Simon, J.C.; Termeer, C. In situ profiling and quantification of cytokines released during ultraviolet B-induced inflammation by combining dermal microdialysis and protein microarrays. Exp. Dermatol. 2006, 15, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Van der Pols, J.C.; Williams, G.M.; Pandeya, N.; Logan, V.; Green, A.C. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2006, 15, 2546–2548. [Google Scholar] [CrossRef] [PubMed]
- Hughes-Formella, B.; Wunderlich, O.; Williams, R. Anti-inflammatory and skin-hydrating properties of a dietary supplement and topical formulations containing oligomeric proanthocyanidins. Skin Pharmacol. Physiol. 2007, 20, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.V.; Kim, S.K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef] [PubMed]
- Gianeti, M.D.; Maia Campos, P.M. Efficacy evaluation of a multifunctional cosmetic formulation: The benefits of a combination of active antioxidant substances. Molecules 2014, 19, 18268–18282. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, D.G.; Wagemaker, T.A.; Alves, V.M.; Benevenuto, C.G.; Gaspar, L.R.; Maia Campos, P.M. In vivo photoprotective effects of cosmetic formulations containing UV filters, vitamins, ginkgo biloba and red algae extracts. J. Photochem. Photobiol. B 2015, 153, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Ratz-Lyko, A.; Arct, J.; Pytkowska, K. Moisturizing and antiinflammatory properties of cosmetic formulations containing centella asiatica extract. Indian J. Pharm. Sci. 2016, 78, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Leite e Silva, V.R.; Schulman, M.A.; Ferelli, C.; Gimenis, J.M.; Ruas, G.W.; Baby, A.R.; Velasco, M.V.; Taqueda, M.E.; Kaneko, T.M. Hydrating effects of moisturizer active compounds incorporated into hydrogels: In vivo assessment and comparison between devices. J. Cosmet. Dermatol. 2009, 8, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Wynne, A.; Whitefield, M.; Dixon, A.J.; Anderson, S. An effective, cosmetically acceptable, novel hydro-gel emollient for the management of dry skin conditions. J. Dermatol. Treat. 2002, 13, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.; Javed, F.; Ahmad, Z.; Md Akil, H. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Kim, S.R.; Cho, H.I.; Kang, M.H.; Yeom, D.W.; Lee, S.H.; Lee, S.; Choi, Y.W. Hydrogel-based ultra-moisturizing cream formulation for skin hydration and enhanced dermal drug delivery. Biol. Pharm. Bull. 2014, 37, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guan, J. Thermosensitive hydrogels for drug delivery. Expert Opin. Drug Deliv. 2011, 8, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, Y.; Ye, H.; Yu, S.; He, C.; Chen, X. Interleukin-15 and cisplatin co-encapsulated thermosensitive polypeptide hydrogels for combined immuno-chemotherapy. J. Control. Release 2017, 255, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Alcorn, D.; Fraser, J.R. Absorption of hyaluronan applied to the surface of intact skin. J. Investig. Dermatol. 1999, 113, 740–746. [Google Scholar] [CrossRef] [PubMed]
- World Medical, A. World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- The International Conference on Harmonisation of Technical Requirements for the Registration of Pharmaceuticals for Human Use (ICH). Good Clinical Practice. Available online: http://ec.europa.eu/health//sites/health/files/files/eudralex/vol-10/3cc1aen_en.pdf (accessed on 28 May 2017).
