Cosmetic Functional Ingredients from Botanical Sources for Anti-Pollution Skincare Products
Abstract
:1. Introduction
2. Effects of Air Pollution on Human Health
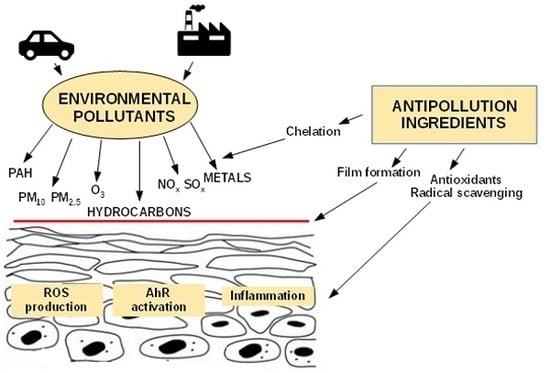
3. Pollution-Induced Skin Damage
4. Cosmetic Strategies for Anti-Pollution Skin Defense
5. Anti-Pollution Ingredients from Algae
6. Anti-Pollution Ingredients from Spermatophytae
6.1. Eriodictyon Californicum
6.2. Camellia Sinensis
6.3. Marrubium Vulgare
6.4. Schinus Molle
6.5. Camellia Japonica
6.6. Schisandra Chinensis
7. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Krutman, J.; Liu, W.; Li, L.; Pan, X.; Crawford, M.; Sore, G.; Seite, S. Pollution and skin: From epidemiological and mechanistic studies to clinical implications. J. Dermatol. Sci. 2014, 76, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.A. Particulate air pollution, systemic oxidative stress, inflammation and atherosclerosis. Air Qual. Atmos. Health 2011, 4, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Van Donkelaar, A.; Martin, R.V.; Brauer, M.; Kahn, R.; Levy, R.; Verduzco, C.; Villeneuve, P.J. Global estimates of ambient fine particulate matter concentrations from satellite-based aerosol optical depth: Development and application. Environ. Health Perspect. 2010, 118, 847–855. [Google Scholar] [CrossRef] [PubMed]
- WHO’s Ambient Air Pollution Database—Update 2014. Available online: http://www.who.int/phe/health_topics/outdoorair/databases/AAP_database_results_2014.pdf (accessed on 31 January 2018).
- Lee, B.-J.; Kin, B.; Lee, K. Air pollution exposure and cardiovascular disease. Toxicol. Res. 2014, 30, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Genc, S.; Zadeoglulari, Z.; Fuss, S.H.; Genc, K. The adverse effects of air pollution on the nervous system. J. Toxicol. 2012, 2012, 23. [Google Scholar] [CrossRef] [PubMed]
- Ghorani-Azam, A.; Riahi-Zanjani, B.; Balali-Mood, M. Effects of air pollution on human health and practical measures for prevention in Iran. J. Res. Med. Sci. 2016, 21, 65. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Nel, A. The role of reactive oxygen species and oxidative stress in mediating particulate matter injury. Clin. Occup. Environ. Med. 2006, 5, 817–836. [Google Scholar] [PubMed]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xia, T.; Nel, A. The role of the oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic. Biol. Med. 2008, 44, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Sioutas, C.; Cho, A.; Schmitz, D.; Misra, C.; Sempf, J.; Wang, M.; Oberley, T.; Froines, J.; Nel, A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003, 111, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Justiniano, R.; Wondrack, G.T. The aryl hydrocarbon receptor (AhR) as an environmental stress sensor and regulator of skin barrier function: Molecular mechanisms and therapeutic opportunities. In Skin Stress Response Pathways: Environmental Factors and Molecular Opportunities; Wondrack, G.T., Ed.; Springer: Cham, Switzerland, 2016; p. 16. [Google Scholar]
- Pan, T.-L.; Wang, P.-W.; Aljuffali, I.A.; Huang, C.-T.; Lee, C.-W.; Fang, J.-Y. The impact of urban particulate pollution on skin barrier function and the subsequent drug absorption. J. Dermatol. Sci. 2015, 78, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Badouin, C.; Charveron, M.; Tarroux, R.; Gall, Y. Environmental pollutants and skin cancer. Cell Biol. Toxicol. 2002, 18, 341–348. [Google Scholar] [CrossRef]
- Puri, P.; Nandar, S.K.; Kathuria, S.; Ramesh, V. Effects of air pollution on the skin: A review. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 415–423. [Google Scholar] [PubMed]
