Macroalgae-Derived Ingredients for Cosmetic Industry—An Update
Abstract
:1. Introduction
1.1. Cosmetics Industry
1.2. Macroalgae in the World—Global Numbers
2. Macroalgae as a Source of Functional and Technological Ingredients
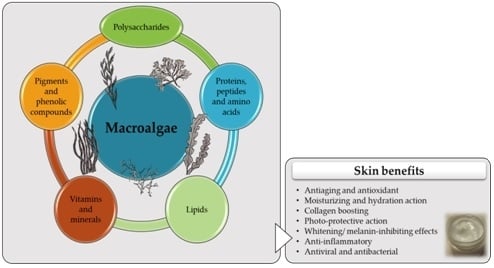
3. Macroalgae as a Source of Bioactive Skin Care Compounds
3.1. Polysaccharides
3.2. Proteins, Peptides, and Amino Acids
3.2.1. Protein
3.2.2. Peptides
3.2.3. Amino Acids
3.3. Lipids
3.4. Vitamins and Minerals
3.5. Pigments and Phenolic Compounds
4. Skin Benefits
4.1. Antiaging and Antioxidant Effects
4.2. Moisturizing/Hydration Action
4.3. Collagen-Boosting Effects
4.4. Photo-Protective Action
4.5. UV-Absorbing Compounds
4.6. Whitening/Melanin-Inhibiting Effects
4.7. Anti-Inflammatory Effect
4.8. Anti-Cellulite and Slimming Effects
4.9. Antiviral and Antibacterial Effects
5. Final Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Department of Economic and Social Affairs of the United Nations. Population Division World Population Prospects: The 2015 Revision, Key Findings and Advance Tables; Working Paper No. ESA/P/WP.241; Department of Economic and Social Affairs of the United Nations: New York, NY, USA, 2015.
- Sieck, G.C. Physiology in perspective: Aging and underlying pathophysiology. Physiology 2017, 32, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Chatterji, S.; Byles, J.; Cutler, D.; Seeman, T.; Verdes, E. Health, functioning, and disability in older adults—Present status and future implications. Lancet 2015, 385, 563–575. [Google Scholar] [CrossRef]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Baierle, M.; Nascimento, S.N.; Moro, A.M.; Brucker, N.; Freitas, F.; Gauer, B.; Durgante, J.; Bordignon, S.; Zibetti, M.; Trentini, C.M.; et al. Relationship between inflammation and oxidative stress and cognitive decline in the institutionalized elderly. Oxidative Med. Cell. Longev. 2015, 2015, 804198. [Google Scholar] [CrossRef] [PubMed]
- Brand, R.M.; Epperly, M.W.; Stottlemyer, J.M.; Skoda, E.M.; Gao, X.; Li, S.; Huq, S.; Wipf, P.; Kagan, V.E.; Greenberger, J.S. A topical mitochondria-targeted redox-cycling nitroxide mitigates oxidative stress-induced skin damage. J. Investig. Dermatol. 2017, 137, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Xia, N.; Li, H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Mathes, S.H.; Ruffner, H.; Graf-Hausner, U. The use of skin models in drug development. Adv. Drug Deliv. Rev. 2014, 69, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.; Yoong, C.; Robertson, T.A.; Soyer, H.P. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef] [PubMed]
- Franzen, L.; Windbergs, M. Applications of Raman spectroscopy in skin research—From skin physiology and diagnosis up to risk assessment and dermal drug delivery. Adv. Drug Deliv. Rev. 2015, 89, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.; Matts, P.; Anderson, C.; Roberts, M. Skin biology, xerosis, barrier repair and measurement. Drug Discov. Today Dis. Mech. 2008, 5, e127–e136. [Google Scholar] [CrossRef]
- Rawlings, A.V.; Matts, P.J. Stratum corneum moisturization at the molecular level: An update in relation to the dry skin cycle. J. Investig. Dermatol. 2005, 124, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Wendtner, M.-H.; Korting, H.C. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.-M.D.; Chen, C.-C.; Huynh, P.; Chang, J.-S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, G.J.; Antignac, E.; Re, T.; Toutain, H. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol. Appl. Pharmacol. 2010, 243, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Official Journal of the European Union. European Parliament, Regulation (EC) No 1223/2009 of the European Parliement and of the Council of 30 November 2009 on Cosmetic Products; European Union: Brussels, Belgium, 2009; pp. L-342/59–L-342/209. [Google Scholar]
- Vermeer, B.J.; Gilchrest, B.A.; Friedel, S.L. Cosmeceuticals: A proposal for rational definition, evaluation, and regulation. Arch. Dermatol. 1996, 132, 337–340. [Google Scholar] [CrossRef] [PubMed]
- FDA. Cosmeceutical. Available online: https://www.fda.gov/Cosmetics/Labeling/Claims/ucm127064.htm (accessed on 31 August 2017).