- Lazar, A.P.; Lazar, P. Dry skin, water, and lubrication. Dermatol. Clin. 1991, 9, 45–51. [Google Scholar] [PubMed]
- Draelos, Z.D. Therapeutic moisturizers. Dermatol. Clin. 2000, 18, 597–607. [Google Scholar] [CrossRef]
- Korponyai, C.; Szel, E.; Behany, Z.; Varga, E.; Mohos, G.; Dura, A.; Dikstein, S.; Kemeny, L.; Eros, G. Effects of locally applied glycerol and xylitol on the hydration, barrier function and morphological parameters of the skin. Acta Derm. Venereol. 2017, 97, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Alber, C.; Buraczewska-Norin, I.; Kocherbitov, V.; Saleem, S.; Loden, M.; Engblom, J. Effects of water activity and low molecular weight humectants on skin permeability and hydration dynamics—A double-blind, randomized and controlled study. Int. J. Cosmet. Sci. 2014, 36, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, S.; Engblom, J.; Thuresson, K.; Sparr, E. Glycerol and urea can be used to increase skin permeability in reduced hydration conditions. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2013, 50, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.A.; Kasting, G.B. Dynamics of glycerine and water transport across human skin from binary mixtures. Int. J. Cosmet. Sci. 2016, 39, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.B.; Jones, S.A. Hyaluronic acid: A unique topical vehicle for the localized delivery of drugs to the skin. Eur. Acad. Dermatol. Venereol. 2005, 19, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Weindl, G.; Schaller, M.; Schafer-Korting, M.; Korting, H.C. Hyaluronic acid in the treatment and prevention of skin diseases: Molecular biological, pharmaceutical and clinical aspects. Skin Pharmacol. Physiol. 2004, 17, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Laugier, J.P.; Shuster, S.; Rosdy, M.; Csoka, A.B.; Stern, R.; Maibach, H.I. Topical hyaluronidase decreases hyaluronic acid and CD44 in human skin and in reconstituted human epidermis: Evidence that hyaluronidase can permeate the stratum corneum. Br. J. Dermatol. 2000, 142, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Pavicic, T.; Gauglitz, G.G.; Lersch, P.; Schwach-Abdellaoui, K.; Malle, B.; Korting, H.C.; Farwick, M. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J. Drugs Dermatol. 2011, 10, 990–1000. [Google Scholar] [PubMed]
- Lee, D.H.; Oh, I.Y.; Koo, K.T.; Suk, J.M.; Jung, S.W.; Park, J.O.; Kim, B.J.; Choi, Y.M. Improvement in skin wrinkles using a preparation containing human growth factors and hyaluronic acid serum. J. Cosmet. Laser Ther. 2015, 17, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Chen, C.R.; Young, T.H. Pearl extract enhances the migratory ability of fibroblasts in a wound healing model. Pharm. Biol. 2013, 51, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Jian-Ping, D.; Jun, C.; Yu-Fei, B.; Bang-Xing, H.; Shang-Bin, G.; Li-Li, J. Effects of pearl powder extract and its fractions on fibroblast function relevant to wound repair. Pharm. Biol. 2010, 48, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Chang, C.H.; Huang, C.C.; Liu, H.W. Anti-inflammation and anti-apoptosis effects of pearl extract gel on UVB irradiation HaCaT cells. Biomed. Mater. Eng. 2015, 26 (Suppl. S1), S139–S145. [Google Scholar] [CrossRef] [PubMed]
- Lijun You, Y.L.; Zhao, H.; Regenstein, J.; Zhao, M.; Ren, J. Purification and characterization of an antioxidant protein from pearl oyster (Pinctada fucata martensii). J. Aquat. Food Prod. Technol. 2014, 24, 661–671. [Google Scholar]