- Valacchi, G.; Sticozzi, C.; Belmonte, G.; Cervellati, F.; Demaude, J.; Chen, N.; Krol, Y.; Oresajo, C. Vitamin C compound mixtures prevent ozone-induced oxidative damage in human keratinocytes as initial assessment of pollution protection. PLoS ONE 2015, 10, e0131097. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Abu Zaid, M.; Pelle, E.; Khan, N.; Syed, D.N.; Matsui, M.S.; Maes, D. Aryl hydrocarbon receptor is an ozone sensor in human skin. J. Investig. Dermatol. 2009, 129, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.J.; Traber, M.G.; Podda, M.; Tsang, K.; Cross, C.E.; Packer, L. Ozone depletes tocopherols and tocotrienols topically applied on murine skin. FEBS Lett. 1997, 401, 167–170. [Google Scholar] [CrossRef]
- Weber, S.U.; Thiele, J.J.; Cross, C.E.; Packer, L. Vitamin C, uric acid and glutathione gradients in murine stratum corneum and their susceptibility to ozone exposure. J. Investig. Dermatol. 1999, 113, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- He, Q.C.; Tavakkol, A.; Wietecha, K.; Begum-Gafur, R.; Ansari, S.A.; Polefkat, T. Effects of environmentally realistic levels of ozone on stratum corneum function. Int. J. Cosmet. Sci. 2006, 28, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Pagnin, E.; Corbacho, A.M.; Olano, E.; Davis, P.A.; Packer, L.; Cross, C.E. In vivo ozone exposure induces antioxidant/stress-related responses in murine lung and skin. Free Radic. Biol. Med. 2004, 36, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Sticozzi, C.; Pecorelli, A.; Cervellati, F.; Cervellati, C.; Maioli, E. Cutaneous responsiveness to environmental stressors. Ann. N. Y. Acad. Sci. 2012, 1271, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Muresan, X.M.; Sticozzi, C.; Belmonte, G.; Pecorelli, A.; Cervellati, F.; Demaude, J.; Krol, Y.; Oresajo, C. Ozone-induced damage in 3D-skin model is prevented by topical vitamin C and vitamin E compound mixtures application. J. Dermatol. Sci. 2016, 82, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Poljšak, B.; Dahamane, R. Free radicals and extrinsic skin aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef] [PubMed]
- Portugal-Cohen, M.; Oron, M.; Cohen, D.; Ma’or, Z. Antipollution skin protection—A new paradigm and its demonstration on two active compounds. Clin. Cosmet. Investig. Dermatol. 2017, 10, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Vanzo, A.C.; Torloni, L.B.O.; Lage, R.; Pereira, A.F.C.; Mercuri, M.; Francielli Pereira, A.; da Silva, M.S.; Facchini, G.; Eberlin, S. Photoprotective and antipollution assessment of a skin care formulation. J. Am. Acad. Dermatol. 2017, 76 (Suppl. 1), AB197. [Google Scholar] [CrossRef]
- Vierkötter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Krämer, U.; Krutman, J. Airborne particle exposure and extrinsic skin aging. J. Investig. Dermatol. 2010, 130, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, M.-A.; Pham, D.-M.; Boussouira, B.; Bernard, D.; Camus, C.; Nguyen, Q.-L. Evaluation of the impact of urban pollution on the quality of skin: A multicentre study in Mexico. Int. J. Cosmet. Sci. 2015, 37, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.-M.; Boussouira, B.; Moyal, D.; Nguyen, Q.L. Oxidization of squalene, a human skin lipid: A new and reliable marker of environmental pollution studies. Int. J. Cosmet. Sci. 2015, 37, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, E.-H.; Oh, I.; Jung, K.; Han, Y.; Cheong, H.-K.; Ahn, K. Symptoms of atopic dermatitis are influenced by outdoor air pollution. J. Allergy Clin. Immunol. 2013, 132, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K. The role of air pollutants in atopic dermatitis. Clin. Rev. Allergy Immunol. 2014, 134, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Kim, K. Influences of environmental chemicals on atopic dermatitis. Toxicol. Res. 2015, 31, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Dorni, A.I.C.; Amalraj, A.; Gopi, S.; Varma, K.; Anjana, S.N. Novel cosmeceuticals from plants—An industry guided review. J. Appl. Res. Med. Aromat. Plants 2017, 7, 1–26. [Google Scholar]