- Brandt, F.S.; Cazzaniga, A.; Hann, M. Cosmeceuticals: Current Trends and Market Analysis, Seminars in Cutaneous Medicine and Surgery, 2011; Frontline Medical Communications: Parsippany, NJ, USA; pp. 141–143.
- Kim, S.K. Marine cosmeceuticals. J. Cosmet. Dermatol. 2014, 13, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Zappelli, C.; Barbulova, A.; Apone, F.; Colucci, G. Effective active ingredients obtained through Biotechnology. Cosmetics 2016, 3, 39. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Seaweed Application in Cosmetics. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 423–441. [Google Scholar]
- Europe Cosmetics. Cosmetics and Personal Care Industry Overview. Available online: https://www.cosmeticseurope.eu/cosmetics-industry/ (accessed on 28 August 2017).
- Nunes, M.A.; Rodrigues, F.; Oliveira, M.B.P.P. Grape processing by-products as active ingredients for cosmetic proposes. In Handbook of Grape Processing By-Products; Galanakis, C.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 267–292. [Google Scholar]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Thornfeldt, C. Botanicals. Cosmetic Dermatology; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 267–280. Available online: https://www.wiley.com/en-us/Cosmetic+Dermatology%3A+Products+and+Procedures-p-9781444359510 (accessed on 25 December 2017).
- Harnedy, P.A.; FitzGerald, R.J. Bioactive proteins, peptides, and amino acids from macroalgae. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Bixler, H.J.; Porse, H. A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 2011, 23, 321–335. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. The State of World Fisheries and Aquaculture 2014; Fisheries and Aquaculture Department of the Food and Agricultural Organization of the United Nations: Rome, Italy, 2014.
- Kolanjinathan, K.; Ganesh, P.; Saranraj, P. Pharmacological importance of seaweeds: A review. World J. Fish Mar. Sci. 2014, 6, 1–15. [Google Scholar]
- Dhargalkar, V.; Pereira, N. Seaweed: Promising plant of the millennium. Sci. Cult. 2005, 71, 60–66. [Google Scholar]
- Pereira, L. A review of the nutrient composition of selected edible seaweeds. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Pomin, V.H., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 15–47. [Google Scholar]
- Baweja, P.; Kumar, S.; Sahoo, D.; Levine, I. Biology of Seaweeds. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 41–106. [Google Scholar]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.H.; Pereira, R.; Yarish, C.; Buschmann, A.H.; Sousa-Pinto, I. IMTA with Gracilaria vermiculophylla: Productivity and nutrient removal performance of the seaweed in a land-based pilot scale system. Aquaculture 2011, 312, 77–87. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Ortiz, J.; Uquiche, E.; Robert, P.; Romero, N.; Quitral, V.; Llantén, C. Functional and nutritional value of the Chilean seaweeds Codium fragile, Gracilaria chilensis and Macrocystis pyrifera. Eur. J. Lipid Sci. Technol. 2009, 111, 320–327. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Yarish, C.; Sousa-Pinto, I. The influence of stocking density, light and temperature on the growth, production and nutrient removal capacity of Porphyra dioica (Bangiales, Rhodophyta). Aquaculture 2006, 252, 66–78. [Google Scholar] [CrossRef]
- Lee, J.-C.; Hou, M.-F.; Huang, H.-W.; Chang, F.-R.; Yeh, C.-C.; Tang, J.-Y.; Chang, H.-W. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Kılınç, B.; Cirik, S.; Turan, G.; Tekogul, H.; Koru, E. Seaweeds for food and industrial applications. In Food Industry; Muzzalupo, I., Ed.; InTech: Rijeka, Croatia, 2013; pp. 735–748. [Google Scholar]
- Glicksman, M. Utilization of seaweed hydrocolloids in the food industry. Hydrobiologia 1987, 151, 31–47. [Google Scholar] [CrossRef]
- Personal Care—Global Portfolio. Available online: https://www.cargill.com/doc/1432075967907/personale-care-product-portfolio.pdf (accessed on 24 December 2017).