- Hong, Y.H.; Jung, E.Y.; Shin, K.S.; Yu, K.W.; Chang, U.J.; Suh, H.J. Tannase-converted green tea catechins and their anti-wrinkle activity in humans. J. Cosmet. Dermatol. 2013, 12, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Gianeti, M.D.; Mercurio, D.G.; Campos, P.M. The use of green tea extract in cosmetic formulations: Not only an antioxidant active ingredient. Dermatol. Ther. 2013, 26, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Akhtar, N.; Khan, B.A.; Shoaib Khan, H.M.; Saeed, T. Changes in skin mechanical properties after long-term application of cream containing green tea extract. Aging Clin. Exp. Res. 2011, 23, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Hajiaghaalipour, F.; Kanthimathi, M.S.; Abdulla, M.A.; Sanusi, J. The effect of camellia sinensis on wound healing potential in an animal model. Evid. Based Complement. Altern. Med. 2013, 2013, 386734. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Chen, F. Isolation and purification of baicalein, wogonin and oroxylin a from the medicinal plant scutellaria baicalensis by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1074, 107–110. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Gao, S.; Jin, H.; Li, W.; Zhang, H.; Yu, A. On-line continuous flow ultrasonic extraction coupled with high performance liquid chromatographic separation for determination of the flavonoids from root of scutellaria baicalensis georgi. J. Chromatogr. A 2010, 1217, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ma, J.; Chen, Y.; Tian, Q.; Shen, Y.; Wang, X.; Chen, B.; Yao, S. Investigation of flavonoid profile of scutellaria bacalensis georgi by high performance liquid chromatography with diode array detection and electrospray ion trap mass spectrometry. J. Chromatogr. A 2009, 1216, 4809–4814. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, L.; Lin, G.; Zuo, Z. Contents of major bioactive flavones in proprietary traditional chinese medicine products and reference herb of radix scutellariae. J. Pharm. Biomed. Anal. 2009, 50, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.P.; Park, J.B.; Bae, K.H. Pharmacological effects of methanolic extract from the root of scutellaria baicalensis and its flavonoids on human gingival fibroblast. Planta Medica 1995, 61, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Son, K.H.; Chang, H.W.; Kang, S.S.; Kim, H.P. Inhibition of chronic skin inflammation by topical anti-inflammatory flavonoid preparation, ato formula. Arch. Pharmacal Res. 2006, 29, 503–507. [Google Scholar] [CrossRef]
- Huang, W.H.; Lee, A.R.; Yang, C.H. Antioxidative and anti-inflammatory activities of polyhydroxyflavonoids of scutellaria baicalensis GEORGI. Biosci. Biotechnol. Biochem. 2006, 70, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.J.; Huang, W.C.; Hsieh, M.C.; Sung, P.J.; Kuo, Y.H.; Wu, W.H. Flavones isolated from scutellariae radix suppress propionibacterium acnes-induced cytokine production in vitro and in vivo. Molecules 2016, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.S.; Lim, H.; Park, H.; Kim, H.P. Effects of wogonin, a plant flavone from scutellaria radix, on skin inflammation: In vivo regulation of inflammation-associated gene expression. Biochem. Pharmacol. 2003, 66, 1271–1278. [Google Scholar] [CrossRef]
- Gao, Z.; Huang, K.; Yang, X.; Xu, H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of scutellaria baicalensis georgi. Biochim. Biophys. Acta 1999, 1472, 643–650. [Google Scholar] [CrossRef]