- Wang, H.-M.; Chen, C.-C.; Huynh, P.; Chang, J.-S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.D.; Li, X.-C.; Lee, D.-J.; Chang, J.-S. Potential biomedical applications of marine algae. Bioresour. Technol. 2017, 244, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Ariede, M.B.; Candido, T.M.; Morocho Jacome, A.L.; Robles Velasco, M.V.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.-K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Escrig, A.; Jiménez-Jiménez, I.; Pulido, R.; Saura-Calixto, F. Antioxidant activity of fresh and processed edible seaweeds. J. Sci. Food Agric. 2001, 81, 530–534. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Walsh, N.A. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem. Toxicol. 2006, 44, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Dominguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, N.; Smyth, T.J.; Soler-Villa, A.; Fitzgerald, R.J.; Brunton, N.P. Phenolic content and antioxidant activity of fractions obtained from selected Irish macroalgae species (Laminaria digitata, Fucus serratus, Gracilaria gracilis and Codium fragile). J. Appl. Phycol. 2015, 27, 519–530. [Google Scholar] [CrossRef]
- Sathya, R.; Kanaga, N.; Sankar, P.; Jeeva, S. Antioxidant properties of phlorotannins from brown seaweed Cystoseira trinodis (Forsskål) C. Agardh. Arab. J. Chem. 2017, 10, S2608–S2614. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Kim, S.-K. Sulfated polysaccharides as bioactive agents from marine algae. Int. J. Biol. Macromol. 2013, 62, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Pangestuti, R.; Kim, S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Yaich, H.; Amira, A.B.; Abbes, F.; Bouaziz, M.; Besbes, S.; Richel, A.; Blecker, C.; Attia, H.; Garna, H. Effect of extraction procedures on structural, thermal and antioxidant properties of ulvan from Ulva lactuca collected in Monastir coast. Int. J. Biol. Macromol. 2017, 105, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R.; Zhang, H.; Niw, X.; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharides extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Sun, Y. Antioxidant activity of high sulfate content derivatives of ulvan in hyperlipidemic rats. Int. J. Biol. Macromol. 2015, 76, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Macquairre, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.-K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Aida, W.M.W.; Maskat, M.Y.; Mamot, S.; Ropien, J.; Mohd, D.M. Isolation and antioxidant capacity of fucoidan from selected Malaysian seaweeds. Food Hydrocoll. 2014, 42, 280–288. [Google Scholar] [CrossRef]
- Li, G.-Y.; Luo, Z.-C.; Yuan, F.; Yu, X.-B. Combined process of high-pressure homogenization and hydrothermal extraction of fucoidan with good antioxidant properties from Nemacystus decipients. Food Bioprod. Process. 2017, 106, 35–42. [Google Scholar] [CrossRef]
- Phull, A.-R.; Majid, M.; Haq, I.; Khan, M.R.; Kim, S.J. In vitro and in vivo evaluation of anti-arthritic, antioxidant efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol. 2017, 97, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Park, K.S.; Ku, M.J.; Lee, M.S.; Jeong, S.H.; Imbs, T.I.; Zvyagintseva, T.N.; Ermakova, S.P.; Lee, Y.H. Effect of Costaria costata fucoidan on expression of matrix metalloproteinase-1 promoter, mRNA, and protein. J. Nat. Prod. 2009, 72, 1731–1734. [Google Scholar] [CrossRef] [PubMed]
- Le Tutour, B.; Benslimane, F.; Gouleau, M.P.; Gouygou, J.P.; Saadan, B.; Quemeneur, F. Antioxidant and pro-oxidant activities of the brown algae, Laminaria digitata, Himanthalia elongata, Fucus vesiculosus, Fucus serratus and Ascophyllum nodosum. J. Appl. Phycol. 1998, 10, 121–129. [Google Scholar] [CrossRef]