- SpecialChem—The Universal Selection Source: Cosmetics Ingredients. Available online: https://cosmetics.specialchem.com/ (accessed on 30 November 2017).
- SEPPIC. SEPPIC Launches XLYLISHINE™ the New Hair Repairing and Moisturizing Active Ingredient. 2017. Available online: https://worldnews.today/news/25/453/seppic-launches-a-new-hair-repairing-and-moisturizing-active-ingredient.html (accessed on 22 December 2017).
- Bowe, W.P.; Pugliese, S. Cosmetic benefits of natural ingredients. J. Drugs Dermatol. 2014, 13, 1021–1025. [Google Scholar] [PubMed]
- Agatonovic-Kustrin, S.; Morton, D. Cosmeceuticals derived from bioactive substances found in marine algae. Oceanography 2013, 1, 1–11. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Wijesekara, I.; Vo, T.-S.; Van Ta, Q.; Kim, S.-K. Marine food-derived functional ingredients as potential antioxidants in the food industry: An overview. Food Res. Int. 2011, 44, 523–529. [Google Scholar] [CrossRef]
- Athukorala, Y.; Kim, K.-N.; Jeon, Y.-J. Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem. Toxicol. 2006, 44, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Nepalia, A.; Singh, A.; Mathur, N.; Pareek, S. An overview of the harmful additives and contaminants possibly present in baby cosmetic products. Int. J. Chem. Sci. 2017, 15, 127. [Google Scholar]
- Jo, J.-H.; Kim, D.; Lee, S.; Lee, T.K. Total phenolic contents and biological activities of Korean seaweed extracts. Food Sci. Biotechnol. 2005, 14, 798–802. [Google Scholar]
- Da Costa, E.; Melo, T.; Moreira, A.; Bernardo, C.; Helguero, L.; Ferreira, I.; Cruz, M.; Rego, A.; Domingues, P.; Calado, R.; et al. Valorization of lipids from Gracilaria sp. through lipidomics and decoding of antiproliferative and anti-inflammatory activity. Mar. Drugs 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, C.L.F.; Falcão, D.S.; Lima, D.M.; Gedson, R.; Montenegro, D.A.; Lira, N.S.; De Athayde-Filho, P.F.; Rodrigues, L.C.; de Souza, M.D.V.; Barbosa-Filho, J.M.; et al. Bioactivities from marine algae of the genus Gracilaria. Int. J. Mol. Sci. 2011, 12, 4550–4573. [Google Scholar] [CrossRef] [PubMed]
- Le Lann, K.; Surget, G.; Couteau, C.; Coiffard, L.; Cérantola, S.; Gaillard, F.; Larnicol, M.; Zubia, M.; Guérard, F.; Poupart, N.; et al. Sunscreen, antioxidant, and bactericide capacities of phlorotannins from the brown macroalga Halidrys siliquosa. J. Appl. Phycol. 2016, 28, 3547–3559. [Google Scholar] [CrossRef]
- Siahaan, E.A.; Pangestuti, R.; Munandar, H.; Kim, S.-K. Cosmeceuticals properties of Sea Cucumbers: Prospects and trends. Cosmetics 2017, 4, 26. [Google Scholar] [CrossRef]
- Hardouin, K.; Burlot, A.-S.; Umami, A.; Tanniou, A.; Stiger-Pouvreau, V.; Widowati, I.; Bedoux, G.; Bourgougnon, N. Biochemical and antiviral activities of enzymatic hydrolysates from different invasive French seaweeds. J. Appl. Phycol. 2014, 26, 1029–1042. [Google Scholar] [CrossRef]
- De Souza, M.C.R.; Marques, C.T.; Dore, C.M.G.; da Silva, F.R.F.; Rocha, H.A.O.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Fleita, D.; El-Sayed, M.; Rifaat, D. Evaluation of the antioxidant activity of enzymatically-hydrolyzed sulfated polysaccharides extracted from red algae Pterocladia capillacea. Food Sci. Technol. 2015, 63, 1236–1244. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, F.; Wang, X.; Liu, X.; Hou, Y.; Zhang, Q. Extraction of the polysaccharides from five algae and their potential antioxidant activity in vitro. Carbohydr. Polym. 2010, 82, 118–121. [Google Scholar] [CrossRef]