- Gabrielska, J.; Oszmianski, J.; Zylka, R.; Komorowska, M. Antioxidant activity of flavones from scutellaria baicalensis in lecithin liposomes. Z. Naturforschung C J. Biosci. 1997, 52, 817–823. [Google Scholar]
- Bochorakova, H.; Paulova, H.; Slanina, J.; Musil, P.; Taborska, E. Main flavonoids in the root of scutellaria baicalensis cultivated in europe and their comparative antiradical properties. Phytother. Res. 2003, 17, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, D.; Lamer-Zarawska, E.; Matkowski, A. Antimutagenic and antiradical properties of flavones from the roots of scutellaria baicalensis georgi. Die Nahr. 2004, 48, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.K.; Kwak, J.Y.; Choi, G.W.; An, S.M.; Kwak, J.H.; Seo, H.H.; Suh, H.J.; Boo, Y.C. Scutellaria radix extract as a natural UV protectant for human skin. Phytother. Res. 2016, 30, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Bing-Rong, Z.; Song-Liang, J.; Xiao, E.C.; Xiang-Fei, L.; Bao-Xiang, C.; Jie, G.; Dan, L. Protective effect of the baicalin against DNA damage induced by ultraviolet B irradiation to mouse epidermis. Photodermatol. Photoimmunol. Photomed. 2008, 24, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Duan, D.D.; Zhang, J.Q.; Zhou, Y.Z.; Qin, X.M.; Du, G.H. A bioinformatic approach for the discovery of antiaging effects of baicalein from scutellaria baicalensis GEORGI. Rejuvenation Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Nemudzivhadi, V.; Masoko, P. In vitro assessment of cytotoxicity, antioxidant, and anti-inflammatory activities of ricinus communis (euphorbiaceae) leaf extracts. Evid. Based Complement. Altern. Med. 2014, 2014, 625961. [Google Scholar] [CrossRef] [PubMed]
- Lomash, V.; Parihar, S.K.; Jain, N.K.; Katiyar, A.K. Effect of solanum nigrum and ricinus communis extracts on histamine and carrageenan-induced inflammation in the chicken skin. Cell. Mol. Biol. 2010, 56 (Suppl. 56), OL1239–OL1251. [Google Scholar] [PubMed]
- Diez-Pascual, A.M.; Diez-Vicente, A.L. Wound healing bionanocomposites based on castor oil polymeric films reinforced with chitosan-modified ZnO nanoparticles. Biomacromolecules 2015, 16, 2631–2644. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Mitoma, C.; Nakahara, T.; Uchi, H.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Nakahara, M.; Furue, M. Antioxidant houttuynia cordata extract upregulates filaggrin expression in an aryl hydrocarbon-dependent manner. Hukuoka Acta Medica 2014, 105, 205–213. [Google Scholar] [PubMed]
- Kim, S.Y.; Shin, K.S. Evaluation of physiological activities of the citron (Citrus junos Sieb. Ex TANAKA) seed extracts. Prev. Nutr. Food Sci. 2013, 18, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Minamisawa, M.; Yoshida, S.; Uzawa, A. The functional evaluation of waste yuzu (Citrus junos) seeds. Food Funct. 2014, 5, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Hirota, R.; Roger, N.N.; Nakamura, H.; Song, H.S.; Sawamura, M.; Suganuma, N. Anti-inflammatory effects of limonene from yuzu (Citrus junos Tanaka) essential oil on eosinophils. J. Food Sci. 2010, 75, H87–H92. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.; Jung, Y.; Chun, W.; Yang, B.; Ryu, J.; Lim, C.; Kim, J.H.; Kim, H.; Cho, S.I. Anti-inflammatory effects of artemisia leaf extract in mice with contact dermatitis in vitro and in vivo. Mediat. Inflam. 2016, 2016, 8027537. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.; Akhtar, N.; Khan, M.S.; Menaa, A.; Menaa, B.; Khan, B.A.; Menaa, F. Formulation and evaluation on human skin of a water-in-oil emulsion containing muscat hamburg black grape seed extract. Int. J. Cosmet. Sci. 2015, 37, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.A.; Akhtar, N.; Menaa, A.; Menaa, F. A novel Cassia fistula (L.)-based emulsion elicits skin anti-aging benefits in humans. Cosmetics 2015, 2, 368–383. [Google Scholar] [CrossRef]