- Cox, S.; Abu-Ghannam, N.; Gupta, S. An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. Int. Food Res. J. 2010, 17, 205–220. [Google Scholar]
- Rajauria, G.; Jaiswal, A.K.; Abu-Ghannam, N.; Gupta, S. Effect of hydrothermal processing on colour, antioxidant and free radical scavenging capacities of edible brown seaweeds. Int. J. Food Sci. Technol. 2010, 45, 2485–2493. [Google Scholar] [CrossRef]
- Blanco-Pascual, N.; Montero, M.P.; Gómez-Guillén, M.C. Antioxidant film development from unrefined extracts of brown seaweeds Laminaria digitata and Ascophyllum nodosum. Food Hydrocoll. 2014, 37, 100–110. [Google Scholar] [CrossRef]
- Hu, T.; Liu, D.; Chen, Y.; Wu, J.; Wang, S. Antioxidant activity of sulfated polysaccharide fractions extracted from Undaria pinnatifida in vitro. Int. J. Biol. Macromol. 2010, 46, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.; Hamid, N.; Lu, J. Fucoxanthin content and antioxidant properties of Undaria pinnatifida. Food Chem. 2013, 136, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Colliec, S.; Boisson-Vidal, C.; Jozefonvicz, J. A low molecular weight fucoidan fraction from the brown seaweed Pelvetia canaliculata. Phytochemistry 1994, 35, 697–700. [Google Scholar] [CrossRef]
- Hupel, M.; Lecointre, C.; Meudec, A.; Poupart, N.; Gall, E.A. Comparison of photoprotective responses to UV radiation in the brown seaweed Pelvetia canaliculata and the marine angiosperm Salicornia ramosissima. J. Exp. Mar. Biol. Ecol. 2011, 401, 36–47. [Google Scholar] [CrossRef]
- Bhatangar, A.; Vilar, V.J.P.; Santos, J.C.; Botelho, C.M.S.; Boaventura, R.A.R. Valorisation of marine Pelvetia canaliculata Ochrophyta for separation and recovery of nickel from water: Equilibrium and kinetics modeling on Na-loaded algae. Chem. Eng. J. 2012, 200–202, 365–372. [Google Scholar] [CrossRef]
- Hackbarth, F.V.; Girardi, F.; De Souza, S.M.; De Souza, A.A.U.; Boaventura, R.A.R.; Vilar, V.J.P. Marine macroalgae Pelvetia canaliculata (Phaeophyceae) as a natural cation exchanger for cadmium and lead ions separation in aqueous solutions. Chem. Eng. J. 2014, 242, 294–305. [Google Scholar] [CrossRef]
- Hackbarth, F.V.; Maass, D.; De Souza, A.A.U.; Vilar, V.J.P.; De Souza, S.M.A.G.U. Removal of hexavalent chromium from electroplating wastewaters using marine macroalga Pelvetia canaliculata as natural electron donor. Chem. Eng. J. 2016, 290, 477–489. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.; Domínguez, R.; Carballo, J.; Franco, D.; Lorenzo, J.M. Proximate composition, phenolic content and in vitro antioxidant activity of aqueous extracts of the seaweeds Ascophyllum nodosum, Bifurcaria bifurcata and Fucus vesiculosus. Effect of addition of the extracts on the oxidative stability of canola oil under accelerated storage conditions. Food Res. Int. 2017, 99, 986–994. [Google Scholar] [PubMed]
- Abu, R.; Jiang, Z.; Ueno, M.; Okimura, T.; Yamaguchi, K.; Oda, T. In vitro antioxidant activities of sulfated polysaccharide ascophyllan isolated from Ascophyllum nodosum. Int. J. Biol. Macromol. 2013, 59, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Breton, F.; Cérantola, S.; Gall, E.A. Distribution and radical scavenging activity of phenols in Ascophyllum nodosum (Phaeophyceae). J. Exp. Mar. Biol. Ecol. 2011, 399, 167–172. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.; Franco, D.; Domínguez, R.; Carballo, J.; Lorenzo, J.M. Phenolic compounds from three brown seaweed species using LC-DAD-ESI-MS/MS. Food Res. Int. 2017, 99, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Invincity. The Secret Weapon against Polluaging. Available online: http://www.in-cosmetics.com/__novadocuments/230150?v=635950341834400000 (accessed on 31 January 2018).