- Leelapornpisid, P.; Mungmai, L.; Sirithunyalug, B.; Jiranusornkul, S.; Peerapornpisal, Y. A novel moisturizer extracted from freshwater macroalga [Rhizoclonium hieroglyphicum (C.Agardh) Kützing] for skin care cosmetic. Chiang Mai J. Sci. 2014, 41, 1195–1207. [Google Scholar]
- Choi, J.-S.; Moon, W.S.; Choi, J.N.; Do, K.H.; Moon, S.H.; Cho, K.K.; Han, C.-J.; Choi, I.S. Effects of seaweed Laminaria japonica extracts on skin moisturizing activity in vivo. J. Cosmet. Sci. 2013, 64, 193–209. [Google Scholar] [PubMed]
- Bedoux, G.; Hardouin, K.; Burlot, A.S.; Bourgougnon, N. Bioactive components from seaweeds: Cosmetic applications and future development. Adv. Bot. Res. 2014, 71, 345–378. [Google Scholar]
- Chojnacka, K.; Saeid, A.; Witkowska, Z.; Tuhy, L. Biologically active compounds in seaweed extracts—The prospects for the application. Open Conf. Proc. J. 2012, 3, 20–28. [Google Scholar] [CrossRef]
- Fleurence, J.; Ar Gall, E. Antiallergic Properties. In Seaweed in Health and Disease Prevention; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 389–406. [Google Scholar]
- Dumay, J.; Morançais, M. Proteins and Pigments. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 275–318. [Google Scholar]
- Sá, A.G.A.; Meneses, A.C.; Araújo, P.H.H.; Oliveira, D. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food. Sci. Technol. 2017, 69, 95–105. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed proteins. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Fonseca, P.; Carneiro, M.; Moreira, W. Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour. Technol. 2006, 97, 2402–2406. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, K.; Jeon, Y.-J. Bio-functionalities of proteins derived from marine algae—A review. Food Res. Int. 2012, 48, 948–960. [Google Scholar] [CrossRef]
- Malerich, S.; Berson, D. Next generation Cosmeceuticals. Dermatol. Clin. 2014, 32, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Seo, C.H.; Park, Y. Marine peptides and their anti-infective activities. Mar. Drugs 2015, 13, 618–654. [Google Scholar] [CrossRef] [PubMed]
- Schagen, S.K. Topical peptide treatments with effective anti-aging results. Cosmetics 2017, 4, 16. [Google Scholar] [CrossRef]
- Cardozo, K.H.; Marques, L.G.; Carvalho, V.M.; Carignan, M.O.; Pinto, E.; Marinho-Soriano, E.; Colepicolo, P. Analyses of photoprotective compounds in red algae from the Brazilian coast. Braz. J. Pharmacogn. 2011, 21, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.; Pandey, S.; Pathak, J.; Ahmed, H.; Singh, V.; Singh, S.P.; Sinha, R.P. Mycosporine-Like Amino Acids (MAAs) Profile of Two Marine Red Macroalgae, Gelidium sp. and Ceramium Sp. Int. J. Appl. Biotechnol. Biochem. 2017, 5, 12–21. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.; Jha, B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Misurcova, L.; Ambrozova, J.; Samek, D. Seaweed lipids as nutraceuticals. Adv. Food Nutr. Res. 2011, 64, 339–355. [Google Scholar] [PubMed]
- Tapiero, H.; Nguyen Ba, G.; Couvreur, P.; Tew, K.D. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed. Pharmacother. 2002, 56, 215–222. [Google Scholar] [CrossRef]
- Osman, N.A.R.; Abdo, B.; Mohamed, S.E.-T. Assessment of the nutritional value and native agar content of the red alga Gracilaria foliifera (Forsskal) Borgesen from the Red Sea coast of Sudan. J. Algal Biomass Utln. 2017, 8, 48–63. [Google Scholar]