- Popa, E.G.; Carvalho, P.P.; Dias, A.F.; Santos, T.C.; Santo, V.E.; Marques, A.P.; Viegas, C.A.; Dias, I.R.; Gomes, M.E.; Reis, R.L. Evaluation of the in vitro and in vivo biocompatibility of carrageenan-based hydrogels. J. Biomed. Mater. Res. A 2014, 102, 4087–4097. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Final Report on the Safety Assessment of Ricinus Communis (Castor) Seed Oil, Hydrogenated Castor Oil, Glyceryl Ricinoleate, Glyceryl Ricinoleate SE, Ricinoleic Acid, Potassium Ricinoleate, Sodium Ricinoleate, Zinc Ricinoleate, Cetyl Ricinoleate, Ethyl Ricinoleate, Glycol Ricinoleate, Isopropyl Ricinoleate, Methyl Ricinoleate, and Octyldodecyl Ricinoleate. Int. J. Toxicol. 2007, 26 (Suppl. S3), 31–77. [Google Scholar]
- Kim, T.W.; Song, I.B.; Lee, H.K.; Kim, M.S.; Ham, S.H.; Cho, J.H.; Lim, J.H.; Yun, H.I. Assessment of dermal safety of scutellaria baicalensis aqueous extract topical application on skin hypersensitivity. Planta Med. 2013, 79, 959–962. [Google Scholar] [CrossRef] [PubMed]







| Ingredients |
|---|
| Water |
| Glycerin |
| 1,2-Hexanediol |
| Ceratonia Siliqua Gum |
| Chondrus Crispus (Carrageenan) Extract |
| Lactobacillus Ferment |
| Scutellaria Baicalensis Root Extract |
| Camellia Sinensis Leaf Extract |
| Artemisia Princeps Leaf Extract |
| Houttuynia Cordata Extract |
| Citrus Junos Fruit Extract |
| Ricinus Communis (Castor) Seed Oil |
| Caprylyl Glycol |
| Butylene Glycol |
| Phenoxyethanol |
| Pearl Extract |
| Sodium Hyaluronate |
| Dipotassium Glycyrrhizate |
| PEG-60 Hydrogenated Castor Oil |
| Disodium EDTA |
| Arginine |
| Fragrance |
| Ethnicity | n° | % |
|---|---|---|
| Caucasian | 44 | 70.9 |
| Asian | 7 | 11.3 |
| Black | 4 | 6.5 |
| Latin-American | 4 | 6.5 |
| Middle-Eastern | 3 | 4.8 |
| Skin Type | % | |
|---|---|---|
| Hydration | Oily | 9.7 |
| Normal | 16.1 | |
| Dry | 21 | |
| Mixed | 53.2 | |
| Sensitivity | Sensitive | 33.8 |
| Resistant | 6.5 | |
| Normal | 59.7 | |
| Wrinkles | Tight | 61.3 |
| Normal | 35.5 | |
| Wrinkled | 3.2 | |
| Pigmentation | Normal | 53.2 |
| Pigmented | 22.6 | |
| Non-pigmented | 24.2 | |
| Skin Colour/ Fitzpatrick Scale | % |
|---|---|
| I | 1.6 |
| II | 22.6 |
| III | 43.5 |
| IV | 25.8 |
| V | 1.6 |
| VI | 4.9 |
| Photo-ageing | % |
|---|---|
| Mild | 89 |
| Moderate | 8 |
| Obvious | 3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quattrone, A.; Czajka, A.; Sibilla, S. Thermosensitive Hydrogel Mask Significantly Improves Skin Moisture and Skin Tone; Bilateral Clinical Trial. Cosmetics 2017, 4, 17. https://doi.org/10.3390/cosmetics4020017
Quattrone A, Czajka A, Sibilla S. Thermosensitive Hydrogel Mask Significantly Improves Skin Moisture and Skin Tone; Bilateral Clinical Trial. Cosmetics. 2017; 4(2):17. https://doi.org/10.3390/cosmetics4020017
Chicago/Turabian StyleQuattrone, Anna, Anna Czajka, and Sara Sibilla. 2017. "Thermosensitive Hydrogel Mask Significantly Improves Skin Moisture and Skin Tone; Bilateral Clinical Trial" Cosmetics 4, no. 2: 17. https://doi.org/10.3390/cosmetics4020017
APA StyleQuattrone, A., Czajka, A., & Sibilla, S. (2017). Thermosensitive Hydrogel Mask Significantly Improves Skin Moisture and Skin Tone; Bilateral Clinical Trial. Cosmetics, 4(2), 17. https://doi.org/10.3390/cosmetics4020017