- Margret, R.J.; Kumaresan, S.; Ravikumar, S. A preliminary study on the anti-inflammatory activity of methanol extract of Ulva lactuca in rat. J. Environ. Biol. 2009, 30, 899–902. [Google Scholar] [PubMed]
- Hassan, S.; Abd El-Twab, S.; Hetta, M.; Mahmoud, B. Improvement of lipid profile and antioxidant of hypercholesterolemic albino rats by polysaccharides extracted from the green alga Ulva lactuca Linnaeus. Saudi J. Biol. Sci. 2011, 18, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.D. Flavonoid aglycones from Eriodictyon californicum resin and their implication for herbivory and UV screening. Biochem. Syst. Ecol. 1983, 11, 211–215. [Google Scholar] [CrossRef]
- Fletcher, J.N.; Kinghorn, A.D.; Slack, J.P.; Scott McCluskey, T.; Odley, A.; Jia, Z. In vitro evaluation of flavonoids from Eriodictyon californicum for antagonist activity against the bitterness receptor hTAS2R31. J. Agric. Food Chem. 2011, 59, 13117–13121. [Google Scholar] [CrossRef] [PubMed]
- Rossato, M.F.; Trevisan, G.; Banderó Walker, C.I.; Klafke, J.Z.; de Oliveira, A.P.; Gomes Villarinho, J.; Basso Zanon, R.; Freire Royes, L.F.; Athayde, M.L.; Gomez, M.V.; et al. Eriodictyol: A flavonoid antagonist of the TRPV1 receptor with antioxidant activity. Biochem. Pharmacol. 2011, 8, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Chobot, V.; Hadacek, F.; Bachmann, G.; Weckwerth, W.; Kubicova, L. Pro- and antioxidant activity of three selected flavan type flavonoids: Catechin, eriodictyol and taxifolin. Int. J. Mol. Sci. 2016, 17, 1986. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mo, H.; Zhao, L.; Gao, W.; Wang, S.; Cromie, M.M.; Lu, C.; Wang, J.-S.; Shen, C.-L. Therapeutic properties of green tea against environmental insults. J. Nutr. Biochem. 2017, 40, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.; Giménez, R.; Carmen-López, M. Determination of tea components with antioxidant activity. J. Agric. Food Chem. 2003, 51, 4427–4435. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K. Skin photoprotection by green tea: Antioxidant and immunomodulatory effects. Curr. Drug Targets Immune Endocr. Metab. Disord. 2003, 3, 234–242. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Bachrach, U. The specific anti-cancer activity of green tea (-)-epigallocatechin-3-gallate. Amino Acids 2002, 22, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Roginsky, V.; Alegria, A.E. Oxidation of tea extracts and tea catechins by molecular oxygen. J. Agric. Food Chem. 2005, 53, 4529–4535. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Bioavailable Polyphenols for Anti-Ageing Cosmetics. Personal Care Magazine Online 2011. Available online: https://www.personalcaremagazine.com/story/8392/bioavailable-polyphenols-for-anti-ageing-cosmetics (accessed on 31 January 2018).