- Kendel, M.; Wielgosz-Collin, G.; Bertrand, S.; Roussakis, C.; Bourgougnon, N.; Bedoux, G. Lipid composition, fatty acids and sterols in the seaweeds Ulva armoricana, and Solieria chordalis from Brittany (France): An analysis from nutritional, chemotaxonomic, and antiproliferative activity perspectives. Mar. Drugs 2015, 13, 5606–5628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegazi, M.M. Separation, identification and quantification of photosynthetic pigments from three red sea seaweeds using reversed-phase high-performance liquid chromatography. Egypt. J. Biol. 2002, 4, 1–6. [Google Scholar]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Lipids. Food Chemistry; Springer: Berlin, Germany, 2009; pp. 158–247. [Google Scholar]
- Lyons, N.M.; O’Brien, N.M. Modulatory effects of an algal extract containing astaxanthin on UVA-irradiated cells in culture. J. Dermatol. Sci. 2002, 30, 73–84. [Google Scholar] [CrossRef]
- Kelman, D.; Posner, E.K.; McDermid, K.J.; Tabandera, N.K.; Wright, P.R.; Wright, A.D. Antioxidant activity of Hawaiian marine algae. Mar. Drugs 2012, 10, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Aneiros, A.; Garateix, A. Bioactive peptides from marine sources: Pharmacological properties and isolation procedures. J. Chromatogr. B 2004, 803, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Sonani, R.R.; Singh, N.K.; Kumar, J.; Thakar, D.; Madamwar, D. Concurrent purification and antioxidant activity of phycobiliproteins from Lyngbya sp. A09DM: An antioxidant and anti-aging potential of phycoerythrin in Caenorhabditis elegans. Process Biochem. 2014, 49, 1757–1766. [Google Scholar] [CrossRef]
- Guillerme, J.-B.; Couteau, C.; Coiffard, L. Applications for marine resources in cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef]
- Lecas, S.; Boursier, E.; Fitoussi, R.; Vié, K.; Momas, I.; Seta, N.; Achard, S. In vitro model adapted to the study of skin ageing induced by air pollution. Toxicol. Lett. 2016, 259, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Farris, P.K. Natural ingredients and their applications in dermatology. In Practical Dermatology; Rick Ehrlich: New York, NY, USA, 2010; pp. 51–54. [Google Scholar]
- Pillai, S.; Cornell, M.; Oresajo, C. Part 1: Skin Physiology Pertinent to Cosmetic Dermatology. Epidermal barrier. In Cosmetic Dermatology—Products and Procedures; Draelos, Z.D., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2010; pp. 3–12. [Google Scholar]
- Wang, J.; Jin, W.; Hou, Y.; Niu, X.; Zhang, H.; Zhang, Q. Chemical composition and moisture-absorption/retention ability of polysaccharides extracted from five algae. Int. J. Biol. Macromol. 2013, 57, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Robert, L.; Labat-Robert, J.; Robert, A.-M. Physiology of skin aging. Pathol. Biol. 2009, 57, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- Heo, S.-J.; Jeon, Y.-J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B 2009, 95, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kindred, C.; Halder, R.M. Pigmentation and skin of color. In Cosmetic Dermatology; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 27–37. [Google Scholar]
- Cha, S.H.; Ko, S.C.; Kim, D.; Jeon, Y.J. Screening of marine algae for potential tyrosinase inhibitor: Those inhibitors reduced tyrosinase activity and melanin synthesis in zebrafish. J. Dermatol. 2011, 38, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Ko, S.-C.; Kang, S.-M.; Cha, S.-H.; Lee, S.-H.; Kang, D.-H.; Jung, W.-K.; Affan, A.; Oh, C.; Jeon, Y.-J. Inhibitory effect of diphlorethohydroxycarmalol on melanogenesis and its protective effect against UV-B radiation-induced cell damage. Food Chem. Toxicol. 2010, 48, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Senevirathne, M.; Ahn, C.-B.; Je, J.-Y. Enzymatic extracts from edible red algae, Porphyra tenera, and their antioxidant, anti-acetylcholinesterase, and anti-inflammatory activities. Food Sci. Biotechnol. 2010, 19, 1551–1557. [Google Scholar] [CrossRef]