- Prusakiewicz, J.J.; Ackerman, C.; Voorman, R. Comparison of skin esterase activities from different species. Pharm. Res. 2006, 23, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Karioti, A.; Protopappa, A.; Megoulas, N.; Skaltsa, H. Identification of tyrosinase inhibitors from Marrubium velutinum and Marrubium cylleneum. Bioorg. Med. Chem. 2007, 15, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Tepe, B.; Daferera, D.; Polissiou, M.; Harmandar, M. Studies on the antioxidant activity of the essential oil and methanol extract of Marrubium globosum subsp. globosum (Lamiaceae) by three different chemical assays. Bioresour. Technol. 2008, 99, 4239–4246. [Google Scholar] [CrossRef] [PubMed]
- Edziri, H.; Mastouri, M.; Aouni, M.; Verschaeve, L. Polyphenol content, antioxidant and antiviral activities of leaf extracts of Marrubium deserti growing in Tunisia. S. Afr. J. Bot. 2012, 80, 104–109. [Google Scholar] [CrossRef]
- Telek, E.; Töth, L.; Botz, L.; Máthé, I. Chemical tests with Marrubium species. Official data on Marrubii herba in Pharmacopoeia Hungarica VII. Acta Pharm. Hung. 1997, 67, 31–37. [Google Scholar] [PubMed]
- EMA. Assessment Report on Marrubium vulgare L., Herba. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2013/08/WC500147016.pdf (accessed on 31 January 2018).
- Ettaya, A.; Dhibi, S.; Samout, N.; Elfeki, A.; Hfaiedh, N. Hepatoprotective activity of white horehound (Marrubium vulgare) extract against cyclophosphamide toxicity in male rats. Can. J. Physiol. Pharmacol. 2016, 94, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Eldberry, A.A.; Harraz, F.M.; Ghareib, S.A.; Gabr, S.A.; Nagy, A.A.; Abdel-Sattar, E. Methanolic extract of Marrubium vulgare ameliorates hyperglicemia and dislipidemia in streptozotocin-induced diabetic rats. Int. J. Diabetes Mellit. 2015, 3, 37–44. [Google Scholar] [CrossRef]
- Masoodi, M.; Ahmad, B.; Zargar, I.M.; Khan, S.A.; Khan, S.; Sing, P. Antibacterial activity of whole plant extract of Marrubium vulgare. Afr. J. Biotechnol. 2008, 7, 86–87. [Google Scholar]
- Amessis-Ouchemoukh, N.; Madani, K.; Falé, P.L.V.; Serralheiro, M.L.; Araújo, M.E.M. Antioxidant capacity and phenolic contents of some Mediterranean medicinal plants and their potential role in the inhibition of cyclooxygenase-1 and acetylcholinesterase activities. Ind. Crops Prod. 2014, 53, 6–15. [Google Scholar] [CrossRef]
- Amessis-Ouchemoukh, N.; Abu-Reidah, I.A.; Quirantes-Piné, R.; Madani, K.; Segura-Carretera, A. Phytochemical profiling, in vitro evaluation of total phenolic contents and antioxidant properties of Marrubium vulgare (horehound) leaves of plants growing in Algeria. Ind. Crops Prod. 2014, 61, 120–129. [Google Scholar] [CrossRef]
- Boulila, A.; Sanaa, A.; Ben Salem, I.; Rokbeni, N.; M’rabet, Y.; Hosni, K.; Fernandez, X. Antioxidant properties and phenolic variation in wild populations of Marrubium vulgare L. (Lamiaceae). Ind. Crops Prod. 2015, 76, 616–622. [Google Scholar] [CrossRef]
- Bouterfas, K.; Mehdadi, Z.; Elaoufi, M.M.; Latreche, A.; Benchiha, W. Antioxidant activity and total phenolic and flavonoids content variations of leaves extracts of white Horehound (Marrubium vulgare Linné) from three geographical origins. Ann. Pharm. Fr. 2016, 74, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Pukalskas, A.; Rimantas Venskutonis, P.; Salido, S.; de Waard, P.; van Beek, T.A. Isolation, identification and activity of natural antioxidants from horehound (Marrubium vulgare L.) cultivated in Lithuania. Food Chem. 2012, 130, 695–701. [Google Scholar] [CrossRef]
- Popoola, O.K.; Elbagory, A.M.; Ameer, F.; Hussein, A.A. Marrubiin. Molecules 2013, 18, 9049–9060. [Google Scholar] [CrossRef] [PubMed]
- Sahpaz, S.; Garbacki, N.; Tits, M.; Bailleul, F. Isolation and pharmacological activity of phenilpropanoid esters from Marrubium vulgare. J. Ethopharmacol. 2002, 79, 389–392. [Google Scholar] [CrossRef]
- Seidel, V.; Verholle, M.; Malard, Y.; Tillequin, F.; Fruchart, J.-C.; Duriez, P.; Bailleul, F.; Teissier, E. Phenylpropanoids from Ballota nigra L. inhibit in vitro LDL peroxidation. Phytother. Res. 2000, 14, 93–98. [Google Scholar] [CrossRef]
- Martin-Nizard, F.; Sahpaz, S.; Furman, C.; Fruchart, J.-C.; Duriez, P.; Bailleul, F. Natural phenylpropanoids protect endothelial cells against oxidized LDL-induced cytotoxicity. Planta Med. 2003, 69, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Hosni, K.; Jemli, M.; Dziri, S.; M’rabet, Y.; Ennigrou, A.; Sgaier, A.; Casabianca, H.; Vulliet, E.; Ben Brahim, N.; Sebei, H. Changes in phytochemical, antimicrobial and free radical scavenging activities of the Peruvian pepper tree (Schinus molle L.) as influenced by fruit maturation. Ind. Crops Prod. 2011, 34, 1622–1628. [Google Scholar] [CrossRef]
- Bendaoud, H.; Romdhane, M.; Souchard, J.P.; Cazaux, S.; Bouajila, J. Chemical composition and antioxidant activities of Schinus molle L. and Schinus terebinthifolius Raddi berries essential oils. J. Food Sci. 2010, 75, C466–C472. [Google Scholar] [CrossRef] [PubMed]
- Do Rosário Martins, M.; Arantes, S.; Candeias, F.; Tinoco, M.T.; Cruz-Morais, J. Antioxidant, antimicrobial and toxicological properties of Schinus molle L. essential oils. J. Ethnopharmacol. 2014, 151, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Aranda, R.; Pérez-Lopez, L.A.; López-Arroyo, J.; Alanís-Garza, B.A.; Waksman de Torres, N. Antimicrobial and antioxidant activities of plants from Northeast of Mexico. J. Evid. Based Complement. Altern. Med. 2011, 2011, 536139. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, M.S.; Moharram, F.A.; Haggag, E.G.; Ibrahim, M.T.; Badary, O.A. Antioxidant flavonol glycosides from Schinus molle. Phytother. Res. 2006, 20, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.I.; Yoo, E.S.; Koh, Y.S.; Kang, H.K.; Kim, J.; Kim, Y.J.; Kang, H.H.; Hyun, J.W. Antioxidant effects of the ethanol extracts from flower of Camellia japonica via scavenging of reactive oxygen species and induction of antioxidant enzymes. Int. J. Mol. Sci. 2011, 12, 2618–2630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-I.; Yin, C.-P.; Kong, L.-C.; Jiang, D.-H. Extraction optimisation, purification and major antioxidant component of red pigments extracted from Camellia japonica. Food Chem. 2011, 129, 660–664. [Google Scholar] [CrossRef]
- Akanda, M.R.; Park, B.-Y. Involvement of MAPK/NF-κB signal transduction pathways: Camellia japonica mitigates inflammation and gastric ulcer. Biomed. Pharmacother. 2017, 95, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Li, W.; Higai, K.; Takemoto, M.; Sasaki, T.; Onoda, T.; Suzuki, T.; Koike, K. Flavonoid glycosides from Japanese Camellia oil cakes and their inhibitory activity against advanced glycation end-products formation. J. Funct. Foods 2017, 35, 159–165. [Google Scholar] [CrossRef]