- Rahimpour, Y.; Hamishehkar, H. Liposomes in cosmeceutics. Expert Opin. Drug Deliv. 2012, 9, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Al-Bader, T.; Byrne, A.; Gillbro, J.; Mitarotonda, A.; Metois, A.; Vial, F.; Rawlings, A.V.; Laloeuf, A. Effect of cosmetic ingredients as anticellulite agents: Synergistic action of actives with in vitro and in vivo efficacy. J. Cosmet. Dermatol. 2012, 11, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.S.; Choung, S.Y. Antiobesity effects of the ethanol extract of Laminaria japonica Areshoung in high-fat-diet-induced obese rat. J. Evid. Based Complement. Altern. Med. 2013, 2013, 492807. [Google Scholar]
- Westby, T.; Cadogan, A.; Duignan, G. In vivo uptake of iodine from a Fucus serratus Linnaeus seaweed bath: Does volatile iodine contribute? Environ. Geochem. Health 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Berardesca, E.; Abril, E.; Rona, C.; Vesnaver, R.; Cenni, A.; Oliva, M. An effective night slimming topical treatment. Int. J. Cosmet. Sci. 2012, 34, 263–272. [Google Scholar] [CrossRef] [PubMed]


| Ingredient | Species | Technological Function | Application | Reference |
|---|---|---|---|---|
| Carrageenan | Not specified | Thickening and gelling agent, binder, sensory enhancer | Bath and shower gel | [45] |
| Carrageenan | Not specified | Thickening and suspending agent, stabilizer, sensory enhancer | Skin care | [45] |
| Carrageenan | Not specified | Thickening and suspending agent, stabilizer, sensory enhancer | Sun care | [45] |
| Carrageenan | Not specified | Thickening agent, film former, fixative agent, sensory enhancer | Hair care | [45] |
| Alginate | Not specified | Interface vitalization | Shampoo | [45] |
| Carrageenan | Not specified | Thickening and suspending agent, stabilizer, binder | Oral care | [45] |
| Alginate | Not specified | Form retention | Dental moulds | [45] |
| Alginate | Not specified | Emulsification, viscosity | Lipstick | [45] |
| Gelcarin® PC 379 | Chondrus crispus | Exfoliant | Decorative cosmetic care applications | [46] |
| Gelcarin® PC 812 | Chondrus crispus | Emulsifier and thickener | Lipsticks and deodorants | [46] |
| Wakamine 1% (peptidic extract) | Undaria pinnatifida | Whitening/lightening agent | Skin care products | [46] |
| Wakamine XP | Undaria pinnatifida | Whitening/lightening agent | Skin care products | [46] |
| EPHEMER™ | Undaria pinnatifida | Antioxidant and anti-aging agent | Skin care products | [46] |
| Akomarine® Fucus | Fucus vesiculosus | Skin softness and elasticity | Slimming and anti-cellulitis cosmetic formulations | [46] |
| DENTACTIVE® | Fucus serratus | Protecting agent (reduces gingivorrhagia) | Oral-care products | [46] |
| Gracilaria Hydrogel | Gracilaria conferta | Humectant, nourishing and conditioning agent | Skin care products | [46] |
| Hijiki Extract | Hizikia Fusiforme | Whitening agent | Whitening preparations | [46] |
| Chlorofiltrat® Ulva HG | Ulva lactuca | Moisturizing and anti-inflammatory agent | Skin care products | [46] |
| AT UV PROTECTOR P | Porphyra tenera | Photo-protection | Skin and sun care | [46] |
| XYLISHINE ™ | Pelvetia canaliculata | Hair moisturizer | Hair formulations | [47] |
| Bioactive Compounds | Species | Assays | Health Benefits | Reference |
|---|---|---|---|---|
| Polysaccharides | ||||