- Yoon, I.S.; Park, D.H.; Kim, J.E.; Yoo, J.C.; Bae, M.S.; Oh, D.S.; Shim, J.H.; Choi, C.Y.; An, K.W.; Kim, E.I.; et al. Identification of the biologically active constituents of Camellia japonica leaf and anti-hyperuricemic effect in vitro and in vivo. Int. J. Mol. Med. 2017, 39, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-H.; Cho, J.-Y.; Moon, J.-H.; Park, K.-H. Isolation and identification of antioxidative phenolic acids and flavonoid glycosides from Camellia japonica flowers. Hortic. Environ. Biotechnol. 2011, 52, 270–277. [Google Scholar] [CrossRef]
- Shin, S.; Kim, M.; Jung, E. Anti-aging effects of Camellia japonica flower extract on a pollutant-induced stress. J. Dermatol. Sci. 2016, 84, e138–e139. [Google Scholar] [CrossRef]
- Mocan, A.; Schafberg, M.; Crisan, G.; Rohn, S. Determination of lignans and phenolic components of Schisandra chinensis (Turcz.) Baill. Using HPLC-ESI-ToF-MS and HPLC-online TEAC: Contribution of individual components to overall antioxidant activity and comparison with traditional antioxidant assays. J. Funct. Foods 2016, 24, 579–594. [Google Scholar] [CrossRef]
- Cheng, Z.; Cheng, L.; Song, H.; Yu, L.; Zhong, F.; Shen, Q.; Hu, H. Aqueous two-phase system for preliminary purification of lignans from fruits of Schisandra chinensis Baill. Sep. Purif. Technol. 2016, 166, 16–25. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, H.; Tian, J.; Chen, S. Antioxidant and antiproliferative activities of five compounds from Schisandra chinensis fruit. Ind. Crops. Prod. 2013, 50, 690–693. [Google Scholar] [CrossRef]
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Crisan, G.; Vlase, L.; Crisan, O.; Vodnar, D.C.; Raita, O.; Gheldiu, A.-M.; Toiu, A.; Oprean, R.; Tilea, I. Comparative studies on polyphenolic composition, antioxidant and antimicrobial activities of Schisandra chinensis leaves and fruits. Molecules 2014, 19, 15162–15179. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.Y.; Hung, T.M.; Bae, K.H.; Shin, E.M.; Zhou, H.Y.; Hong, Y.N.; Kang, S.S.; Kim, H.P.; Kim, Y.S. Anti-inflammatory effects of schisandrin isolated from the fruit of Schisandra chinensis Baill. Eur. J. Pharmacol. 2008, 591, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Checker, R.; Patwardhan, R.S.; Sharma, D.; Menon, J.; Thoh, M.; Bhilwade, H.N.; Konishi, T.; Sandur, S.K. Schisandrin B exhibits anti-inflammatory activity through modulation of the redox-sensitive transcription factors Nrf2 and NF-κB. Free Radic. Biol. Med. 2012, 53, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Lu, Y.; Yang, J.; Zhao, X.; Lu, X. Inhibitory effects of Schisandra chinensis extract on acne-related inflammation and UVB-induced photoageing. Pharm. Biol. 2016, 54, 2987–2994. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ulprospector.com/en/la/PersonalCare/Detail/2064/736210/URBALYS (accessed on 31 January 2018).

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juliano, C.; Magrini, G.A. Cosmetic Functional Ingredients from Botanical Sources for Anti-Pollution Skincare Products. Cosmetics 2018, 5, 19. https://doi.org/10.3390/cosmetics5010019
Juliano C, Magrini GA. Cosmetic Functional Ingredients from Botanical Sources for Anti-Pollution Skincare Products. Cosmetics. 2018; 5(1):19. https://doi.org/10.3390/cosmetics5010019
Chicago/Turabian StyleJuliano, Claudia, and Giovanni Antonio Magrini. 2018. "Cosmetic Functional Ingredients from Botanical Sources for Anti-Pollution Skincare Products" Cosmetics 5, no. 1: 19. https://doi.org/10.3390/cosmetics5010019
APA StyleJuliano, C., & Magrini, G. A. (2018). Cosmetic Functional Ingredients from Botanical Sources for Anti-Pollution Skincare Products. Cosmetics, 5(1), 19. https://doi.org/10.3390/cosmetics5010019