| Sulphated oligosaccharides or polysaccharides | Solieria chordalis (1) | In vitro assays against the Herpes simplex virus in African green monkey kidney cells (Vero, ATCC CCL-81); cell viability study by neutral red assay, using Vero cell/HSV-1 model | Some fractions obtained from Solieria chordalis with good antiherpetic activities; no cytotoxicity observed | [59] |
| Sulphated polysaccharides (carrageenans) | Gigartina acicularis (1) Gigartina pistillata (1) Eucheuma cottonii (1) Euchema spinosa (1) | Antioxidant activity assays—superoxide anion and hydroxyl radical scavenge capacity, and in vitro study in liver microsomal lipid peroxidation | High antioxidant activity and free radical scavenging activity, especially shown by lambda carrageenan | [60] |
| Sulphated polysaccharides | Pterocladia capillacea (1) | In vitro assays for antioxidant capacity and antibacterial effect against Escherichia coli and Staphylococcus aureus | Antioxidant and antibacterial activity | [61] |
| Sulphated polysaccharides | Porphyra haitanensi (1) Laminaria japonica (2) Ulva pertusa (3) Enteromorpha linza (3) Bryopsis plumose (3) | Antioxidant activity assays—superoxide and hydroxyl radical scavenging effects, and reducing power | Antioxidant response is dependent on the type of polysaccharides, which differs among red, brown, and green species | [62] |
| Not specified | Ecklonia cava (2) | In vitro study in murine colon cancer cell line (CT-26), mouse melanoma cell line (B-16), hamster fibroblast cell line/normal cell line (V79-4) and human leukaemia cell lines (U-937 and THP-1) | Strong selective cell proliferation inhibition on all cancer cell lines tested, high antioxidant activity, and low cell toxicity | [51] |
| Sulphated polysaccharides: homofucans | Fucus vesiculosus (2) Padina gymnospora (2) | Antioxidant activity assays—superoxide anion and hydroxyl radical scavenge capacity, and in vitro study in liver microsomal lipid peroxidation | High antioxidant activity and free radical scavenging activity | [60] |
| Not specified | Rhizoclonium hieroglyphicum (3) | Moisturizing effect in pig skin model and in human skin (human volunteers) | Increased moisturizing effect, comparable to hyaluronic acid; no skin irritation observed | [63] |
| Not specified | Laminaria japonica (2) | In vivo skin moisturizing activity | Increased moisturizing skin effect | [64] |
| Sulphated oligosaccharides or polysaccharides | Ulva sp. (3) | In vitro assays against the Herpes simplex virus in African green monkey kidney cells (Vero, ATCC CCL-81); cell viability study by neutral red assay, using Vero cell/HSV-1 model | Some fractions obtained from Solieria chordalis with good antiherpetic activities; no cytotoxicity observed | [59] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimentel, F.B.; Alves, R.C.; Rodrigues, F.; P. P. Oliveira, M.B. Macroalgae-Derived Ingredients for Cosmetic Industry—An Update. Cosmetics 2018, 5, 2. https://doi.org/10.3390/cosmetics5010002
Pimentel FB, Alves RC, Rodrigues F, P. P. Oliveira MB. Macroalgae-Derived Ingredients for Cosmetic Industry—An Update. Cosmetics. 2018; 5(1):2. https://doi.org/10.3390/cosmetics5010002
Chicago/Turabian StylePimentel, Filipa B., Rita C. Alves, Francisca Rodrigues, and M. Beatriz P. P. Oliveira. 2018. "Macroalgae-Derived Ingredients for Cosmetic Industry—An Update" Cosmetics 5, no. 1: 2. https://doi.org/10.3390/cosmetics5010002
APA StylePimentel, F. B., Alves, R. C., Rodrigues, F., & P. P. Oliveira, M. B. (2018). Macroalgae-Derived Ingredients for Cosmetic Industry—An Update. Cosmetics, 5(1), 2. https://doi.org/10.3390/cosmetics5010002